Abstract
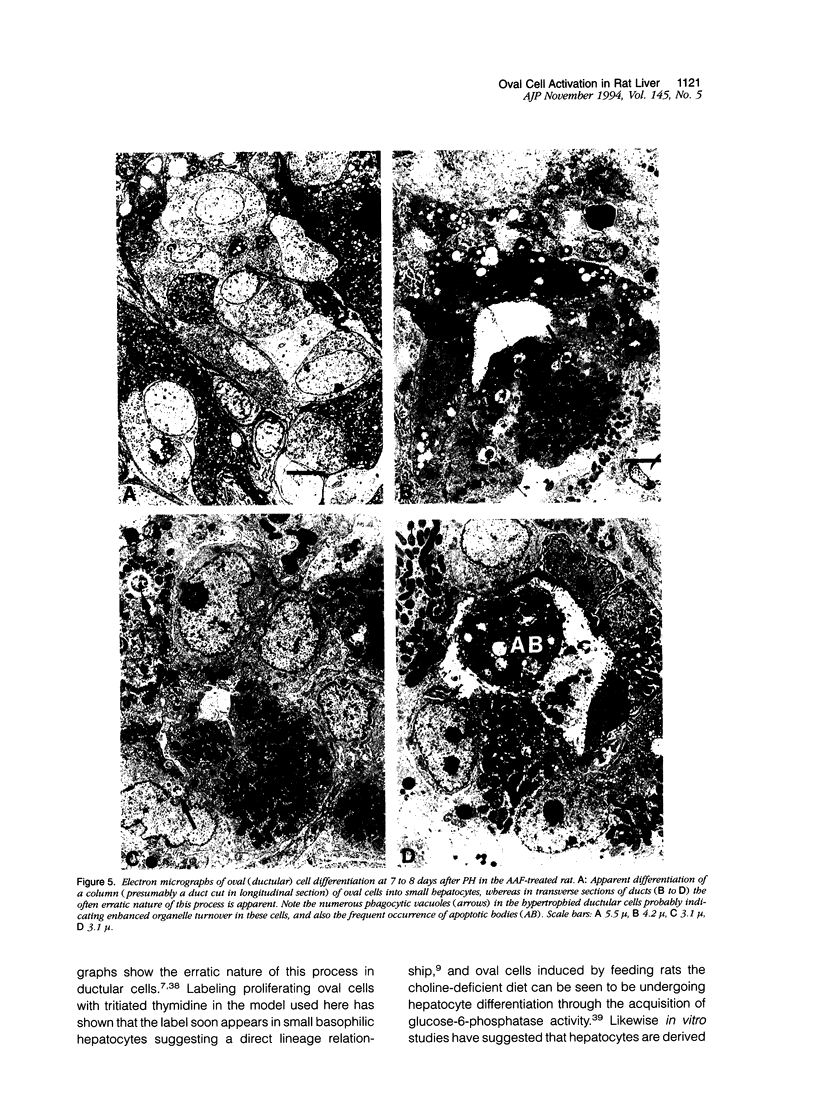
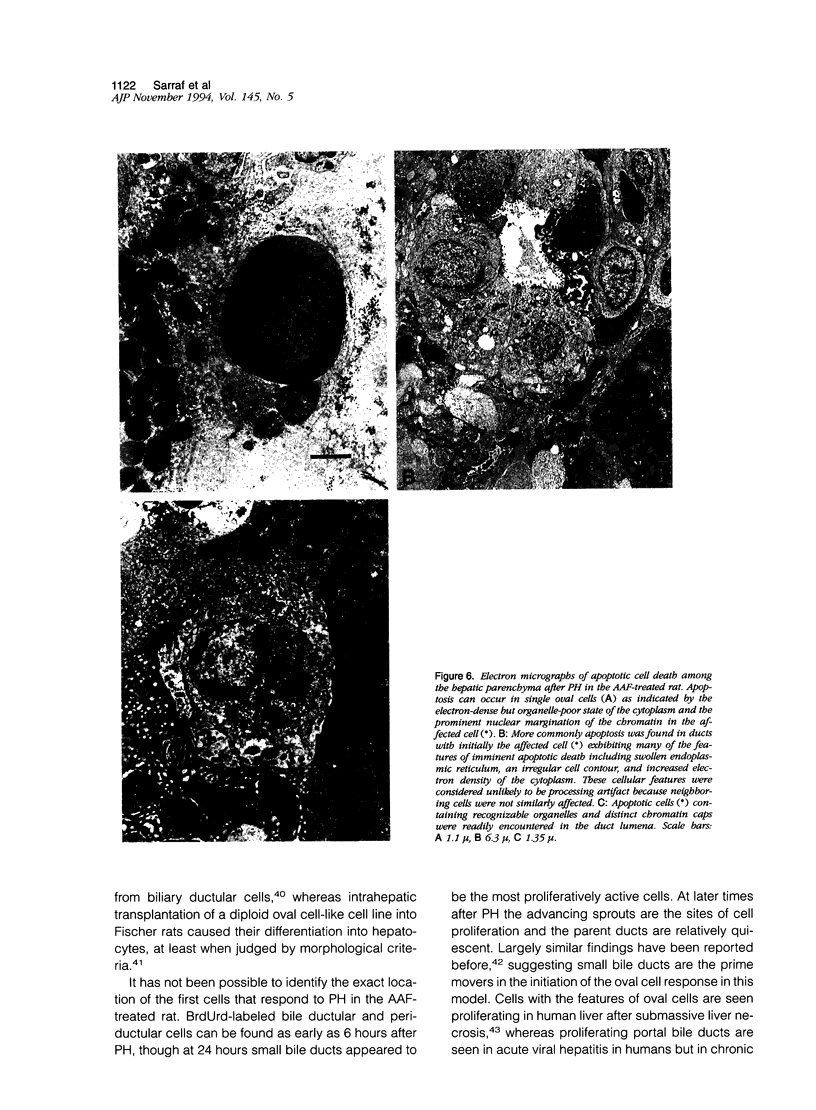
When hepatocyte regeneration is impaired, facultative stem cells and their descendants, also called oval cells, become activated and produce cell progeny that eventually differentiate. We have observed these cells in the rat liver after partial hepatectomy when the animals have been fed 2-acetylaminofluorene. Oval cells emerge from the portal areas and stain strongly with monoclonal antibodies raised against cytokeratins 8 and 19 and vimentin, the intermediate filament traditionally associated with mesenchymal cells. The majority of oval cells appeared to be part of a bile ductular reaction, manifest by their cytokeratin expression, and the bile duct injection of pigmented gelatin confirmed that these oval cells were essentially tortuous, arborizing duct-like structures (cholangioles) branched from and continuous with preexisting bile ducts. In situ hybridization studies showed that hepatocyte growth factor mRNA-expressing sinusoid lining cells were most numerous in the periportal areas during the period of ductular proliferation. At 1 week after partial hepatectomy, we observed morphological evidence of areas of in situ focal differentiation in the ductular structures, either to a columnar intestinal-type epithelia or to a hepatocyte phenotype, with abundant large mitochondria and membranous cytokeratin 8 immunoreactivity contrasting with the diffuse staining of the ductular cells. By following the fate of oval cells the authors conclude that in this model proliferated bile ductules represent the oval cell compartment capable of producing pluripotential progenitor cells.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alison M. R., Nasim M. M., Anilkumar T. V., Sarraf C. E. Transforming growth factor-alpha immunoreactivity in a variety of epithelial tissues. Cell Prolif. 1993 Sep;26(5):449–460. doi: 10.1111/j.1365-2184.1993.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Alison M. R., Poulsom R., Jeffery R., Anilkumar T. V., Jagoe R., Sarraf C. E. Expression of hepatocyte growth factor mRNA during oval cell activation in the rat liver. J Pathol. 1993 Dec;171(4):291–299. doi: 10.1002/path.1711710410. [DOI] [PubMed] [Google Scholar]
- Alison M. R. Regulation of hepatic growth. Physiol Rev. 1986 Jul;66(3):499–541. doi: 10.1152/physrev.1986.66.3.499. [DOI] [PubMed] [Google Scholar]
- Alison M. R., Sarraf C. E. Apoptosis: a gene-directed programme of cell death. J R Coll Physicians Lond. 1992 Jan;26(1):25–35. [PMC free article] [PubMed] [Google Scholar]
- Alpini G., Aragona E., Dabeva M., Salvi R., Shafritz D. A., Tavoloni N. Distribution of albumin and alpha-fetoprotein mRNAs in normal, hyperplastic, and preneoplastic rat liver. Am J Pathol. 1992 Sep;141(3):623–632. [PMC free article] [PubMed] [Google Scholar]
- Aterman K. The stem cells of the liver--a selective review. J Cancer Res Clin Oncol. 1992;118(2):87–115. doi: 10.1007/BF01187498. [DOI] [PubMed] [Google Scholar]
- Baffet G., Loyer P., Glaise D., Corlu A., Etienne P. L., Guguen-Guillouzo C. Distinct effects of cell-cell communication and corticosteroids on the synthesis and distribution of cytokeratins in cultured rat hepatocytes. J Cell Sci. 1991 Jul;99(Pt 3):609–615. doi: 10.1242/jcs.99.3.609. [DOI] [PubMed] [Google Scholar]
- Baribault H., Blouin R., Bourgon L., Marceau N. Epidermal growth factor-induced selective phosphorylation of cultured rat hepatocyte 55-kD cytokeratin before filament reorganization and DNA synthesis. J Cell Biol. 1989 Oct;109(4 Pt 1):1665–1676. doi: 10.1083/jcb.109.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhathal P. S., Christie G. S. A fluorescence microscopic study of bile duct proliferation induced in guinea pigs by alpha-naphthyl isothiocyanate. Lab Invest. 1969 May;20(5):480–487. [PubMed] [Google Scholar]
- Bisgaard H. C., Thorgeirsson S. S. Evidence for a common cell of origin for primitive epithelial cells isolated from rat liver and pancreas. J Cell Physiol. 1991 May;147(2):333–343. doi: 10.1002/jcp.1041470220. [DOI] [PubMed] [Google Scholar]
- Burt A. D., MacSween R. N. Bile duct proliferation--its true significance? Histopathology. 1993 Dec;23(6):599–602. doi: 10.1111/j.1365-2559.1993.tb01258.x. [DOI] [PubMed] [Google Scholar]
- Coleman W. B., Wennerberg A. E., Smith G. J., Grisham J. W. Regulation of the differentiation of diploid and some aneuploid rat liver epithelial (stemlike) cells by the hepatic microenvironment. Am J Pathol. 1993 May;142(5):1373–1382. [PMC free article] [PubMed] [Google Scholar]
- Connell N. D., Rheinwald J. G. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983 Aug;34(1):245–253. doi: 10.1016/0092-8674(83)90155-1. [DOI] [PubMed] [Google Scholar]
- Dabeva M. D., Shafritz D. A. Activation, proliferation, and differentiation of progenitor cells into hepatocytes in the D-galactosamine model of liver regeneration. Am J Pathol. 1993 Dec;143(6):1606–1620. [PMC free article] [PubMed] [Google Scholar]
- De Vos R., Desmet V. Ultrastructural characteristics of novel epithelial cell types identified in human pathologic liver specimens with chronic ductular reaction. Am J Pathol. 1992 Jun;140(6):1441–1450. [PMC free article] [PubMed] [Google Scholar]
- Dunsford H. A., Maset R., Salman J., Sell S. Connection of ductlike structures induced by a chemical hepatocarcinogen to portal bile ducts in the rat liver detected by injection of bile ducts with a pigmented barium gelatin medium. Am J Pathol. 1985 Feb;118(2):218–224. [PMC free article] [PubMed] [Google Scholar]
- Evarts R. P., Hu Z., Fujio K., Marsden E. R., Thorgeirsson S. S. Activation of hepatic stem cell compartment in the rat: role of transforming growth factor alpha, hepatocyte growth factor, and acidic fibroblast growth factor in early proliferation. Cell Growth Differ. 1993 Jul;4(7):555–561. [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Marsden E., Thorgeirsson S. S. A precursor-product relationship exists between oval cells and hepatocytes in rat liver. Carcinogenesis. 1987 Nov;8(11):1737–1740. doi: 10.1093/carcin/8.11.1737. [DOI] [PubMed] [Google Scholar]
- Evarts R. P., Nakatsukasa H., Marsden E. R., Hu Z., Thorgeirsson S. S. Expression of transforming growth factor-alpha in regenerating liver and during hepatic differentiation. Mol Carcinog. 1992;5(1):25–31. doi: 10.1002/mc.2940050107. [DOI] [PubMed] [Google Scholar]
- Gerber M. A., Thung S. N., Shen S., Stromeyer F. W., Ishak K. G. Phenotypic characterization of hepatic proliferation. Antigenic expression by proliferating epithelial cells in fetal liver, massive hepatic necrosis, and nodular transformation of the liver. Am J Pathol. 1983 Jan;110(1):70–74. [PMC free article] [PubMed] [Google Scholar]
- Grisham J. W. Cell types in long-term propagable cultures of rat liver. Ann N Y Acad Sci. 1980;349:128–137. doi: 10.1111/j.1749-6632.1980.tb29521.x. [DOI] [PubMed] [Google Scholar]
- Hsia C. C., Evarts R. P., Nakatsukasa H., Marsden E. R., Thorgeirsson S. S. Occurrence of oval-type cells in hepatitis B virus-associated human hepatocarcinogenesis. Hepatology. 1992 Dec;16(6):1327–1333. doi: 10.1002/hep.1840160604. [DOI] [PubMed] [Google Scholar]
- Johnson M., Koukoulis G., Matsumoto K., Nakamura T., Iyer A. Hepatocyte growth factor induces proliferation and morphogenesis in nonparenchymal epithelial liver cells. Hepatology. 1993 Jun;17(6):1052–1061. [PubMed] [Google Scholar]
- Koukoulis G., Rayner A., Tan K. C., Williams R., Portmann B. Immunolocalization of regenerating cells after submassive liver necrosis using PCNA staining. J Pathol. 1992 Apr;166(4):359–368. doi: 10.1002/path.1711660407. [DOI] [PubMed] [Google Scholar]
- Lemire J. M., Shiojiri N., Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol. 1991 Sep;139(3):535–552. [PMC free article] [PubMed] [Google Scholar]
- Lenzi R., Liu M. H., Tarsetti F., Slott P. A., Alpini G., Zhai W. R., Paronetto F., Lenzen R., Tavoloni N. Histogenesis of bile duct-like cells proliferating during ethionine hepatocarcinogenesis. Evidence for a biliary epithelial nature of oval cells. Lab Invest. 1992 Mar;66(3):390–402. [PubMed] [Google Scholar]
- MASUKO K., RUBIN E., POPPER H. PROLIFERATION OF BILE DUCTS IN CIRRHOSIS. Arch Pathol. 1964 Oct;78:421–431. [PubMed] [Google Scholar]
- Marceau N., Baribault H., Leroux-Nicollet I. Dexamethasone can modulate the synthesis and organization of cytokeratins in cultured differentiating rat hepatocytes. Can J Biochem Cell Biol. 1985 Jun;63(6):448–457. doi: 10.1139/o85-064. [DOI] [PubMed] [Google Scholar]
- Marceau N. Epithelial cell lineages in developing, restoring, and transforming liver: evidence for the existence of a 'differentiation window'. Gut. 1994 Mar;35(3):294–296. doi: 10.1136/gut.35.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G. K. Liver regeneration: molecular mechanisms of growth control. FASEB J. 1990 Feb 1;4(2):176–187. [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Niedobitek G., Kim K. Y., Stein H. Vimentin expression of newly formed rat bile duct epithelial cells in secondary biliary fibrosis. Virchows Arch A Pathol Anat Histopathol. 1989;415(3):237–242. doi: 10.1007/BF00724910. [DOI] [PubMed] [Google Scholar]
- Onoé T., Kaneko A., Dempo K., Ogawa K., Minase T. Alpha-Fetoprotein and early histological changes of hepatic tissue in DAB-hepatocarcinogenesis. Ann N Y Acad Sci. 1975 Aug 22;259:168–180. doi: 10.1111/j.1749-6632.1975.tb25412.x. [DOI] [PubMed] [Google Scholar]
- Plenat F., Braun L., Fausto N. Demonstration of glucose-6-phosphatase and peroxisomal catalase activity by ultrastructural cytochemistry in oval cells from livers of carcinogen-treated rats. Am J Pathol. 1988 Jan;130(1):91–102. [PMC free article] [PubMed] [Google Scholar]
- Roskams T., Campos R. V., Drucker D. J., Desmet V. J. Reactive human bile ductules express parathyroid hormone-related peptide. Histopathology. 1993 Jul;23(1):11–19. doi: 10.1111/j.1365-2559.1993.tb01178.x. [DOI] [PubMed] [Google Scholar]
- SCHAFFNER F., POPPER H. Electron microscopic studies of normal and proliferated bile ductules. Am J Pathol. 1961 Apr;38:393–410. [PMC free article] [PubMed] [Google Scholar]
- Sasaki H., Schaffner F., Popper H. Bile ductules in cholestasis: morphologic evidence for secretion and absorption in man. Lab Invest. 1967 Jan;16(1):84–95. [PubMed] [Google Scholar]
- Seki S., Sakaguchi H., Kawakita N., Yanai A., Kuroki T., Kobayashi K. Analysis of proliferating biliary epithelial cells in human liver disease using a monoclonal antibody against DNA polymerase alpha. Virchows Arch A Pathol Anat Histopathol. 1993;422(2):133–143. doi: 10.1007/BF01607165. [DOI] [PubMed] [Google Scholar]
- Sell S. Is there a liver stem cell? Cancer Res. 1990 Jul 1;50(13):3811–3815. [PubMed] [Google Scholar]
- Sell S., Salman J. Light- and electron-microscopic autoradiographic analysis of proliferating cells during the early stages of chemical hepatocarcinogenesis in the rat induced by feeding N-2-fluorenylacetamide in a choline-deficient diet. Am J Pathol. 1984 Feb;114(2):287–300. [PMC free article] [PubMed] [Google Scholar]
- Senior P. V., Critchley D. R., Beck F., Walker R. A., Varley J. M. The localization of laminin mRNA and protein in the postimplantation embryo and placenta of the mouse: an in situ hybridization and immunocytochemical study. Development. 1988 Nov;104(3):431–446. doi: 10.1242/dev.104.3.431. [DOI] [PubMed] [Google Scholar]
- Shiojiri N., Lemire J. M., Fausto N. Cell lineages and oval cell progenitors in rat liver development. Cancer Res. 1991 May 15;51(10):2611–2620. [PubMed] [Google Scholar]
- Solt D. B., Medline A., Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol. 1977 Sep;88(3):595–618. [PMC free article] [PubMed] [Google Scholar]
- Tatematsu M., Kaku T., Medline A., Farber E. Intestinal metaplasia as a common option of oval cells in relation to cholangiofibrosis in liver of rats exposed to 2-acetylaminofluorene. Lab Invest. 1985 Apr;52(4):354–362. [PubMed] [Google Scholar]
- Thorgeirsson S. S. Hepatic stem cells. Am J Pathol. 1993 May;142(5):1331–1333. [PMC free article] [PubMed] [Google Scholar]
- Travis J. The search for liver stem cells picks up. Science. 1993 Mar 26;259(5103):1829–1829. doi: 10.1126/science.8456311. [DOI] [PubMed] [Google Scholar]
- Uchida T., Peters R. L. The nature and origin of proliferated bile ductules in alcoholic liver disease. Am J Clin Pathol. 1983 Mar;79(3):326–333. doi: 10.1093/ajcp/79.3.326. [DOI] [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Callea F., Ramaekers F., Schaart G., Desmet V. J. A cytokeratin-immunohistochemical study of hepatoblastoma. Hum Pathol. 1990 Mar;21(3):302–308. doi: 10.1016/0046-8177(90)90231-s. [DOI] [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Desmet V. J. A cytokeratin immunohistochemical study of cholestatic liver disease: evidence that hepatocytes can express 'bile duct-type' cytokeratins. Histopathology. 1989 Aug;15(2):125–135. doi: 10.1111/j.1365-2559.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Desmet V. Intrahepatic bile duct development in the rat: a cytokeratin-immunohistochemical study. Lab Invest. 1988 Jul;59(1):52–59. [PubMed] [Google Scholar]
- Van Eyken P., Sciot R., Paterson A., Callea F., Kew M. C., Desmet V. J. Cytokeratin expression in hepatocellular carcinoma: an immunohistochemical study. Hum Pathol. 1988 May;19(5):562–568. doi: 10.1016/s0046-8177(88)80205-3. [DOI] [PubMed] [Google Scholar]
- Vandersteenhoven A. M., Burchette J., Michalopoulos G. Characterization of ductular hepatocytes in end-stage cirrhosis. Arch Pathol Lab Med. 1990 Apr;114(4):403–406. [PubMed] [Google Scholar]
- WILSON J. W., LEDUC E. H. Role of cholangioles in restoration of the liver of the mouse after dietary injury. J Pathol Bacteriol. 1958 Oct;76(2):441–449. doi: 10.1002/path.1700760213. [DOI] [PubMed] [Google Scholar]
- van Eyken P., Sciot R., van Damme B., de Wolf-Peeters C., Desmet V. J. Keratin immunohistochemistry in normal human liver. Cytokeratin pattern of hepatocytes, bile ducts and acinar gradient. Virchows Arch A Pathol Anat Histopathol. 1987;412(1):63–72. doi: 10.1007/BF00750732. [DOI] [PubMed] [Google Scholar]