Abstract
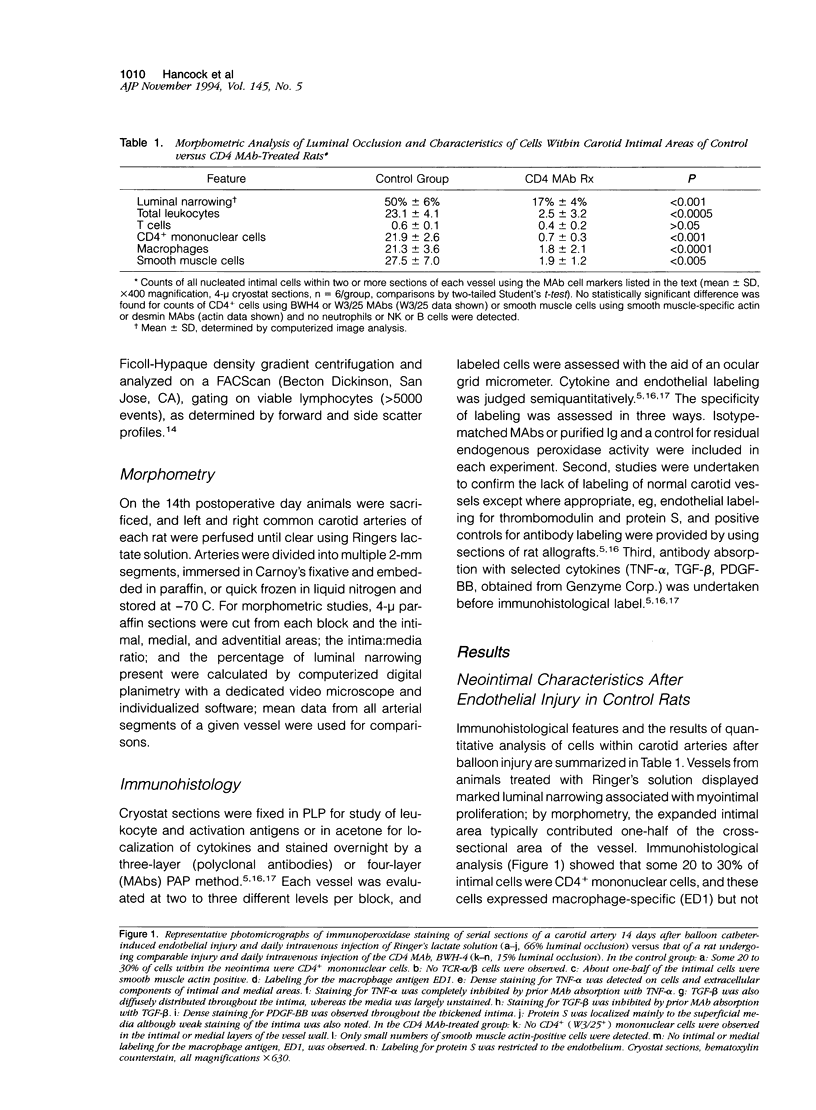
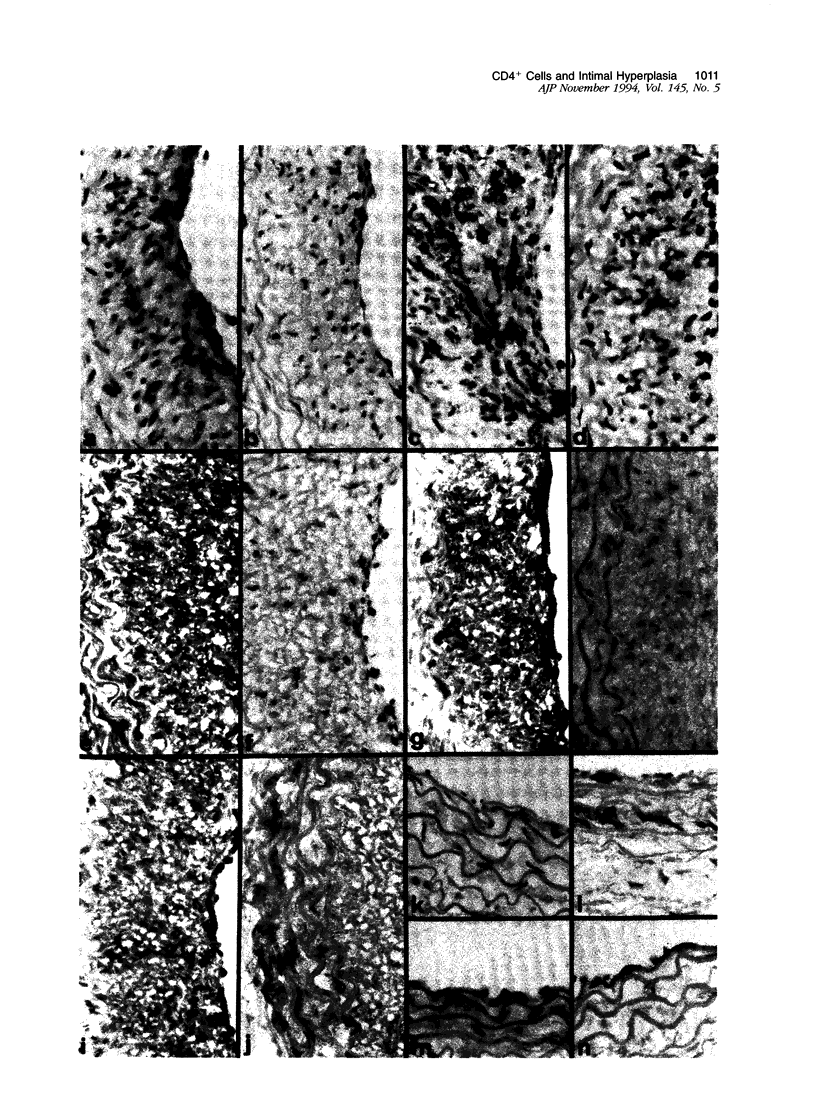
Studies of T cell-deficient or immunosuppressed animals undergoing arterial endothelial denudation have yielded conflicting results as to the contribution of the immune system to neointimal vascular smooth muscle cell accumulation and proliferation. We investigated the cell types and cytokine expression associated with intimal hyperplasia occurring 14 days after balloon angioplasty of the carotid artery in Sprague-Dawley rats. Immunohistological studies using monoclonal antibodies showed that the carotid luminal occlusion observed was associated with smooth muscle cell proliferation and neointimal accumulation of large numbers of CD4+, ED1+ mononuclear cells but no T cells. There was also wide-spread staining for the inflammatory cytokine interleukin-1B (IL-1 beta), tumor necrosis factor-alpha (TNF-alpha), and IL-8, as well as dense expression of the potent smooth muscle mitogens platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-beta), and protein S. The relationship of smooth muscle cell proliferation to monocyte/macrophage accumulation and cytokine expression was tested by daily intraperitoneal administration for 14 days of a rat CD4-specific monoclonal antibody, BWH-4 (500 micrograms/day). Morphometric analysis at day 14 showed that the intimal area of animals treated with CD4 monoclonal antibody comprised 7% +/- 4% of the arterial wall compared with 50% +/- 6% in control animals (n = 6/group, P < 0.001). In addition, immunohistological studies showed that CD4 monoclonal antibody treatment markedly reduced the intimal accumulation of mononuclear and smooth muscle cells and essentially abrogated expression of the cytokines PDGF-BB, TGF-beta, IL-1 beta, TNF-alpha, and IL-8, plus the anticoagulant molecule, protein S. Our results document the extensive expression in vivo of cytokines that in vitro promote vascular smooth muscle cell proliferation, and suggest that CD4+ mononuclear cells or their secreted products play a key role in the pathogenesis of intimal hyperplasia after endothelial injury. Furthermore, these observations may have clinical relevance in the development of novel strategies to prevent arteriosclerosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Russell M. E., Hancock W. W., Sayegh M. H., Wyner L. R., Karnovsky M. J. Chronic rejection in experimental cardiac transplantation: studies in the Lewis-F344 model. Immunol Rev. 1993 Aug;134:5–19. doi: 10.1111/j.1600-065x.1993.tb00637.x. [DOI] [PubMed] [Google Scholar]
- Adams D. H., Tilney N. L., Collins J. J., Jr, Karnovsky M. J. Experimental graft arteriosclerosis. I. The Lewis-to-F-344 allograft model. Transplantation. 1992 May;53(5):1115–1119. [PubMed] [Google Scholar]
- Casscells W. Smooth muscle cell growth factors. Prog Growth Factor Res. 1991;3(3):177–206. doi: 10.1016/0955-2235(91)90006-p. [DOI] [PubMed] [Google Scholar]
- Coughlan A. F., Hau H., Dunlop L. C., Berndt M. C., Hancock W. W. P-selectin and platelet-activating factor mediate initial endotoxin-induced neutropenia. J Exp Med. 1994 Jan 1;179(1):329–334. doi: 10.1084/jem.179.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De S., Zelazny E. T., Souhrada J. F., Souhrada M. Interleukin-1 beta stimulates the proliferation of cultured airway smooth muscle cells via platelet-derived growth factor. Am J Respir Cell Mol Biol. 1993 Dec;9(6):645–651. doi: 10.1165/ajrcmb/9.6.645. [DOI] [PubMed] [Google Scholar]
- Edelman E. R., Adams D. H., Karnovsky M. J. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci U S A. 1990 May;87(10):3773–3777. doi: 10.1073/pnas.87.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman E. R., Pukac L. A., Karnovsky M. J. Protamine and protamine-insulins exacerbate the vascular response to injury. J Clin Invest. 1993 May;91(5):2308–2313. doi: 10.1172/JCI116460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeson E. E., Shen M. L. Accelerated atherosclerosis in hyperlipidemic C57BL/6 mice treated with cyclosporin A. Am J Pathol. 1993 Jun;142(6):1906–1915. [PMC free article] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Reidy M. A., Ross R. Balloon catheter de-endothelialization of the nude rat carotid. Response to injury in the absence of functional T lymphocytes. Am J Pathol. 1991 Apr;138(4):1045–1057. [PMC free article] [PubMed] [Google Scholar]
- Gasic G. P., Arenas C. P., Gasic T. B., Gasic G. J. Coagulation factors X, Xa, and protein S as potent mitogens of cultured aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2317–2320. doi: 10.1073/pnas.89.6.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabbe S., Gallo R. L., Lindgren A., Granstein R. D. Deficient antigen presentation by Langerhans cells from athymic (nu/nu) mice. Restoration with thymic transplantation or administration of cytokines. J Immunol. 1993 Oct 1;151(7):3430–3439. [PubMed] [Google Scholar]
- Gregory C. R., Huie P., Billingham M. E., Morris R. E. Rapamycin inhibits arterial intimal thickening caused by both alloimmune and mechanical injury. Its effect on cellular, growth factor, and cytokine response in injured vessels. Transplantation. 1993 Jun;55(6):1409–1418. doi: 10.1097/00007890-199306000-00037. [DOI] [PubMed] [Google Scholar]
- Grieff M., Loertscher R., Shohaib S. A., Stewart D. J. Cyclosporine-induced elevation in circulating endothelin-1 in patients with solid-organ transplants. Transplantation. 1993 Oct;56(4):880–884. doi: 10.1097/00007890-199310000-00021. [DOI] [PubMed] [Google Scholar]
- Haberstroh U., Zahner G., Disser M., Thaiss F., Wolf G., Stahl R. A. TGF-beta stimulates rat mesangial cell proliferation in culture: role of PDGF beta-receptor expression. Am J Physiol. 1993 Feb;264(2 Pt 2):F199–F205. doi: 10.1152/ajprenal.1993.264.2.F199. [DOI] [PubMed] [Google Scholar]
- Hancock W. H., Whitley W. D., Tullius S. G., Heemann U. W., Wasowska B., Baldwin W. M., 3rd, Tilney N. L. Cytokines, adhesion molecules, and the pathogenesis of chronic rejection of rat renal allografts. Transplantation. 1993 Sep;56(3):643–650. doi: 10.1097/00007890-199309000-00028. [DOI] [PubMed] [Google Scholar]
- Hancock W. W., Khoury S. J., Carpenter C. B., Sayegh M. H. Differential effects of oral versus intrathymic administration of polymorphic major histocompatibility complex class II peptides on mononuclear and endothelial cell activation and cytokine expression during a delayed-type hypersensitivity response. Am J Pathol. 1994 Jun;144(6):1149–1158. [PMC free article] [PubMed] [Google Scholar]
- Hancock W. W., Sayegh M. H., Sablinski T., Kut J. P., Kupiec-Weglinski J. W., Milford E. L. Blocking of mononuclear cell accumulation, cytokine production, and endothelial activation within rat cardiac allografts by CD4 monoclonal antibody therapy. Transplantation. 1992 Jun;53(6):1276–1280. doi: 10.1097/00007890-199206000-00022. [DOI] [PubMed] [Google Scholar]
- Leszczynski D., Zhao Y., Yeagley T. J., Foegh M. L. Direct and endothelial cell-mediated effect of cyclosporin A on the proliferation of rat smooth muscle cells in vitro. Am J Pathol. 1993 Jan;142(1):149–155. [PMC free article] [PubMed] [Google Scholar]
- Poston R. N., Hussain I. F. The immunohistochemical heterogeneity of atheroma macrophages: comparison with lymphoid tissues suggests that recently blood-derived macrophages can be distinguished from longer-resident cells. J Histochem Cytochem. 1993 Oct;41(10):1503–1512. doi: 10.1177/41.10.7504008. [DOI] [PubMed] [Google Scholar]
- Rocha B., Vassalli P., Guy-Grand D. The extrathymic T-cell development pathway. Immunol Today. 1992 Nov;13(11):449–454. doi: 10.1016/0167-5699(92)90074-H. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sablinski T., Hancock W. W., Tilney N. L., Kupiec-Weglinski J. W. CD4 monoclonal antibodies in organ transplantation--a review of progress. Transplantation. 1991 Oct;52(4):579–589. doi: 10.1097/00007890-199110000-00001. [DOI] [PubMed] [Google Scholar]
- Sayegh M. H., Sablinski T., Tanaka K., Kut J. P., Kwok C. A., Tilney N. L., Kupiec-Weglinski J. W., Milford E. L. Effects of BWH-4 anti-CD4 monoclonal antibody on rat vascularized cardiac allografts before and after engraftment. Transplantation. 1991 Feb;51(2):296–299. doi: 10.1097/00007890-199102000-00003. [DOI] [PubMed] [Google Scholar]
- Tazelaar H. D., Edwards W. D. Pathology of cardiac transplantation: recipient hearts (chronic heart failure) and donor hearts (acute and chronic rejection). Mayo Clin Proc. 1992 Jul;67(7):685–696. doi: 10.1016/s0025-6196(12)60726-5. [DOI] [PubMed] [Google Scholar]
- Tipping P. G., Hancock W. W. Production of tumor necrosis factor and interleukin-1 by macrophages from human atheromatous plaques. Am J Pathol. 1993 Jun;142(6):1721–1728. [PMC free article] [PubMed] [Google Scholar]
- Yue T. L., Mckenna P. J., Gu J. L., Feuerstein G. Z. Interleukin-8 is chemotactic for vascular smooth muscle cells. Eur J Pharmacol. 1993 Aug 10;240(1):81–84. doi: 10.1016/0014-2999(93)90549-w. [DOI] [PubMed] [Google Scholar]



