Abstract
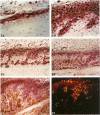
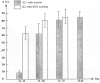
A prerequisite in defining the role of a growth factor in a disease is knowledge of its expression kinetics during the natural course of the disease. We, therefore, used immunohistochemical and immunoblot analyses to examine tissue distribution of basic fibroblast growth factor (bFGF) and platelet-derived growth factor (PDGF-A) during the development of destructive arthropathy in the rat adjuvant arthritis model. In normal joints, bFGF was primarily localized in endothelial cells. In inflamed joints, increased staining for bFGF was found in the invading panni, hyperplastic synovium, and thickened periosteum where bFGF was also co-localized with two cell proliferation markers. Staining for bFGF began to increase at the onset of arthritis (days 11 to 13), reached peak level on days 17 to 24, and gradually declined afterward. In contrast, PDGF-A staining did not change until day 17 and the increased staining was restricted to areas of newly formed bone. The district temporal and spatial distribution pattern of these two growth factors during the destructive arthropathy strongly suggests that they play different roles during arthritis. Although PDGF-A seems to be exclusively related to osteogenesis, bFGF may have a more extensive impact on synovial proliferation and bone destruction as well as bone formation.
Full text
PDF


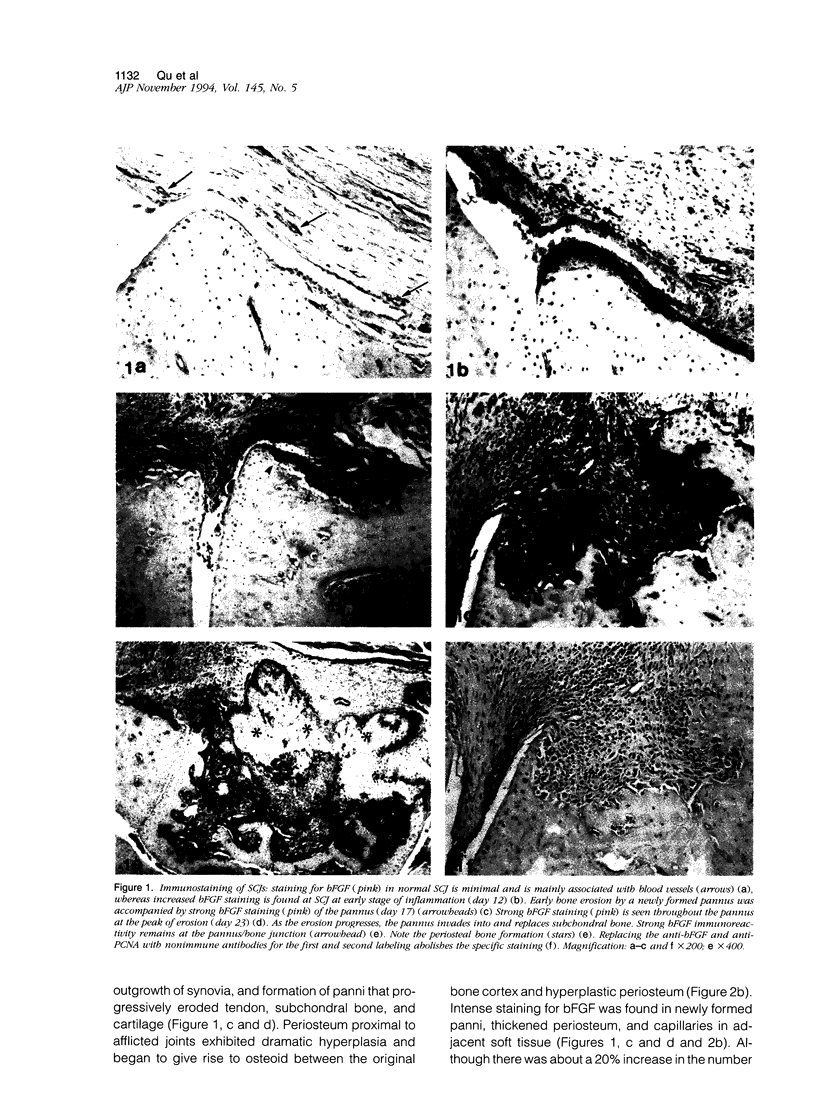

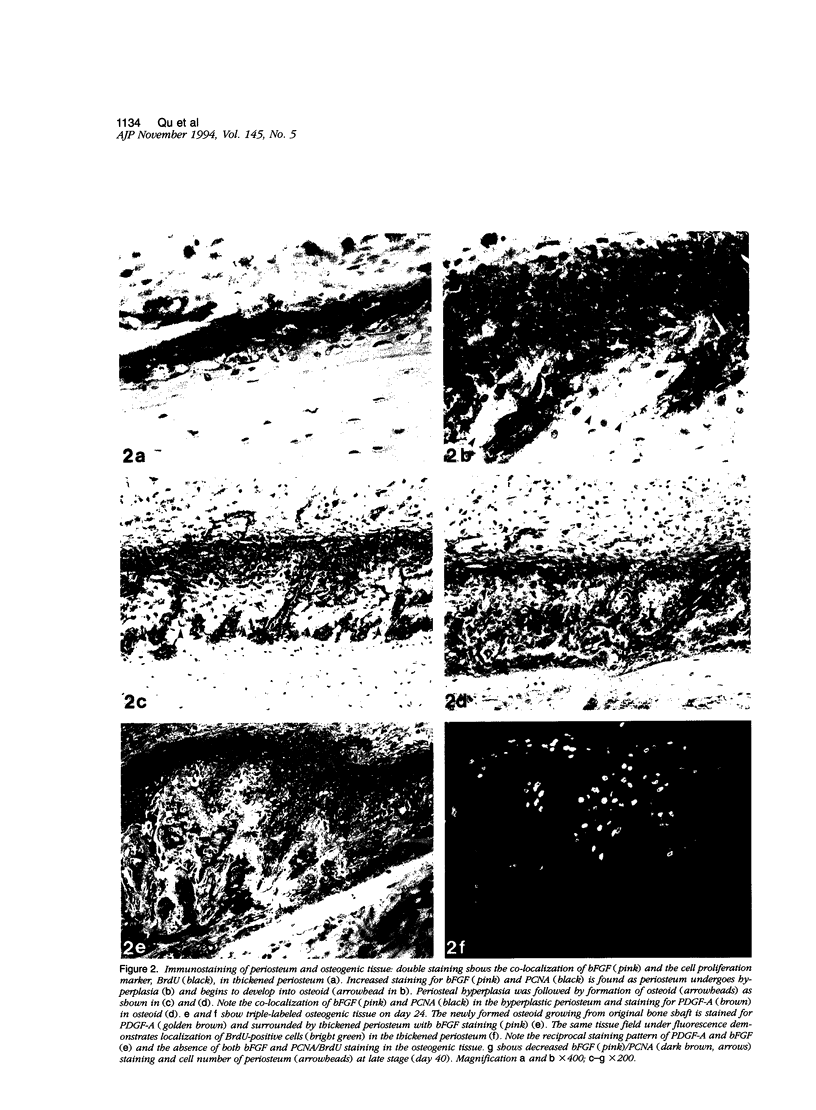
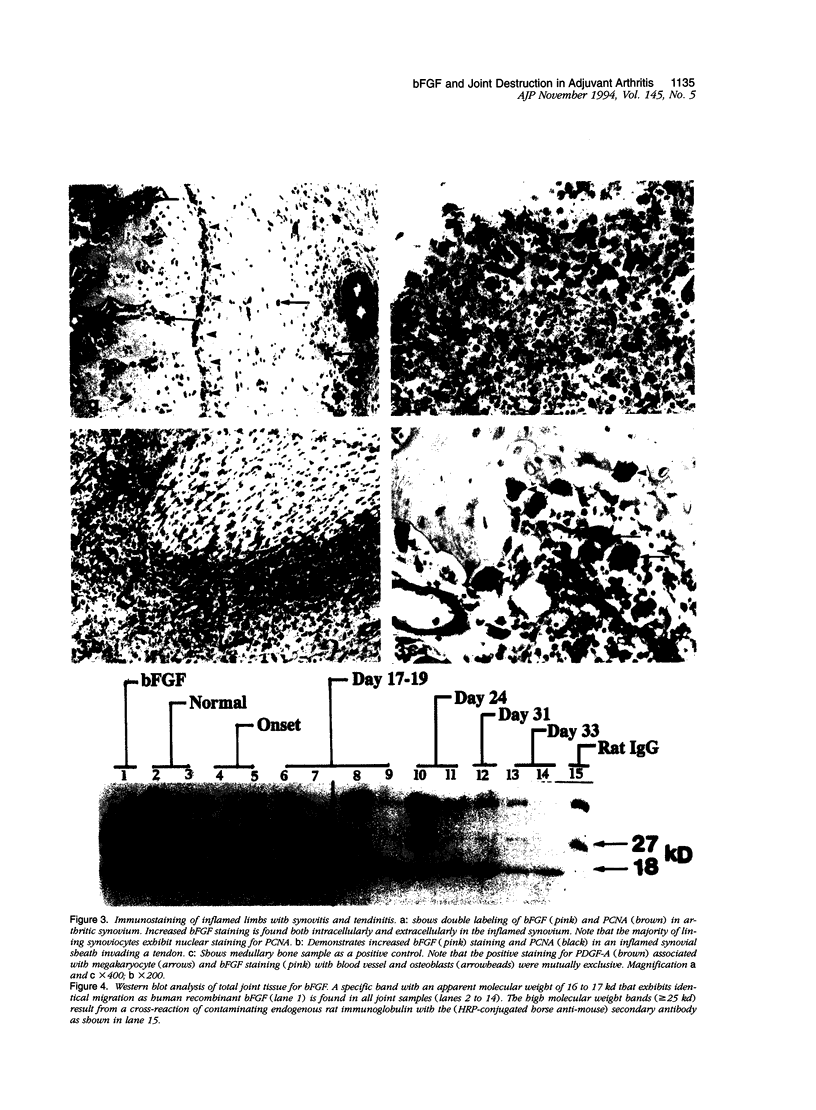
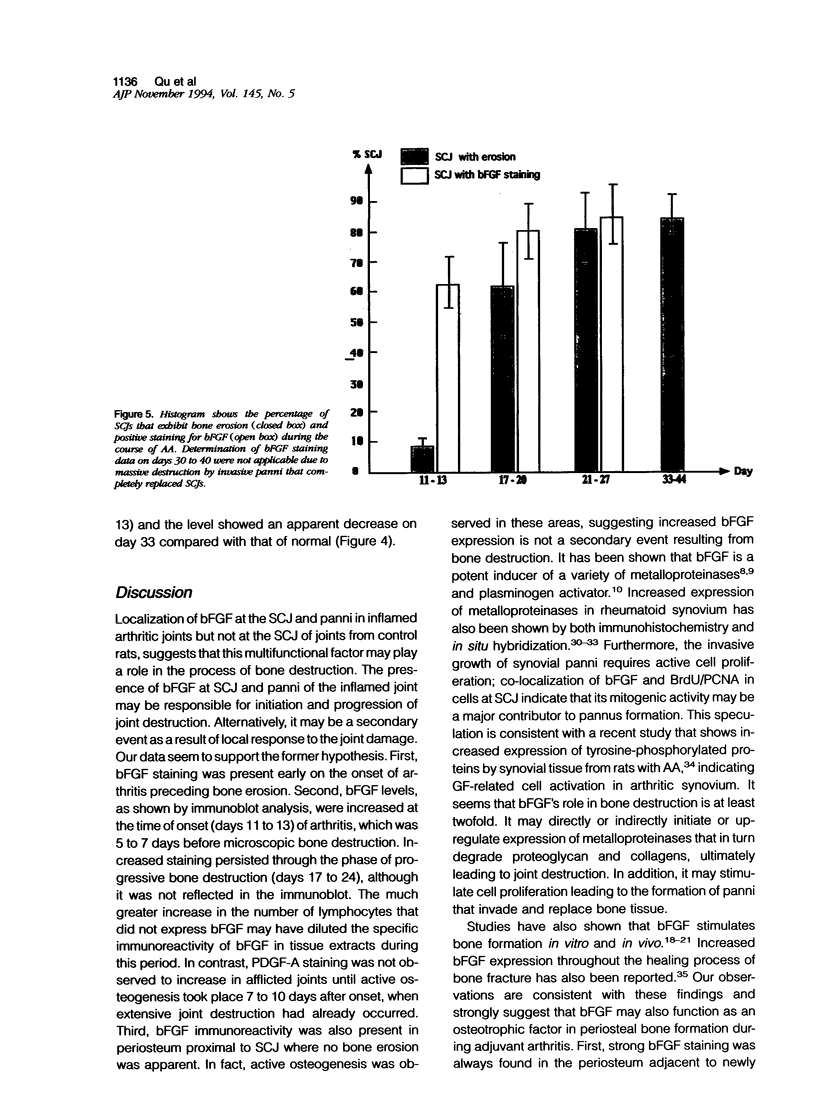



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansel J. C., Tiesman J. P., Olerud J. E., Krueger J. G., Krane J. F., Tara D. C., Shipley G. D., Gilbertson D., Usui M. L., Hart C. E. Human keratinocytes are a major source of cutaneous platelet-derived growth factor. J Clin Invest. 1993 Aug;92(2):671–678. doi: 10.1172/JCI116636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenberg P., Lohmander L. S. Fibroblast growth factor stimulates bone formation. Bone induction studied in rats. Acta Orthop Scand. 1989 Aug;60(4):473–476. doi: 10.3109/17453678909149323. [DOI] [PubMed] [Google Scholar]
- Bandara G., Lin C. W., Georgescu H. I., Mendelow D., Evans C. H. Chondrocyte activation by interleukin-1: analysis of the synergistic properties of fibroblast growth factor and phorbol myristate acetate. Arch Biochem Biophys. 1989 Nov 1;274(2):539–547. doi: 10.1016/0003-9861(89)90468-2. [DOI] [PubMed] [Google Scholar]
- Bolander M. E. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992 Jun;200(2):165–170. doi: 10.3181/00379727-200-43410a. [DOI] [PubMed] [Google Scholar]
- Brennan F. M., Field M., Chu C. Q., Feldmann M., Maini R. N. Cytokine expression in rheumatoid arthritis. Br J Rheumatol. 1991;30 (Suppl 1):76–80. [PubMed] [Google Scholar]
- Broadley K. N., Aquino A. M., Hicks B., Ditesheim J. A., McGee G. S., Demetriou A. A., Woodward S. C., Davidson J. M. Growth factors bFGF and TGB beta accelerate the rate of wound repair in normal and in diabetic rats. Int J Tissue React. 1988;10(6):345–353. [PubMed] [Google Scholar]
- Broadley K. N., Aquino A. M., Woodward S. C., Buckley-Sturrock A., Sato Y., Rifkin D. B., Davidson J. M. Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab Invest. 1989 Nov;61(5):571–575. [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Canalis E., Centrella M., McCarthy T. Effects of basic fibroblast growth factor on bone formation in vitro. J Clin Invest. 1988 May;81(5):1572–1577. doi: 10.1172/JCI113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E., McCarthy T. L., Centrella M. Effects of platelet-derived growth factor on bone formation in vitro. J Cell Physiol. 1989 Sep;140(3):530–537. doi: 10.1002/jcp.1041400319. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar S., Harvey A. K. Induction of interleukin-1 receptors on chondrocytes by fibroblast growth factor: a possible mechanism for modulation of interleukin-1 activity. J Cell Physiol. 1989 Feb;138(2):236–246. doi: 10.1002/jcp.1041380204. [DOI] [PubMed] [Google Scholar]
- Chin J. E., Hatfield C. A., Krzesicki R. F., Herblin W. F. Interactions between interleukin-1 and basic fibroblast growth factor on articular chondrocytes. Effects on cell growth, prostanoid production, and receptor modulation. Arthritis Rheum. 1991 Mar;34(3):314–324. doi: 10.1002/art.1780340309. [DOI] [PubMed] [Google Scholar]
- Chua C. C., Chua B. H., Zhao Z. Y., Krebs C., Diglio C., Perrin E. Effect of growth factors on collagen metabolism in cultured human heart fibroblasts. Connect Tissue Res. 1991;26(4):271–281. doi: 10.3109/03008209109152444. [DOI] [PubMed] [Google Scholar]
- Cuevas P., Burgos J., Baird A. Basic fibroblast growth factor (FGF) promotes cartilage repair in vivo. Biochem Biophys Res Commun. 1988 Oct 31;156(2):611–618. doi: 10.1016/s0006-291x(88)80887-8. [DOI] [PubMed] [Google Scholar]
- Finklestein S. P., Apostolides P. J., Caday C. G., Prosser J., Philips M. F., Klagsbrun M. Increased basic fibroblast growth factor (bFGF) immunoreactivity at the site of focal brain wounds. Brain Res. 1988 Sep 20;460(2):253–259. doi: 10.1016/0006-8993(88)90370-8. [DOI] [PubMed] [Google Scholar]
- Firestein G. S., Paine M. M. Stromelysin and tissue inhibitor of metalloproteinases gene expression in rheumatoid arthritis synovium. Am J Pathol. 1992 Jun;140(6):1309–1314. [PMC free article] [PubMed] [Google Scholar]
- Goddard D. H., Grossman S. L., Newton R., Clark M. A., Bomalaski J. S. Regulation of synovial cell growth: basic fibroblast growth factor synergizes with interleukin 1 beta stimulating phospholipase A2 enzyme activity, phospholipase A2 activating protein production and release of prostaglandin E2 by rheumatoid arthritis synovial cells in culture. Cytokine. 1992 Sep;4(5):377–384. doi: 10.1016/1043-4666(92)90081-2. [DOI] [PubMed] [Google Scholar]
- Goddard D. H., Grossman S. L., Newton R. Polypeptide growth factors augment interleukin 1-induced release of prostaglandin E2 by rheumatoid arthritis synovial cells in vitro. Cytokine. 1990 Jul;2(4):294–299. doi: 10.1016/1043-4666(90)90031-n. [DOI] [PubMed] [Google Scholar]
- Goureau O., Lepoivre M., Becquet F., Courtois Y. Differential regulation of inducible nitric oxide synthase by fibroblast growth factors and transforming growth factor beta in bovine retinal pigmented epithelial cells: inverse correlation with cellular proliferation. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4276–4280. doi: 10.1073/pnas.90.9.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravallese E. M., Darling J. M., Ladd A. L., Katz J. N., Glimcher L. H. In situ hybridization studies of stromelysin and collagenase messenger RNA expression in rheumatoid synovium. Arthritis Rheum. 1991 Sep;34(9):1076–1084. doi: 10.1002/art.1780340903. [DOI] [PubMed] [Google Scholar]
- Harvey A. K., Stack S. T., Chandrasekhar S. Differential modulation of degradative and repair responses of interleukin-1-treated chondrocytes by platelet-derived growth factor. Biochem J. 1993 May 15;292(Pt 1):129–136. doi: 10.1042/bj2920129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafyatis R., Thompson N. L., Remmers E. F., Flanders K. C., Roche N. S., Kim S. J., Case J. P., Sporn M. B., Roberts A. B., Wilder R. L. Transforming growth factor-beta production by synovial tissues from rheumatoid patients and streptococcal cell wall arthritic rats. Studies on secretion by synovial fibroblast-like cells and immunohistologic localization. J Immunol. 1989 Aug 15;143(4):1142–1148. [PubMed] [Google Scholar]
- Lipsky P. E., Davis L. S., Cush J. J., Oppenheimer-Marks N. The role of cytokines in the pathogenesis of rheumatoid arthritis. Springer Semin Immunopathol. 1989;11(2):123–162. doi: 10.1007/BF00197186. [DOI] [PubMed] [Google Scholar]
- Mayahara H., Ito T., Nagai H., Miyajima H., Tsukuda R., Taketomi S., Mizoguchi J., Kato K. In vivo stimulation of endosteal bone formation by basic fibroblast growth factor in rats. Growth Factors. 1993;9(1):73–80. doi: 10.3109/08977199308991583. [DOI] [PubMed] [Google Scholar]
- McCachren S. S. Expression of metalloproteinases and metalloproteinase inhibitor in human arthritic synovium. Arthritis Rheum. 1991 Sep;34(9):1085–1093. doi: 10.1002/art.1780340904. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N., Allen J. B., Mizel D. E., Albina J. E., Xie Q. W., Nathan C. F., Wahl S. M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J Exp Med. 1993 Aug 1;178(2):749–754. doi: 10.1084/jem.178.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk V. O., Shipley G. D., Sternfeld M. D., Sherman L., Rosenbaum J. T. Synoviocytes synthesize, bind, and respond to basic fibroblast growth factor. Arthritis Rheum. 1990 Apr;33(4):493–500. doi: 10.1002/art.1780330405. [DOI] [PubMed] [Google Scholar]
- Noff D., Pitaru S., Savion N. Basic fibroblast growth factor enhances the capacity of bone marrow cells to form bone-like nodules in vitro. FEBS Lett. 1989 Jul 3;250(2):619–621. doi: 10.1016/0014-5793(89)80808-7. [DOI] [PubMed] [Google Scholar]
- Okada Y., Gonoji Y., Nakanishi I., Nagase H., Hayakawa T. Immunohistochemical demonstration of collagenase and tissue inhibitor of metalloproteinases (TIMP) in synovial lining cells of rheumatoid synovium. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(5):305–312. doi: 10.1007/BF02899418. [DOI] [PubMed] [Google Scholar]
- Osborn K. D., Trippel S. B., Mankin H. J. Growth factor stimulation of adult articular cartilage. J Orthop Res. 1989;7(1):35–42. doi: 10.1002/jor.1100070106. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Oechsner M., Naumann A., Gronwald R. G., Minne H. W., Ziegler R. Stimulation of bone matrix apposition in vitro by local growth factors: a comparison between insulin-like growth factor I, platelet-derived growth factor, and transforming growth factor beta. Endocrinology. 1990 Jul;127(1):69–75. doi: 10.1210/endo-127-1-69. [DOI] [PubMed] [Google Scholar]
- Qu Z., Garcia C. H., O'Rourke L. M., Planck S. R., Kohli M., Rosenbaum J. T. Local proliferation of fibroblast-like synoviocytes contributes to synovial hyperplasia. Results of proliferating cell nuclear antigen/cyclin, c-myc, and nucleolar organizer region staining. Arthritis Rheum. 1994 Feb;37(2):212–220. doi: 10.1002/art.1780370210. [DOI] [PubMed] [Google Scholar]
- Remmers E. F., Sano H., Lafyatis R., Case J. P., Kumkumian G. K., Hla T., Maciag T., Wilder R. L. Production of platelet derived growth factor B chain (PDGF-B/c-sis) mRNA and immunoreactive PDGF B-like polypeptide by rheumatoid synovium: coexpression with heparin binding acidic fibroblast growth factor-1. J Rheumatol. 1991 Jan;18(1):7–13. [PubMed] [Google Scholar]
- Risto O., Wahlström O., Abdiu A., Walz T. Effect of platelet derived growth factor on heterotopic bone formation in rats. Acta Orthop Scand. 1991 Feb;62(1):49–51. doi: 10.3109/17453679108993090. [DOI] [PubMed] [Google Scholar]
- Root L. L., Shipley G. D. Human dermal fibroblasts express multiple bFGF and aFGF proteins. In Vitro Cell Dev Biol. 1991 Oct;27A(10):815–822. doi: 10.1007/BF02631248. [DOI] [PubMed] [Google Scholar]
- Saksela O., Rifkin D. B. Cell-associated plasminogen activation: regulation and physiological functions. Annu Rev Cell Biol. 1988;4:93–126. doi: 10.1146/annurev.cb.04.110188.000521. [DOI] [PubMed] [Google Scholar]
- Sano H., Engleka K., Mathern P., Hla T., Crofford L. J., Remmers E. F., Jelsema C. L., Goldmuntz E., Maciag T., Wilder R. L. Coexpression of phosphotyrosine-containing proteins, platelet-derived growth factor-B, and fibroblast growth factor-1 in situ in synovial tissues of patients with rheumatoid arthritis and Lewis rats with adjuvant or streptococcal cell wall arthritis. J Clin Invest. 1993 Feb;91(2):553–565. doi: 10.1172/JCI116235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Forough R., Maier J. A., Case J. P., Jackson A., Engleka K., Maciag T., Wilder R. L. Detection of high levels of heparin binding growth factor-1 (acidic fibroblast growth factor) in inflammatory arthritic joints. J Cell Biol. 1990 Apr;110(4):1417–1426. doi: 10.1083/jcb.110.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Justen J. M., Sam L. M., Rohloff N. A., Ruppel P. L., Brunden M. N., Chin J. E. Platelet-derived growth factor potentiates cellular responses of articular chondrocytes to interleukin-1. Arthritis Rheum. 1991 Jun;34(6):697–706. doi: 10.1002/art.1780340610. [DOI] [PubMed] [Google Scholar]
- Stevens P., Shatzen E. M. Synergism of basic fibroblast growth factor and interleukin-1 beta to induce articular cartilage-degradation in the rabbit. Agents Actions. 1991 Sep;34(1-2):217–219. doi: 10.1007/BF01993284. [DOI] [PubMed] [Google Scholar]
- Stevens T. M., Chin J. E., McGowan M., Giannaras J., Kerr J. S. Phospholipase A2 (PLA2) activity in rabbit chondrocytes. Agents Actions. 1989 Jun;27(3-4):385–387. doi: 10.1007/BF01972829. [DOI] [PubMed] [Google Scholar]
- Stimpson S. A., Dalldorf F. G., Otterness I. G., Schwab J. H. Exacerbation of arthritis by IL-1 in rat joints previously injured by peptidoglycan-polysaccharide. J Immunol. 1988 May 1;140(9):2964–2969. [PubMed] [Google Scholar]
- Taurog J. D., Argentieri D. C., McReynolds R. A. Adjuvant arthritis. Methods Enzymol. 1988;162:339–355. doi: 10.1016/0076-6879(88)62089-1. [DOI] [PubMed] [Google Scholar]
- Unemori E. N., Hibbs M. S., Amento E. P. Constitutive expression of a 92-kD gelatinase (type V collagenase) by rheumatoid synovial fibroblasts and its induction in normal human fibroblasts by inflammatory cytokines. J Clin Invest. 1991 Nov;88(5):1656–1662. doi: 10.1172/JCI115480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]