Abstract
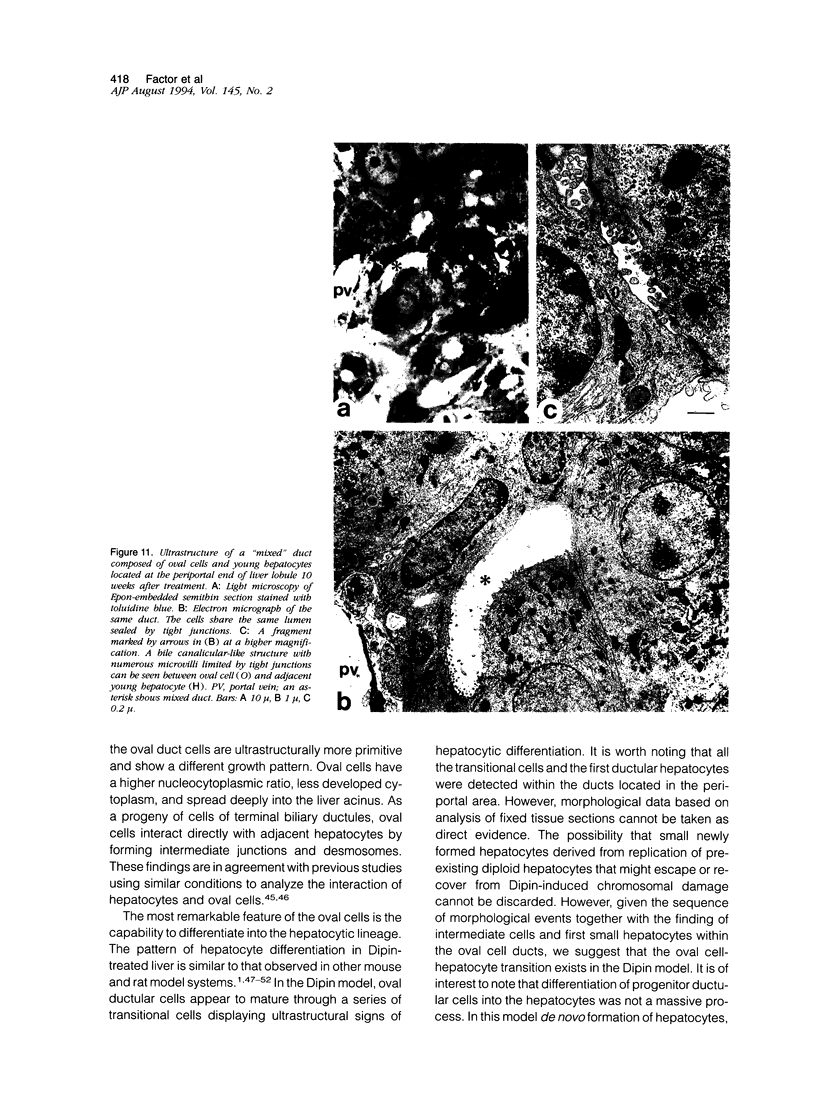
We have studied the development and differentiation of oval cells in the Dipin model of hepatocarcinogenesis in the mouse and compared this process to generation of biliary epithelial cells by bile duct ligation using light and electron microscopy. The Dipin model of hepatocarcinogenesis consists of a single injection of an alkylating drug, Dipin (1,4-bis[N,N'-di(ethylene)-phosphamide]-piperazine), followed by partial hepatectomy. The Dipin treatment resulted in irreversible damage and gradual death of hepatocytes by necrosis and apoptosis. Earlier work provided evidence that regeneration of parenchyma occurred via oval cell proliferation and subsequent differentiation into hepatocytes that replaced the degenerating hepatocytes. Both autoradiographic and morphological data indicated that oval cells were derived from ductular cells of Hering canals. The first oval cells labeled with [3H]thymidine were similar in size and ultrastructure to ductular cells of Hering canals with whom intracellular connections existed. The proliferation of ductular cells of Hering canals gave rise to a new system of oval cell ducts that spread into the liver acinus. In the periportal areas, the transition of oval cells into hepatocytes was observed inside the ducts. Both growth patterns and ultrastructure of oval cells were different from the biliary epithelial cells in bile duct-ligated liver. Also, oval cells retained the property to interact with adjacent hepatocytes through desmosomes and intermediate junctions. Oval cell population was heterogeneous in terms of proliferating potential. A proportion of proliferating cells (38 to 45%) in the Hering canals and small oval cell ducts located in the periportal areas was similar throughout the period of oval cell development. The extent of proliferation of oval cells decreased from 62% at the stage of active migration into the acinus to 22% at maximum formation of oval cell ducts. These data suggest that in the mouse liver cells of the terminal biliary ductules harbor the hepatic stem cell compartment from which oval cells, capable of differentiating into hepatocytes, may be derived.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennoun M., Rissel M., Engelhardt N., Guillouzo A., Briand P., Weber-Benarous A. Oval cell proliferation in early stages of hepatocarcinogenesis in simian virus 40 large T transgenic mice. Am J Pathol. 1993 Nov;143(5):1326–1336. [PMC free article] [PubMed] [Google Scholar]
- Carthew P., Edwards R. E., Hill R. J., Evans J. G. Cytokeratin expression in cells of the rodent bile duct developing under normal and pathological conditions. Br J Exp Pathol. 1989 Dec;70(6):717–725. [PMC free article] [PubMed] [Google Scholar]
- Coleman W. B., Wennerberg A. E., Smith G. J., Grisham J. W. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol. 1993 May;142(5):1373–1382. [PMC free article] [PubMed] [Google Scholar]
- Dempo K., Chisaka N., Yoshida Y., Kaneko A., Onoé T. Immunofluorescent study on alpha-fetoprotein-producing cells in the early stage of 3'-methyl-4-dimethylaminoazobenzene carcinogenesis. Cancer Res. 1975 May;35(5):1282–1287. [PubMed] [Google Scholar]
- Desmet V. J., Van Eyken P., Sciot R. Cytokeratins for probing cell lineage relationships in developing liver. Hepatology. 1990 Nov;12(5):1249–1251. doi: 10.1002/hep.1840120530. [DOI] [PubMed] [Google Scholar]
- Dunsford H. A., Maset R., Salman J., Sell S. Connection of ductlike structures induced by a chemical hepatocarcinogen to portal bile ducts in the rat liver detected by injection of bile ducts with a pigmented barium gelatin medium. Am J Pathol. 1985 Feb;118(2):218–224. [PMC free article] [PubMed] [Google Scholar]
- Dunsford H. A., Sell S. Production of monoclonal antibodies to preneoplastic liver cell populations induced by chemical carcinogens in rats and to transplantable Morris hepatomas. Cancer Res. 1989 Sep 1;49(17):4887–4893. [PubMed] [Google Scholar]
- Engelhardt N. V., Factor V. M., Yasova A. K., Poltoranina V. S., Baranov V. N., Lasareva M. N. Common antigens of mouse oval and biliary epithelial cells. Expression on newly formed hepatocytes. Differentiation. 1990 Oct;45(1):29–37. doi: 10.1111/j.1432-0436.1990.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Hu Z., Fujio K., Marsden E. R., Thorgeirsson S. S. Activation of hepatic stem cell compartment in the rat: role of transforming growth factor alpha, hepatocyte growth factor, and acidic fibroblast growth factor in early proliferation. Cell Growth Differ. 1993 Jul;4(7):555–561. [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Nakatsukasa H., Marsden E., Thorgeirsson S. S. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989 Mar 15;49(6):1541–1547. [PubMed] [Google Scholar]
- FARBER E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956 Feb;16(2):142–148. [PubMed] [Google Scholar]
- Factor V. M., Radaeva S. A. Oval cells--hepatocytes relationships in Dipin-induced hepatocarcinogenesis in mice. Exp Toxicol Pathol. 1993 Aug;45(4):239–244. doi: 10.1016/S0940-2993(11)80399-4. [DOI] [PubMed] [Google Scholar]
- Faktor V. M., Uryvaeva I. V., Sokolova A. S., Chernov V. A., Brodsky W. Y. Kinetics of cellular proliferation in regenerating mouse liver pretreated with the alkylating drug dipin. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;33(2):187–197. doi: 10.1007/BF02899181. [DOI] [PubMed] [Google Scholar]
- Faris R. A., Monfils B. A., Dunsford H. A., Hixson D. C. Antigenic relationship between oval cells and a subpopulation of hepatic foci, nodules, and carcinomas induced by the "resistant hepatocyte" model system. Cancer Res. 1991 Feb 15;51(4):1308–1317. [PubMed] [Google Scholar]
- Fausto N. Hepatocyte differentiation and liver progenitor cells. Curr Opin Cell Biol. 1990 Dec;2(6):1036–1042. doi: 10.1016/0955-0674(90)90153-6. [DOI] [PubMed] [Google Scholar]
- GRISHAM J. W., HARTROFT W. S. Morphologic identification by electron microscopy of "oval" cells in experimental hepatic degeneration. Lab Invest. 1961 Mar-Apr;10:317–332. [PubMed] [Google Scholar]
- GRISHAM J. W., PORTA E. A. ORIGIN AND FATE OF PROLIFERATED HEPATIC DUCTAL CELLS IN THE RAT: ELECTRON MICROSCOPIC AND AUTORADIOGRAPHIC STUDIES. Exp Mol Pathol. 1964 Jun;86:242–261. doi: 10.1016/0014-4800(64)90057-7. [DOI] [PubMed] [Google Scholar]
- Germain L., Blouin M. J., Marceau N. Biliary epithelial and hepatocytic cell lineage relationships in embryonic rat liver as determined by the differential expression of cytokeratins, alpha-fetoprotein, albumin, and cell surface-exposed components. Cancer Res. 1988 Sep 1;48(17):4909–4918. [PubMed] [Google Scholar]
- Germain L., Noël M., Gourdeau H., Marceau N. Promotion of growth and differentiation of rat ductular oval cells in primary culture. Cancer Res. 1988 Jan 15;48(2):368–378. [PubMed] [Google Scholar]
- Grisham J. W. Cell types in long-term propagable cultures of rat liver. Ann N Y Acad Sci. 1980;349:128–137. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- Hixson D. C., Faris R. A., Thompson N. L. An antigenic portrait of the liver during carcinogenesis. Pathobiology. 1990;58(2):65–77. doi: 10.1159/000163565. [DOI] [PubMed] [Google Scholar]
- Hsia C. C., Evarts R. P., Nakatsukasa H., Marsden E. R., Thorgeirsson S. S. Occurrence of oval-type cells in hepatitis B virus-associated human hepatocarcinogenesis. Hepatology. 1992 Dec;16(6):1327–1333. doi: 10.1002/hep.1840160604. [DOI] [PubMed] [Google Scholar]
- Iwasaki T., Dempo K., Kaneko A., Onoe T. Fluctuation of various cell populations and their characteristics during azo-dye carcinogenesis. Gan. 1972 Feb;63(1):21–30. [PubMed] [Google Scholar]
- Lemire J. M., Shiojiri N., Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol. 1991 Sep;139(3):535–552. [PMC free article] [PubMed] [Google Scholar]
- Lenzi R., Liu M. H., Tarsetti F., Slott P. A., Alpini G., Zhai W. R., Paronetto F., Lenzen R., Tavoloni N. Histogenesis of bile duct-like cells proliferating during ethionine hepatocarcinogenesis. Evidence for a biliary epithelial nature of oval cells. Lab Invest. 1992 Mar;66(3):390–402. [PubMed] [Google Scholar]
- Makino Y., Yamamoto K., Tsuji T. Three-dimensional arrangement of ductular structures formed by oval cells during hepatocarcinogenesis. Acta Med Okayama. 1988 Jun;42(3):143–150. doi: 10.18926/AMO/31029. [DOI] [PubMed] [Google Scholar]
- Marceau N., Blouin M. J., Germain L., Noel M. Role of different epithelial cell types in liver ontogenesis, regeneration and neoplasia. In Vitro Cell Dev Biol. 1989 Apr;25(4):336–341. doi: 10.1007/BF02624596. [DOI] [PubMed] [Google Scholar]
- Marceau N. Cell lineages and differentiation programs in epidermal, urothelial and hepatic tissues and their neoplasms. Lab Invest. 1990 Jul;63(1):4–20. [PubMed] [Google Scholar]
- Novikoff P. M., Ikeda T., Hixson D. C., Yam A. Characterizations of and interactions between bile ductule cells and hepatocytes in early stages of rat hepatocarcinogenesis induced by ethionine. Am J Pathol. 1991 Dec;139(6):1351–1368. [PMC free article] [PubMed] [Google Scholar]
- Ogawa K., Medline A., Farber E. Sequential analysis of hepatic carcinogenesis: the comparative architecture of preneoplastic, malignant, prenatal, postnatal and regenerating liver. Br J Cancer. 1979 Nov;40(5):782–790. doi: 10.1038/bjc.1979.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. J., Poucell S. Modern aspects of the morphology of viral hepatitis. Hum Pathol. 1981 Dec;12(12):1060–1084. doi: 10.1016/s0046-8177(81)80328-0. [DOI] [PubMed] [Google Scholar]
- Reid L. M. Stem cell biology, hormone/matrix synergies and liver differentiation. Curr Opin Cell Biol. 1990 Feb;2(1):121–130. doi: 10.1016/s0955-0674(05)80042-0. [DOI] [PubMed] [Google Scholar]
- SCHAFFNER F., POPPER H. Electron microscopic studies of normal and proliferated bile ductules. Am J Pathol. 1961 Apr;38:393–410. [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. N., Page D. L., McKusick K., Hnilica L. S. Cell specificity of rat cytokeratin p39 during azo dye-induced hepatocarcinogenesis. Carcinogenesis. 1985 Aug;6(8):1147–1153. doi: 10.1093/carcin/6.8.1147. [DOI] [PubMed] [Google Scholar]
- Sell S., Dunsford H. A. Evidence for the stem cell origin of hepatocellular carcinoma and cholangiocarcinoma. Am J Pathol. 1989 Jun;134(6):1347–1363. [PMC free article] [PubMed] [Google Scholar]
- Sell S. Is there a liver stem cell? Cancer Res. 1990 Jul 1;50(13):3811–3815. [PubMed] [Google Scholar]
- Sell S., Osborn K., Leffert H. L. Autoradiography of "oval cells" appearing rapidly in the livers of rats fed N-2-fluorenylacetamide in a choline devoid diet. Carcinogenesis. 1981;2(1):7–14. doi: 10.1093/carcin/2.1.7. [DOI] [PubMed] [Google Scholar]
- Sell S., Salman J. Light- and electron-microscopic autoradiographic analysis of proliferating cells during the early stages of chemical hepatocarcinogenesis in the rat induced by feeding N-2-fluorenylacetamide in a choline-deficient diet. Am J Pathol. 1984 Feb;114(2):287–300. [PMC free article] [PubMed] [Google Scholar]
- Sell S. The role of determined stem-cells in the cellular lineage of hepatocellular carcinoma. Int J Dev Biol. 1993 Mar;37(1):189–201. [PubMed] [Google Scholar]
- Shah K. D., Gerber M. A. Development of intrahepatic bile ducts in humans. Possible role of laminin. Arch Pathol Lab Med. 1990 Jun;114(6):597–600. [PubMed] [Google Scholar]
- Shiojiri N., Lemire J. M., Fausto N. Cell lineages and oval cell progenitors in rat liver development. Cancer Res. 1991 May 15;51(10):2611–2620. [PubMed] [Google Scholar]
- Sigal S. H., Brill S., Fiorino A. S., Reid L. M. The liver as a stem cell and lineage system. Am J Physiol. 1992 Aug;263(2 Pt 1):G139–G148. doi: 10.1152/ajpgi.1992.263.2.G139. [DOI] [PubMed] [Google Scholar]
- Spelman L. H., Thompson N. L., Fausto N., Miller K. R. A structural analysis of gap and tight junctions in the rat liver during a dietary treatment that induces oval cell proliferation. Am J Pathol. 1986 Nov;125(2):379–392. [PMC free article] [PubMed] [Google Scholar]
- Steiner J. W., Carruthers J. S. Studies on the Fine Structure of the Terminal Branches of the Biliary Tree: I. The Morphology of Normal Bile Canaliculi, Bile Pre-ductules (Ducts of Hering) and Bile Ductules. Am J Pathol. 1961 Jun;38(6):639–661. [PMC free article] [PubMed] [Google Scholar]
- Stosiek P., Kasper M., Karsten U. Expression of cytokeratin 19 during human liver organogenesis. Liver. 1990 Feb;10(1):59–63. doi: 10.1111/j.1600-0676.1990.tb00436.x. [DOI] [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Desmet V. Intrahepatic bile duct development in the rat: a cytokeratin-immunohistochemical study. Lab Invest. 1988 Jul;59(1):52–59. [PubMed] [Google Scholar]
- WILSON J. W., LEDUC E. H. Role of cholangioles in restoration of the liver of the mouse after dietary injury. J Pathol Bacteriol. 1958 Oct;76(2):441–449. doi: 10.1002/path.1700760213. [DOI] [PubMed] [Google Scholar]