Abstract
The extent of virus replication, tissue distribution, localization of virus within tissues, and the presence of pathological lesions was examined early after experimental infection of rhesus monkeys with simian immunodeficiency virus (SIV). Three strains of SIV were used: molecularly cloned pathogenic SIVmac239; molecularly cloned nonpathogenic SIVmac1A11; and uncloned pathogenic SIVmac. The major targets of infection in all animals at 2 weeks postinoculation were the thymus and spleen. The distribution of virus within lymphoid organs varied with the viral inoculum: nonpathogenic SIVmac1A11 was present primarily within lymphoid follicles and in the thymic cortex; SIVmac239 was present primarily within periarteriolar lymphoid sheaths in the spleen, the paracortex of lymph nodes, and the medulla of the thymus; uncloned SIVmac was present in all these areas but tended to parallel the distribution of SIVmac239. Animals inoculated with nonpathogenic SIVmac1A11 had fewer SIV-positive cells by in situ hybridization and after 13 weeks postinoculation, virus was undetectable in any tissue from these animals. No significant pathological abnormalities were recognized in animals inoculated with this nonpathogenic virus. In contrast, nearly half of the animals inoculated with either SIVmac or SIVmac239 developed significant pathological lesions, including opportunistic infections by 13 weeks postinoculation, highlighting the virulence of these viruses. Our results indicate marked differences in tissue distribution between pathogenic and nonpathogenic molecular clones of SIV during the acute phase of infection. The most striking differences were the absence of SIVmac1A11 from the central nervous system and thymic medulla. The prominent early involvement of the thymus suggests that infection of this organ is a key event in the induction of immune suppression by SIV.
Full text
PDF


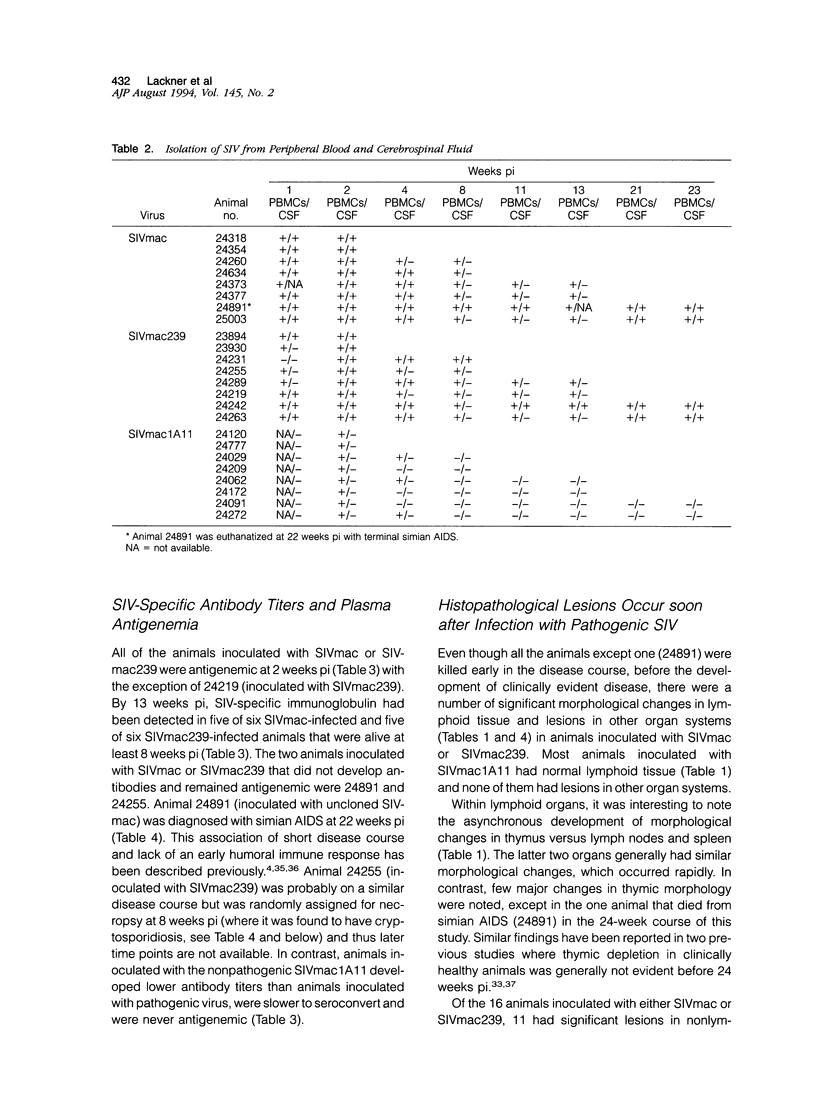
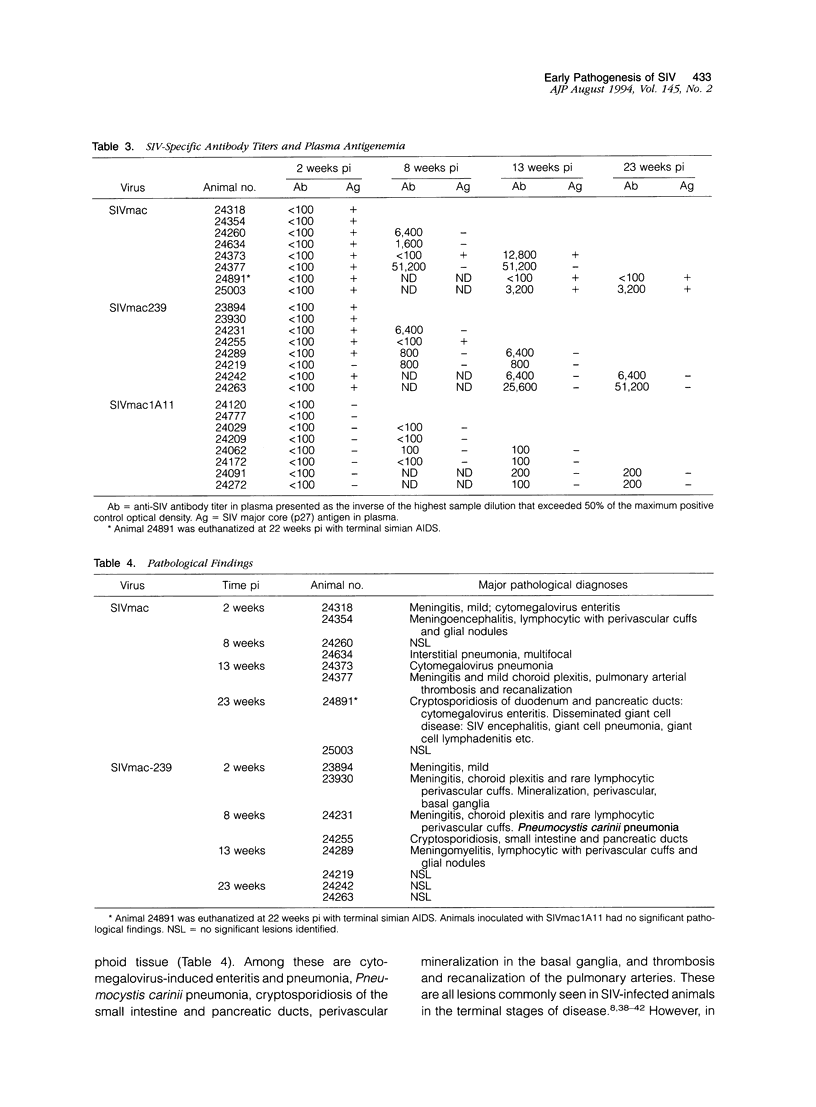
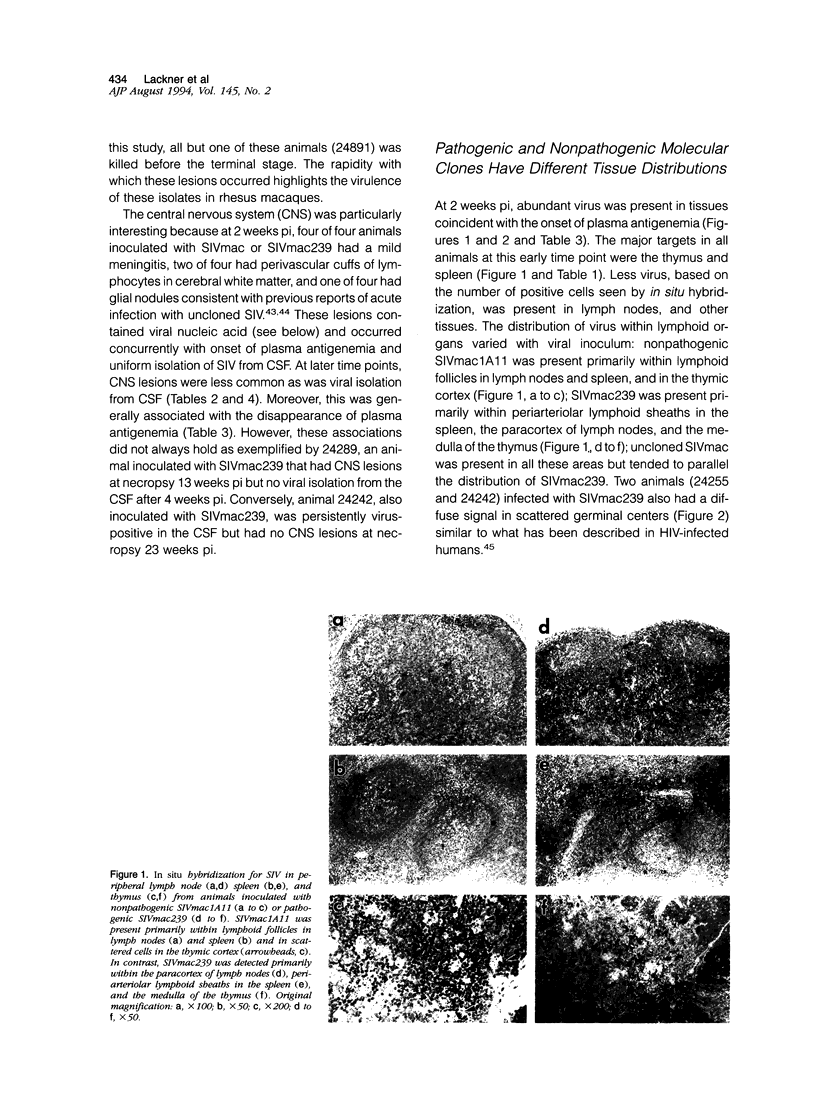




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrovandi G. M., Feuer G., Gao L., Jamieson B., Kristeva M., Chen I. S., Zack J. A. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993 Jun 24;363(6431):732–736. doi: 10.1038/363732a0. [DOI] [PubMed] [Google Scholar]
- Banapour B., Marthas M. L., Munn R. J., Luciw P. A. In vitro macrophage tropism of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). Virology. 1991 Jul;183(1):12–19. doi: 10.1016/0042-6822(91)90113-p. [DOI] [PubMed] [Google Scholar]
- Banapour B., Marthas M. L., Ramos R. A., Lohman B. L., Unger R. E., Gardner M. B., Pedersen N. C., Luciw P. A. Identification of viral determinants of macrophage tropism for simian immunodeficiency virus SIVmac. J Virol. 1991 Nov;65(11):5798–5805. doi: 10.1128/jvi.65.11.5798-5805.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville A., Ramsay A., Cranage M. P., Cook N., Cook R. W., Dennis M. J., Greenaway P. J., Kitchin P. A., Stott E. J. Histopathological changes in simian immunodeficiency virus infection. J Pathol. 1990 Sep;162(1):67–75. doi: 10.1002/path.1711620113. [DOI] [PubMed] [Google Scholar]
- Baskin G. B., Murphey-Corb M., Martin L. N., Davison-Fairburn B., Hu F. S., Kuebler D. Thymus in simian immunodeficiency virus-infected rhesus monkeys. Lab Invest. 1991 Oct;65(4):400–407. [PubMed] [Google Scholar]
- Baskin G. B., Murphey-Corb M., Martin L. N., Soike K. F., Hu F. S., Kuebler D. Lentivirus-induced pulmonary lesions in rhesus monkeys (Macaca mulatta) infected with simian immunodeficiency virus. Vet Pathol. 1991 Nov;28(6):506–513. doi: 10.1177/030098589102800607. [DOI] [PubMed] [Google Scholar]
- Baskin G. B., Murphey-Corb M., Watson E. A., Martin L. N. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet Pathol. 1988 Nov;25(6):456–467. doi: 10.1177/030098588802500609. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti L., Hurtrel M., Maire M. A., Vazeux R., Dormont D., Montagnier L., Hurtrel B. Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol. 1991 Dec;139(6):1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Chalifoux L. V., Simon M. A., Pauley D. R., MacKey J. J., Wyand M. S., Ringler D. J. Arteriopathy in macaques infected with simian immunodeficiency virus. Lab Invest. 1992 Sep;67(3):338–349. [PubMed] [Google Scholar]
- Cranage M. P., Polyanskaya N., McBride B., Cook N., Ashworth L. A., Dennis M., Baskerville A., Greenaway P. J., Corcoran T., Kitchin P. Studies on the specificity of the vaccine effect elicited by inactivated simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1993 Jan;9(1):13–22. doi: 10.1089/aid.1993.9.13. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., Sehgal P. K., Hunsmann G., Schmidt D. K., King N. W., Desrosiers R. C. Long-term persistent infection of macaque monkeys with the simian immunodeficiency virus. J Gen Virol. 1987 Dec;68(Pt 12):3183–3189. doi: 10.1099/0022-1317-68-12-3183. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Hansen-Moosa A., Mori K., Bouvier D. P., King N. W., Daniel M. D., Ringler D. J. Macrophage-tropic variants of SIV are associated with specific AIDS-related lesions but are not essential for the development of AIDS. Am J Pathol. 1991 Jul;139(1):29–35. [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- Fausto N. What's in a Name? The American Society for Investigative Pathology. Am J Pathol. 1993 Jan;142(1):1–1. [PMC free article] [PubMed] [Google Scholar]
- Fox C. H., Tenner-Rácz K., Rácz P., Firpo A., Pizzo P. A., Fauci A. S. Lymphoid germinal centers are reservoirs of human immunodeficiency virus type 1 RNA. J Infect Dis. 1991 Dec;164(6):1051–1057. doi: 10.1093/infdis/164.6.1051. [DOI] [PubMed] [Google Scholar]
- Heise C., Vogel P., Miller C. J., Lackner A., Dandekar S. Distribution of SIV infection in the gastrointestinal tract of rhesus macaques at early and terminal stages of AIDS. J Med Primatol. 1993 Feb-May;22(2-3):187–193. [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., Desrosiers R. C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991 May 17;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kestler H., Kodama T., Ringler D., Marthas M., Pedersen N., Lackner A., Regier D., Sehgal P., Daniel M., King N. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990 Jun 1;248(4959):1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- Lackner A. A., Dandekar S., Gardner M. B. Neurobiology of simian and feline immunodeficiency virus infections. Brain Pathol. 1991 Apr;1(3):201–212. doi: 10.1111/j.1750-3639.1991.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Lackner A. A., Smith M. O., Munn R. J., Martfeld D. J., Gardner M. B., Marx P. A., Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991 Sep;139(3):609–621. [PMC free article] [PubMed] [Google Scholar]
- Lohman B. L., Higgins J., Marthas M. L., Marx P. A., Pedersen N. C. Development of simian immunodeficiency virus isolation, titration, and neutralization assays which use whole blood from rhesus monkeys and an antigen capture enzyme-linked immunosorbent assay. J Clin Microbiol. 1991 Oct;29(10):2187–2192. doi: 10.1128/jcm.29.10.2187-2192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciw P. A., Shaw K. E., Unger R. E., Planelles V., Stout M. W., Lackner J. E., Pratt-Lowe E., Leung N. J., Banapour B., Marthas M. L. Genetic and biological comparisons of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). AIDS Res Hum Retroviruses. 1992 Mar;8(3):395–402. doi: 10.1089/aid.1992.8.395. [DOI] [PubMed] [Google Scholar]
- Marthas M. L., Banapour B., Sutjipto S., Siegel M. E., Marx P. A., Gardner M. B., Pedersen N. C., Luciw P. A. Rhesus macaques inoculated with molecularly cloned simian immunodeficiency virus. J Med Primatol. 1989;18(3-4):311–319. [PubMed] [Google Scholar]
- Marthas M. L., Miller C. J., Sutjipto S., Higgins J., Torten J., Lohman B. L., Unger R. E., Ramos R. A., Kiyono H., McGhee J. R. Efficacy of live-attenuated and whole-inactivated simian immunodeficiency virus vaccines against vaginal challenge with virulent SIV. J Med Primatol. 1992 Feb-May;21(2-3):99–107. [PubMed] [Google Scholar]
- Marthas M. L., Ramos R. A., Lohman B. L., Van Rompay K. K., Unger R. E., Miller C. J., Banapour B., Pedersen N. C., Luciw P. A. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed by using attenuated and pathogenic molecular clones of SIVmac. J Virol. 1993 Oct;67(10):6047–6055. doi: 10.1128/jvi.67.10.6047-6055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthas M. L., Sutjipto S., Higgins J., Lohman B., Torten J., Luciw P. A., Marx P. A., Pedersen N. C. Immunization with a live, attenuated simian immunodeficiency virus (SIV) prevents early disease but not infection in rhesus macaques challenged with pathogenic SIV. J Virol. 1990 Aug;64(8):3694–3700. doi: 10.1128/jvi.64.8.3694-3700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. J., Alexander N. J., Sutjipto S., Lackner A. A., Gettie A., Hendrickx A. G., Lowenstine L. J., Jennings M., Marx P. A. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989 Oct;63(10):4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Ringler D. J., Desrosiers R. C. Restricted replication of simian immunodeficiency virus strain 239 in macrophages is determined by env but is not due to restricted entry. J Virol. 1993 May;67(5):2807–2814. doi: 10.1128/jvi.67.5.2807-2814.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K., Ringler D. J., Kodama T., Desrosiers R. C. Complex determinants of macrophage tropism in env of simian immunodeficiency virus. J Virol. 1992 Apr;66(4):2067–2075. doi: 10.1128/jvi.66.4.2067-2075.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J. G., Krenn V., Schindler C., Czub S., Stahl-Hennig C., Coulibaly C., Hunsmann G., Kneitz C., Kerkau T., Rethwilm A. Alterations of thymus cortical epithelium and interdigitating dendritic cells but no increase of thymocyte cell death in the early course of simian immunodeficiency virus infection. Am J Pathol. 1993 Sep;143(3):699–713. [PMC free article] [PubMed] [Google Scholar]
- Naidu Y. M., Kestler H. W., 3rd, Li Y., Butler C. V., Silva D. P., Schmidt D. K., Troup C. D., Sehgal P. K., Sonigo P., Daniel M. D. Characterization of infectious molecular clones of simian immunodeficiency virus (SIVmac) and human immunodeficiency virus type 2: persistent infection of rhesus monkeys with molecularly cloned SIVmac. J Virol. 1988 Dec;62(12):4691–4696. doi: 10.1128/jvi.62.12.4691-4696.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namikawa R., Kaneshima H., Lieberman M., Weissman I. L., McCune J. M. Infection of the SCID-hu mouse by HIV-1. Science. 1988 Dec 23;242(4886):1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- Niu M. T., Stein D. S., Schnittman S. M. Primary human immunodeficiency virus type 1 infection: review of pathogenesis and early treatment intervention in humans and animal retrovirus infections. J Infect Dis. 1993 Dec;168(6):1490–1501. doi: 10.1093/infdis/168.6.1490. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Demarest J. F., Butini L., Montroni M., Fox C. H., Orenstein J. M., Kotler D. P., Fauci A. S. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature. 1993 Mar 25;362(6418):355–358. doi: 10.1038/362355a0. [DOI] [PubMed] [Google Scholar]
- Pantaleo G., Graziosi C., Fauci A. S. New concepts in the immunopathogenesis of human immunodeficiency virus infection. N Engl J Med. 1993 Feb 4;328(5):327–335. doi: 10.1056/NEJM199302043280508. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Barlough J. E. Clinical overview of feline immunodeficiency virus. J Am Vet Med Assoc. 1991 Nov 15;199(10):1298–1305. [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Regier D. A., Desrosiers R. C. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1221–1231. doi: 10.1089/aid.1990.6.1221. [DOI] [PubMed] [Google Scholar]
- Reimann K. A., Tenner-Racz K., Racz P., Montefiori D. C., Yasutomi Y., Lin W., Ransil B. J., Letvin N. L. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Virol. 1994 Apr;68(4):2362–2370. doi: 10.1128/jvi.68.4.2362-2370.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringler D. J., Wyand M. S., Walsh D. G., MacKey J. J., Chalifoux L. V., Popovic M., Minassian A. A., Sehgal P. K., Daniel M. D., Desrosiers R. C. Cellular localization of simian immunodeficiency virus in lymphoid tissues. I. Immunohistochemistry and electron microscopy. Am J Pathol. 1989 Feb;134(2):373–383. [PMC free article] [PubMed] [Google Scholar]
- Saha K., Wong P. K. Rudimentary thymus of SCID mouse plays an important role in the development of retrovirus-induced neurologic disorders. Virology. 1993 Jul;195(1):211–218. doi: 10.1006/viro.1993.1362. [DOI] [PubMed] [Google Scholar]
- Sasseville V. G., Newman W. A., Lackner A. A., Smith M. O., Lausen N. C., Beall D., Ringler D. J. Elevated vascular cell adhesion molecule-1 in AIDS encephalitis induced by simian immunodeficiency virus. Am J Pathol. 1992 Nov;141(5):1021–1030. [PMC free article] [PubMed] [Google Scholar]
- Sasseville V. G., Newman W., Brodie S. J., Hesterberg P., Pauley D., Ringler D. J. Monocyte adhesion to endothelium in simian immunodeficiency virus-induced AIDS encephalitis is mediated by vascular cell adhesion molecule-1/alpha 4 beta 1 integrin interactions. Am J Pathol. 1994 Jan;144(1):27–40. [PMC free article] [PubMed] [Google Scholar]
- Sharer L. R., Michaels J., Murphey-Corb M., Hu F. S., Kuebler D. J., Martin L. N., Baskin G. B. Serial pathogenesis study of SIV brain infection. J Med Primatol. 1991 Jun;20(4):211–217. [PubMed] [Google Scholar]
- Sheppard H. W., Ascher M. S., McRae B., Anderson R. E., Lang W., Allain J. P. The initial immune response to HIV and immune system activation determine the outcome of HIV disease. J Acquir Immune Defic Syndr. 1991;4(7):704–712. [PubMed] [Google Scholar]
- Simon M. A., Chalifoux L. V., Ringler D. J. Pathologic features of SIV-induced disease and the association of macrophage infection with disease evolution. AIDS Res Hum Retroviruses. 1992 Mar;8(3):327–337. doi: 10.1089/aid.1992.8.327. [DOI] [PubMed] [Google Scholar]
- Stanley S. K., McCune J. M., Kaneshima H., Justement J. S., Sullivan M., Boone E., Baseler M., Adelsberger J., Bonyhadi M., Orenstein J. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993 Oct 1;178(4):1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica G., Floyd E., Illanes O., Wong P. K. Temporal lymphoreticular changes caused by ts1, a paralytogenic mutant of Moloney murine leukemia virus TB. Lab Invest. 1992 Apr;66(4):427–436. [PubMed] [Google Scholar]
- Sutjipto S., Pedersen N. C., Miller C. J., Gardner M. B., Hanson C. V., Gettie A., Jennings M., Higgins J., Marx P. A. Inactivated simian immunodeficiency virus vaccine failed to protect rhesus macaques from intravenous or genital mucosal infection but delayed disease in intravenously exposed animals. J Virol. 1990 May;64(5):2290–2297. doi: 10.1128/jvi.64.5.2290-2297.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenner-Racz K., Racz P., Bofill M., Schulz-Meyer A., Dietrich M., Kern P., Weber J., Pinching A. J., Veronese-Dimarzo F., Popovic M. HTLV-III/LAV viral antigens in lymph nodes of homosexual men with persistent generalized lymphadenopathy and AIDS. Am J Pathol. 1986 Apr;123(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- Unger R. E., Marthas M. L., Pratt-Lowe E., Padrid P. A., Luciw P. A. The nef gene of simian immunodeficiency virus SIVmac1A11. J Virol. 1992 Sep;66(9):5432–5442. doi: 10.1128/jvi.66.9.5432-5442.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. T., Dolatshahi M. First updated and revised survey of worldwide HIV and SIV vaccine challenge studies in nonhuman primates: progress in first and second order studies. J Med Primatol. 1993 Feb-May;22(2-3):203–235. [PubMed] [Google Scholar]
- Wyand M. S., Ringler D. J., Naidu Y. M., Mattmuller M., Chalifoux L. V., Sehgal P. K., Daniel M. D., Desrosiers R. C., King N. W. Cellular localization of simian immunodeficiency virus in lymphoid tissues. II. In situ hybridization. Am J Pathol. 1989 Feb;134(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Yasutomi Y., Reimann K. A., Lord C. I., Miller M. D., Letvin N. L. Simian immunodeficiency virus-specific CD8+ lymphocyte response in acutely infected rhesus monkeys. J Virol. 1993 Mar;67(3):1707–1711. doi: 10.1128/jvi.67.3.1707-1711.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Y., Martin L. N., Watson E. A., Montelaro R. C., West M., Epstein L., Murphey-Corb M. Simian immunodeficiency virus/delta-induced immunodeficiency disease in rhesus monkeys: relation of antibody response and antigenemia. J Infect Dis. 1988 Dec;158(6):1277–1286. doi: 10.1093/infdis/158.6.1277. [DOI] [PubMed] [Google Scholar]