Abstract
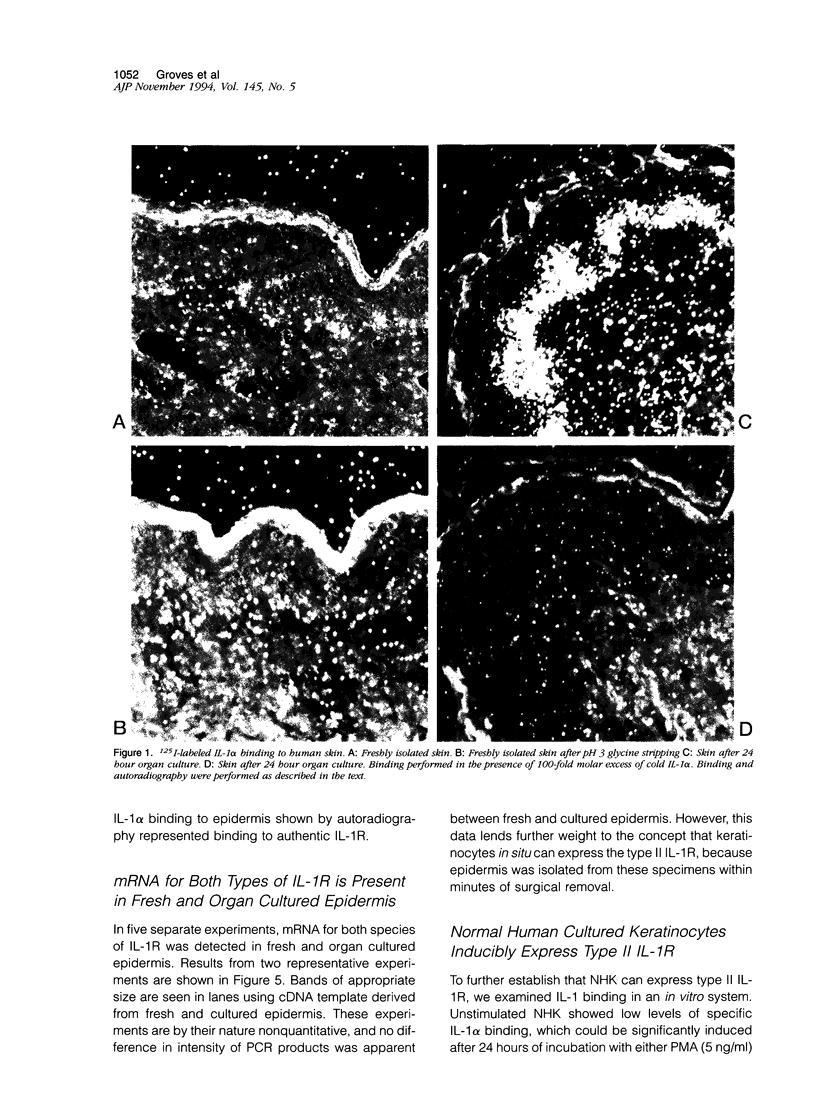
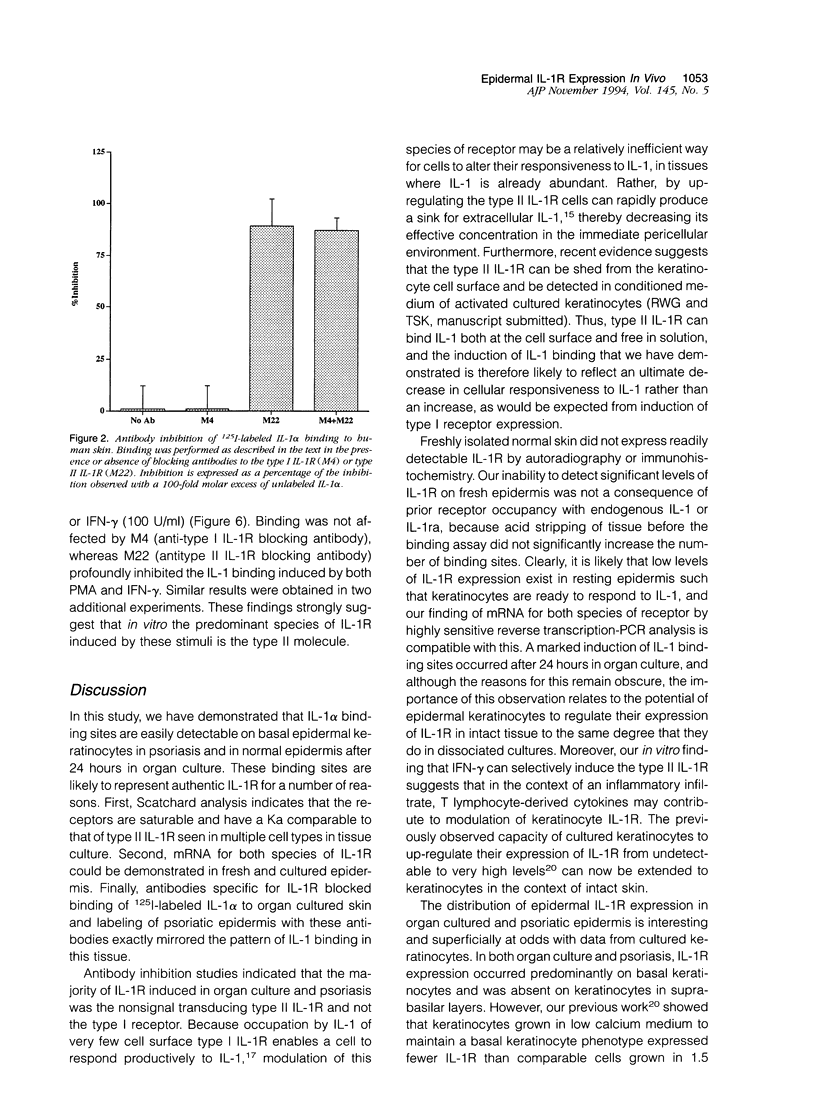
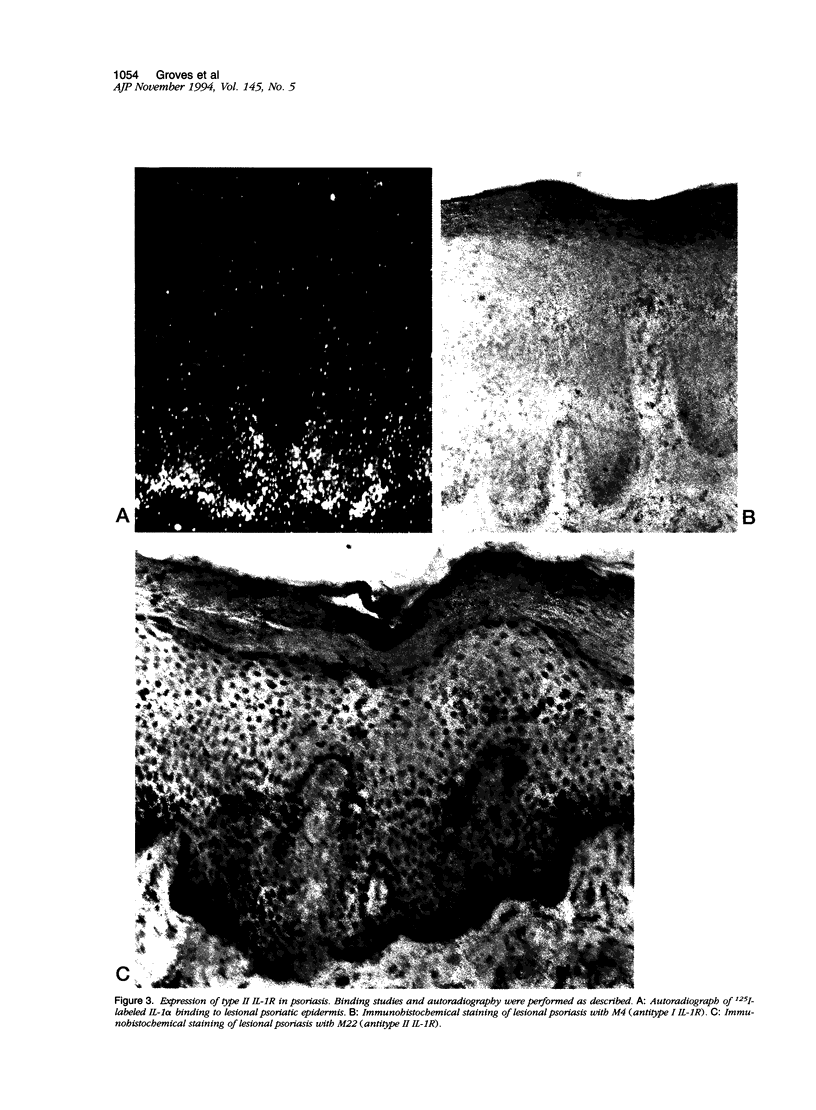
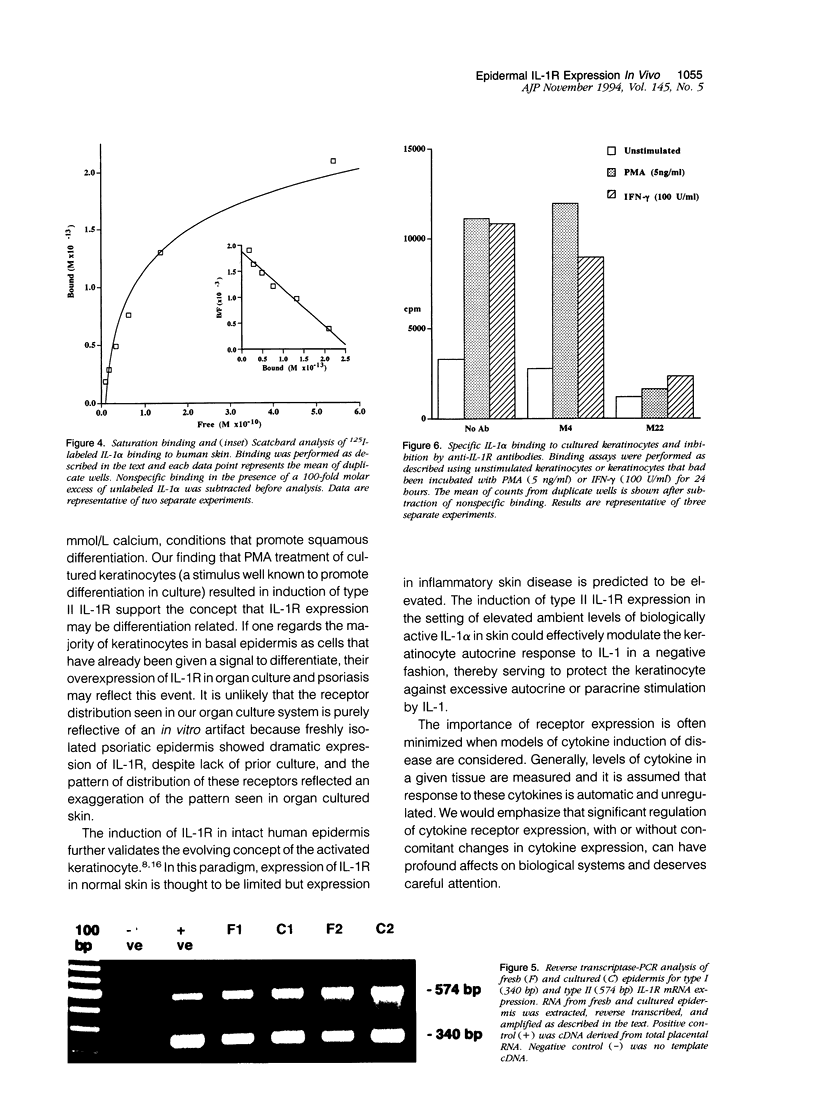
Normal human epidermis is a rich source of biologically active interleukin-1 alpha (IL-1 alpha). Keratinocytes both synthesize this cytokine and respond to it via cell surface receptors (IL-1R), suggesting that the IL-1 system may play an important role in normal epidermal physiology and inflammation. In this study, we have examined the expression of IL-1R in normal and psoriatic epidermis, as judged at a functional level by the capacity to bind 125I-labeled IL-1 alpha (the principal IL-1 species present in epidermis) and by immunostaining with antibodies specific for each species of IL-1R. IL-1R was not readily detectable by either technique in normal, freshly isolated human epidermis. However, in lesional psoriasis or normal epidermis after 24 hours of organ culture, expression of IL-1R was dramatically induced, especially in basal keratinocytes. Immunostaining and antibody blocking studies demonstrated the induced IL-1R to be the type II species, a nonsignal transducing molecule previously demonstrated only on leukocytes. The Ka of this receptor was comparable to that previously demonstrated in vitro. mRNA for both species of IL-1R could be demonstrated by reverse transcriptase-polymerase chain reaction in fresh and cultured epidermis. These in vivo findings were confirmed in culture, where normal human keratinocytes expressed few IL-1R at rest but large numbers of type II IL-1R after activation by phorbol ester or interferon-gamma. We conclude that under resting conditions, epidermal expression of IL-1R is low. However, the potential for keratinocytes in vivo to express large numbers of the nonsignal transducing type II IL-1R is evident from both organ cultured and psoriatic epidermis. The in vitro induction of keratinocyte IL-1R by interferon-gamma suggests that this cytokine may be involved in the induction of type II IL-1R in inflammatory skin disease. The presence of bioactive IL-1 in epidermis, coupled with the inducible expression of the decoy type II IL-1R, indicates the existence of a highly regulated system of autocrine stimulation of keratinocytes by IL-1.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigler C. F., Norris D. A., Weston W. L., Arend W. P. Interleukin-1 receptor antagonist production by human keratinocytes. J Invest Dermatol. 1992 Jan;98(1):38–44. doi: 10.1111/1523-1747.ep12494196. [DOI] [PubMed] [Google Scholar]
- Blanton R. A., Kupper T. S., McDougall J. K., Dower S. Regulation of interleukin 1 and its receptor in human keratinocytes. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1273–1277. doi: 10.1073/pnas.86.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp R., Fincham N., Ross J., Bird C., Gearing A. Potent inflammatory properties in human skin of interleukin-1 alpha-like material isolated from normal skin. J Invest Dermatol. 1990 Jun;94(6):735–741. doi: 10.1111/1523-1747.ep12874591. [DOI] [PubMed] [Google Scholar]
- Colotta F., Re F., Muzio M., Bertini R., Polentarutti N., Sironi M., Giri J. G., Dower S. K., Sims J. E., Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993 Jul 23;261(5120):472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Gallis B., Overell R. W., McMahan C. J., DeRoos P., Ireland R., Eisenman J., Dower S. K., Sims J. E. T-cell interleukin 1 receptor cDNA expressed in Chinese hamster ovary cells regulates functional responses to interleukin 1. Proc Natl Acad Sci U S A. 1989 May;86(9):3045–3049. doi: 10.1073/pnas.86.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Gahring L. C., Buckley A., Daynes R. A. Presence of epidermal-derived thymocyte activating factor/interleukin 1 in normal human stratum corneum. J Clin Invest. 1985 Oct;76(4):1585–1591. doi: 10.1172/JCI112141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves R. W., Ross E., Barker J. N., Ross J. S., Camp R. D., MacDonald D. M. Effect of in vivo interleukin-1 on adhesion molecule expression in normal human skin. J Invest Dermatol. 1992 Mar;98(3):384–387. doi: 10.1111/1523-1747.ep12499816. [DOI] [PubMed] [Google Scholar]
- Hauser C., Saurat J. H., Schmitt A., Jaunin F., Dayer J. M. Interleukin 1 is present in normal human epidermis. J Immunol. 1986 May 1;136(9):3317–3323. [PubMed] [Google Scholar]
- Kupper T. S. Immune and inflammatory processes in cutaneous tissues. Mechanisms and speculations. J Clin Invest. 1990 Dec;86(6):1783–1789. doi: 10.1172/JCI114907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S., Lee F., Birchall N., Clark S., Dower S. Interleukin 1 binds to specific receptors on human keratinocytes and induces granulocyte macrophage colony-stimulating factor mRNA and protein. A potential autocrine role for interleukin 1 in epidermis. J Clin Invest. 1988 Nov;82(5):1787–1792. doi: 10.1172/JCI113792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T. S. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J Invest Dermatol. 1990 Jun;94(6 Suppl):146S–150S. doi: 10.1111/1523-1747.ep12876130. [DOI] [PubMed] [Google Scholar]
- Larsen C. G., Anderson A. O., Oppenheim J. J., Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989 Sep;68(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- McMahan C. J., Slack J. L., Mosley B., Cosman D., Lupton S. D., Brunton L. L., Grubin C. E., Wignall J. M., Jenkins N. A., Brannan C. I. A novel IL-1 receptor, cloned from B cells by mammalian expression, is expressed in many cell types. EMBO J. 1991 Oct;10(10):2821–2832. doi: 10.1002/j.1460-2075.1991.tb07831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney L. B., Stoscheck C. M., Magid M., King L. E., Jr Altered [125I]epidermal growth factor binding and receptor distribution in psoriasis. J Invest Dermatol. 1986 Mar;86(3):260–265. doi: 10.1111/1523-1747.ep12285389. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Sims J. E., March C. J., Cosman D., Widmer M. B., MacDonald H. R., McMahan C. J., Grubin C. E., Wignall J. M., Jackson J. L., Call S. M. cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science. 1988 Jul 29;241(4865):585–589. doi: 10.1126/science.2969618. [DOI] [PubMed] [Google Scholar]
- Slack J., McMahan C. J., Waugh S., Schooley K., Spriggs M. K., Sims J. E., Dower S. K. Independent binding of interleukin-1 alpha and interleukin-1 beta to type I and type II interleukin-1 receptors. J Biol Chem. 1993 Feb 5;268(4):2513–2524. [PubMed] [Google Scholar]