Abstract
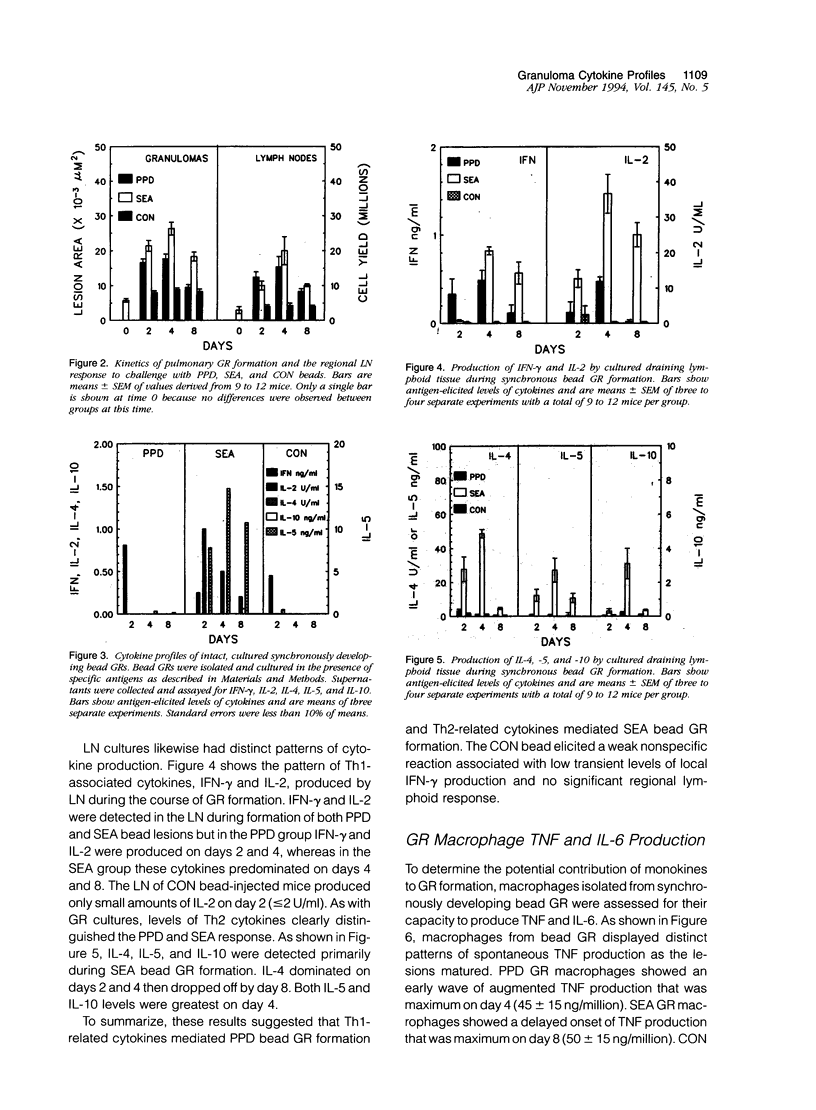
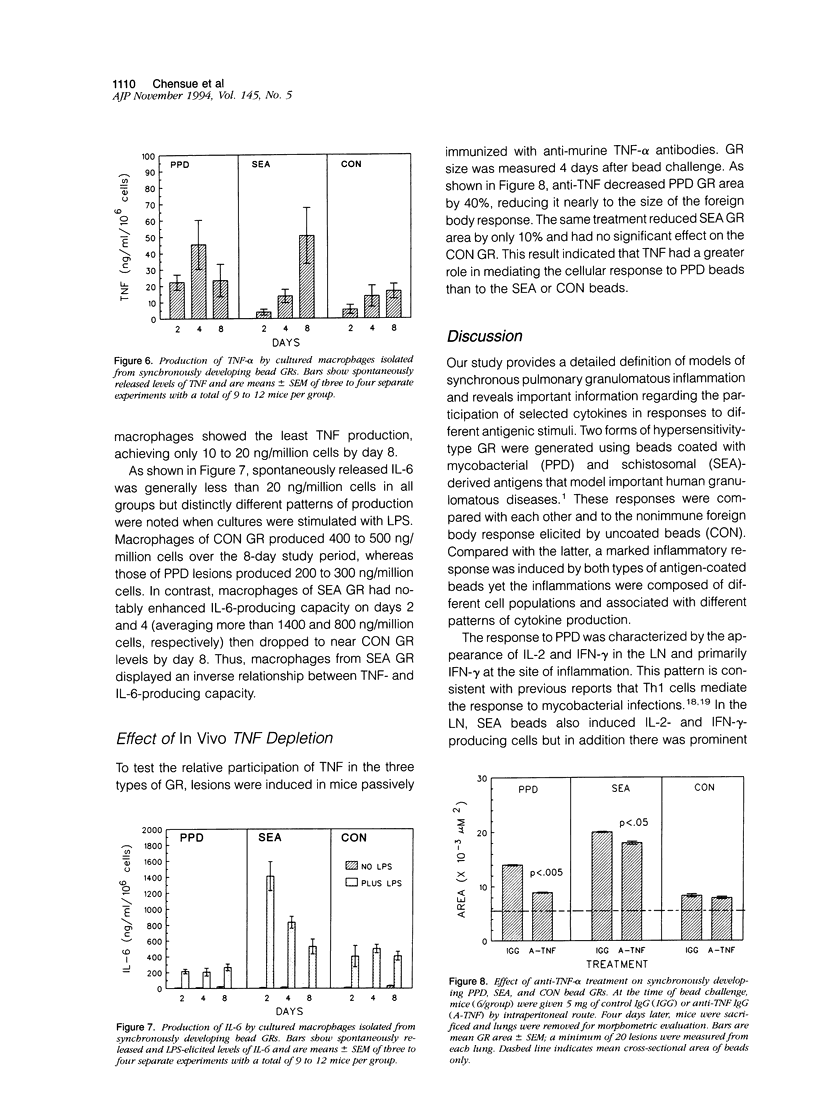
Synchronized pulmonary granulomas (GRs) were induced in presensitized mice by intravenous embolization of polymer beads bound with purified protein derivative (PPD) of Mycobacteria tuberculosis or soluble antigens derived from Schistosoma mansoni eggs (SEA). Uncoated beads served as a foreign body control (CON). Antigen-coated beads elicited GRs with characteristic epithelioid macrophages and multinucleate giant cells by 4 days after embolization. Unlike PPD GR, SEA bead lesions contained eosinophils, whereas CON beads elicited only a limited mononuclear infiltrate. GRs and draining lymph nodes (LN) were assessed on days 2, 4, and 8 for Th1-(interleukin-2 [IL-2], interferon-gamma[IFN] and Th2-type (IL-4, IL-5, and IL-10) cytokines. CON GR produced only a small amount of IFN-gamma on day 2 and failed to induce a significant response in draining LN. In contrast, both PPD and SEA antigen-coated beads induced reactive lymphoid hyperplasia but differed greatly in local and regional cytokine profiles. PPD GR produced IFN-gamma on day 2 and the draining LN produced predominantly Th1 cytokines on days 2 and 4. In contrast, SEA beads GRs were dominated by Th2 cytokines. The corresponding LN produced IL-2 and IL-4 on day 2; IL-2, IL-4, IFN-gamma, and IL-10 on day 4; then IL-2, IFN-gamma, and IL-4 on day 8, probably reflecting maturational changes of T cells. Macrophages (MP) from bead GR also showed different patterns of IL-6 and tumor necrosis factor (TNF) production. Compared with CON GR, MPs from PPD GR were weak sources of IL-6, whereas those of SEA GR showed enhanced and accelerated production. In contrast, MP of PPD GR had augmented TNF-producing capacity, whereas those of SEA GR showed delayed TNF production. In vivo depletion of TNF, respectively, caused 40 and 10% decreases in PPD GR and SEA GR but had no effect on CON GR area, indicating that TNF contributed to a greater degree to the PPD response. These data show that depending on the inciting agent, GR can be mediated by different cytokines. Characterization of inflammatory lesions by cytokine profiles should allow design of more rational therapeutic interventions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiri P., Locksley R. M., Parslow T. G., Sadick M., Rector E., Ritter D., McKerrow J. H. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992 Apr 16;356(6370):604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Barnes P. F., Fong S. J., Brennan P. J., Twomey P. E., Mazumder A., Modlin R. L. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990 Jul 1;145(1):149–154. [PubMed] [Google Scholar]
- Boros D. L. Granulomatous inflammations. Prog Allergy. 1978;24:183–267. doi: 10.1159/000401230. [DOI] [PubMed] [Google Scholar]
- COKER C. M., LICHTENBERG F. A revised method for isolation of Schistosoma mansoni eggs for biological experimentation. Proc Soc Exp Biol Med. 1956 Aug-Sep;92(4):780–782. doi: 10.3181/00379727-92-22612. [DOI] [PubMed] [Google Scholar]
- Chensue S. W., Otterness I. G., Higashi G. I., Forsch C. S., Kunkel S. L. Monokine production by hypersensitivity (Schistosoma mansoni egg) and foreign body (Sephadex bead)-type granuloma macrophages. Evidence for sequential production of IL-1 and tumor necrosis factor. J Immunol. 1989 Feb 15;142(4):1281–1286. [PubMed] [Google Scholar]
- Chensue S. W., Remick D. G., Shmyr-Forsch C., Beals T. F., Kunkel S. L. Immunohistochemical demonstration of cytoplasmic and membrane-associated tumor necrosis factor in murine macrophages. Am J Pathol. 1988 Dec;133(3):564–572. [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Terebuh P. D., Remick D. G., Scales W. E., Kunkel S. L. In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia. Kinetics, Kupffer cell expression, and glucocorticoid effects. Am J Pathol. 1991 Feb;138(2):395–402. [PMC free article] [PubMed] [Google Scholar]
- Chensue S. W., Terebuh P. D., Warmington K. S., Hershey S. D., Evanoff H. L., Kunkel S. L., Higashi G. I. Role of IL-4 and IFN-gamma in Schistosoma mansoni egg-induced hypersensitivity granuloma formation. Orchestration, relative contribution, and relationship to macrophage function. J Immunol. 1992 Feb 1;148(3):900–906. [PubMed] [Google Scholar]
- Chensue S. W., Warmington K. S., Hershey S. D., Terebuh P. D., Othman M., Kunkel S. L. Evolving T cell responses in murine schistosomiasis. Th2 cells mediate secondary granulomatous hypersensitivity and are regulated by CD8+ T cells in vivo. J Immunol. 1993 Aug 1;151(3):1391–1400. [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., de Kossodo S., Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986 Dec 1;164(6):2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry R. C., Kiener P. A., Spitalny G. L. A sensitive immunochemical assay for biologically active MuIFN-gamma. J Immunol Methods. 1987 Nov 23;104(1-2):137–142. doi: 10.1016/0022-1759(87)90497-2. [DOI] [PubMed] [Google Scholar]
- Essner R., Rhoades K., McBride W. H., Morton D. L., Economou J. S. IL-4 down-regulates IL-1 and TNF gene expression in human monocytes. J Immunol. 1989 Jun 1;142(11):3857–3861. [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Fong T. A., Mosmann T. R. The role of IFN-gamma in delayed-type hypersensitivity mediated by Th1 clones. J Immunol. 1989 Nov 1;143(9):2887–2893. [PubMed] [Google Scholar]
- Grzych J. M., Pearce E., Cheever A., Caulada Z. A., Caspar P., Heiny S., Lewis F., Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991 Feb 15;146(4):1322–1327. [PubMed] [Google Scholar]
- Henderson G. S., Lu X., McCurley T. L., Colley D. G. In vivo molecular analysis of lymphokines involved in the murine immune response during Schistosoma mansoni infection. II. Quantification of IL-4 mRNA, IFN-gamma mRNA, and IL-2 mRNA levels in the granulomatous livers, mesenteric lymph nodes, and spleens during the course of modulation. J Immunol. 1992 Apr 1;148(7):2261–2269. [PubMed] [Google Scholar]
- Hu-Li J., Ohara J., Watson C., Tsang W., Paul W. E. Derivation of a T cell line that is highly responsive to IL-4 and IL-2 (CT.4R) and of an IL-2 hyporesponsive mutant of that line (CT.4S). J Immunol. 1989 Feb 1;142(3):800–807. [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Lukacs N. W., Chensue S. W., Strieter R. M., Warmington K., Kunkel S. L. Inflammatory granuloma formation is mediated by TNF-alpha-inducible intercellular adhesion molecule-1. J Immunol. 1994 Jun 15;152(12):5883–5889. [PubMed] [Google Scholar]
- Mathew R. C., Ragheb S., Boros D. L. Recombinant IL-2 therapy reverses diminished granulomatous responsiveness in anti-L3T4-treated, Schistosoma mansoni-infected mice. J Immunol. 1990 Jun 1;144(11):4356–4361. [PubMed] [Google Scholar]
- Mutis T., Kraakman E. M., Cornelisse Y. E., Haanen J. B., Spits H., De Vries R. R., Ottenhoff T. H. Analysis of cytokine production by Mycobacterium-reactive T cells. Failure to explain Mycobacterium leprae-specific nonresponsiveness of peripheral blood T cells from lepromatous leprosy patients. J Immunol. 1993 May 15;150(10):4641–4651. [PubMed] [Google Scholar]
- Müller K. M., Jaunin F., Masouyé I., Saurat J. H., Hauser C. Th2 cells mediate IL-4-dependent local tissue inflammation. J Immunol. 1993 Jun 15;150(12):5576–5584. [PubMed] [Google Scholar]
- Orme I. M., Roberts A. D., Griffin J. P., Abrams J. S. Cytokine secretion by CD4 T lymphocytes acquired in response to Mycobacterium tuberculosis infection. J Immunol. 1993 Jul 1;151(1):518–525. [PubMed] [Google Scholar]
- Perrin P. J., Phillips S. M. The molecular basis of granuloma formation in schistosomiasis. III. In vivo effects of a T cell-derived suppressor effector factor and IL-2 on granuloma formation. J Immunol. 1989 Jul 15;143(2):649–654. [PubMed] [Google Scholar]
- Remick D. G., Chensue S. W., Hiserodt J. C., Higashi G. I., Kunkel S. L. Flow-cytometric evaluation of lymphocyte subpopulations in synchronously developing Schistosoma mansoni egg and Sephadex bead pulmonary granulomas. Am J Pathol. 1988 May;131(2):298–307. [PMC free article] [PubMed] [Google Scholar]
- Robinson D. S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A. M., Corrigan C., Durham S. R., Kay A. B. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992 Jan 30;326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- Seder R. A., Paul W. E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Scott P., Cheever A. W. Interleukin 5 is required for the blood and tissue eosinophilia but not granuloma formation induced by infection with Schistosoma mansoni. Proc Natl Acad Sci U S A. 1990 Jan;87(1):61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H., Shiho O., Kuroshima K., Koyama M., Tsukamoto K. An improved colorimetric assay for interleukin 2. J Immunol Methods. 1986 Nov 6;93(2):157–165. doi: 10.1016/0022-1759(86)90183-3. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Matsumoto-Kobayashi M., Clark S. C., Seehra J., London L., Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984 Oct 1;160(4):1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren K. S. A functional classification of granulomatous inflammation. Ann N Y Acad Sci. 1976;278:7–18. doi: 10.1111/j.1749-6632.1976.tb47011.x. [DOI] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]



