Abstract
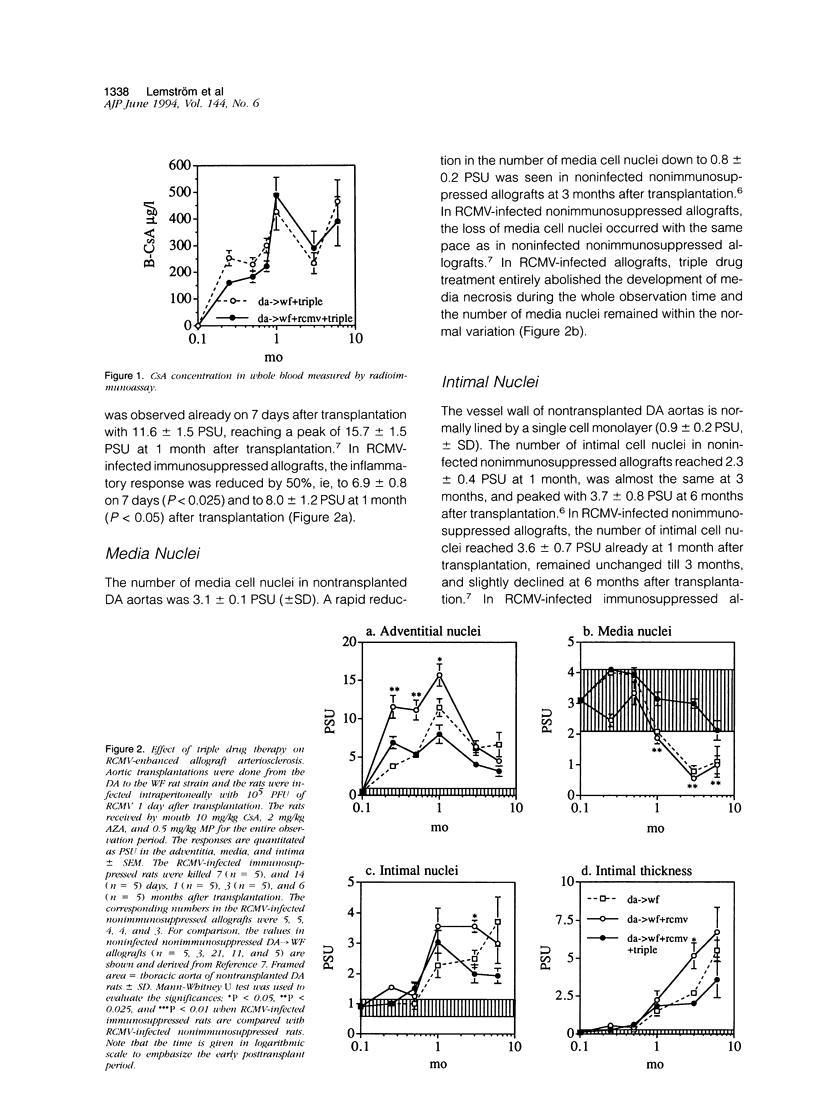
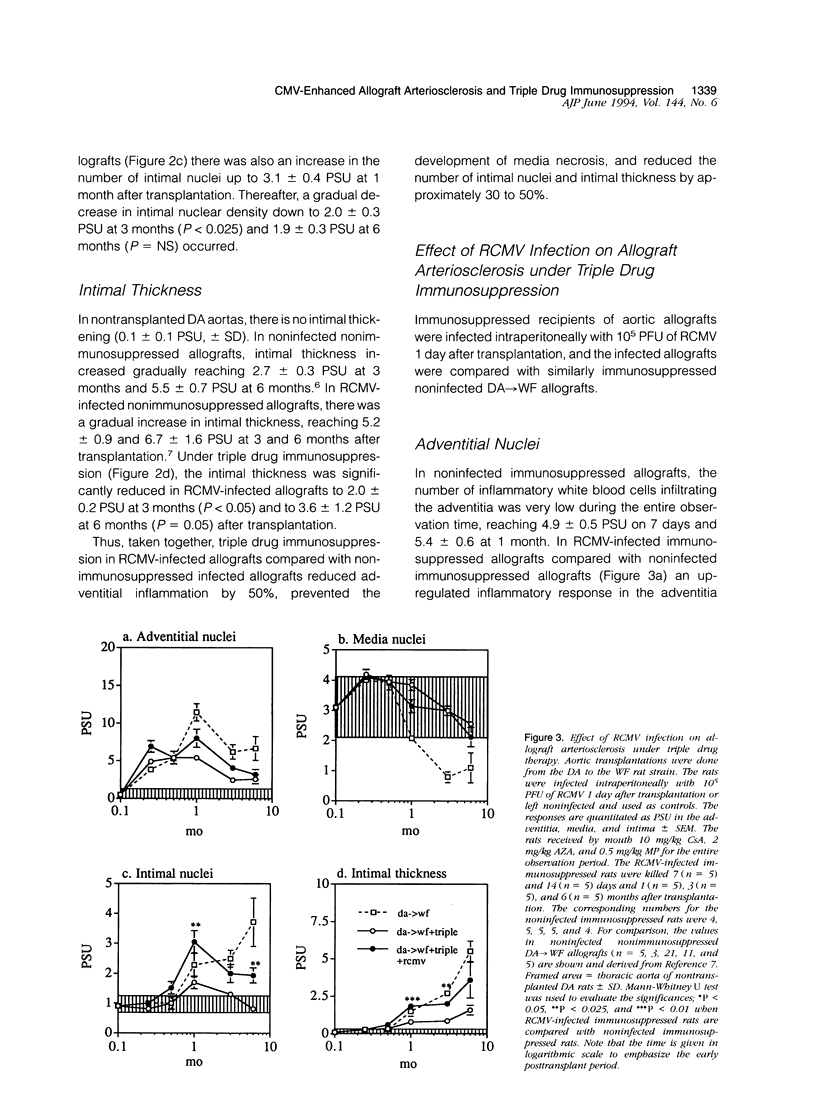
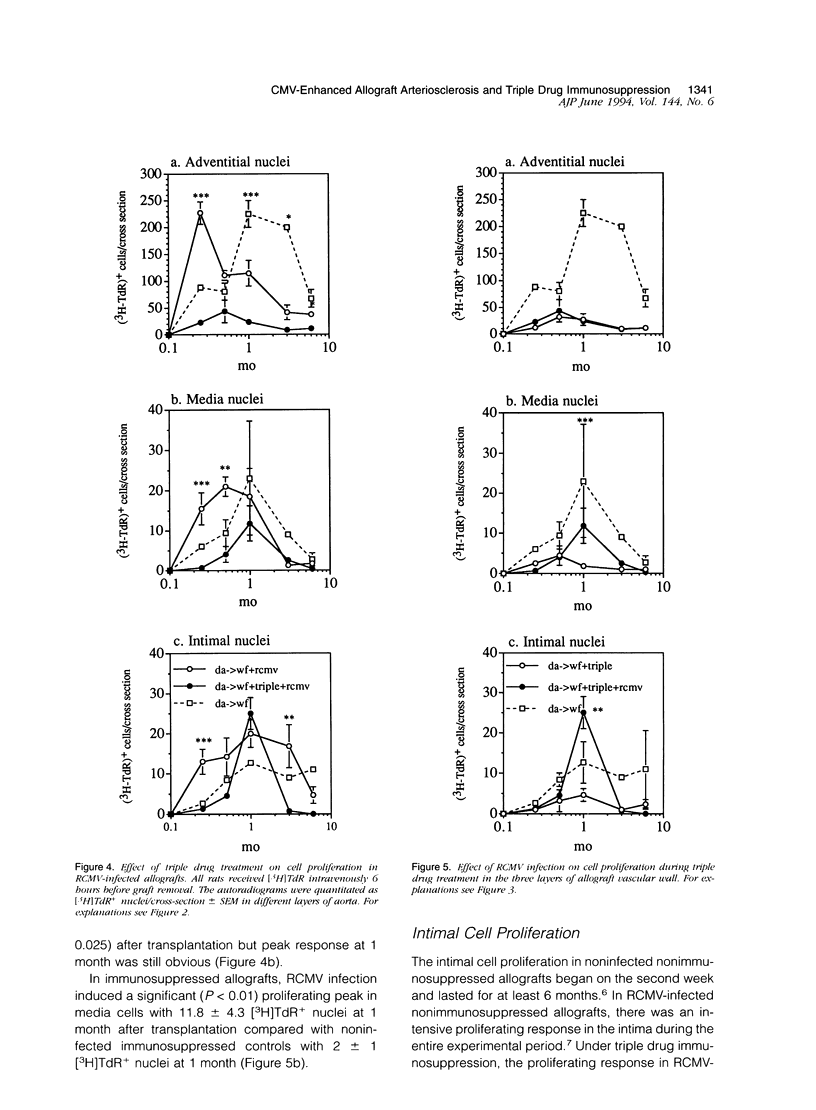
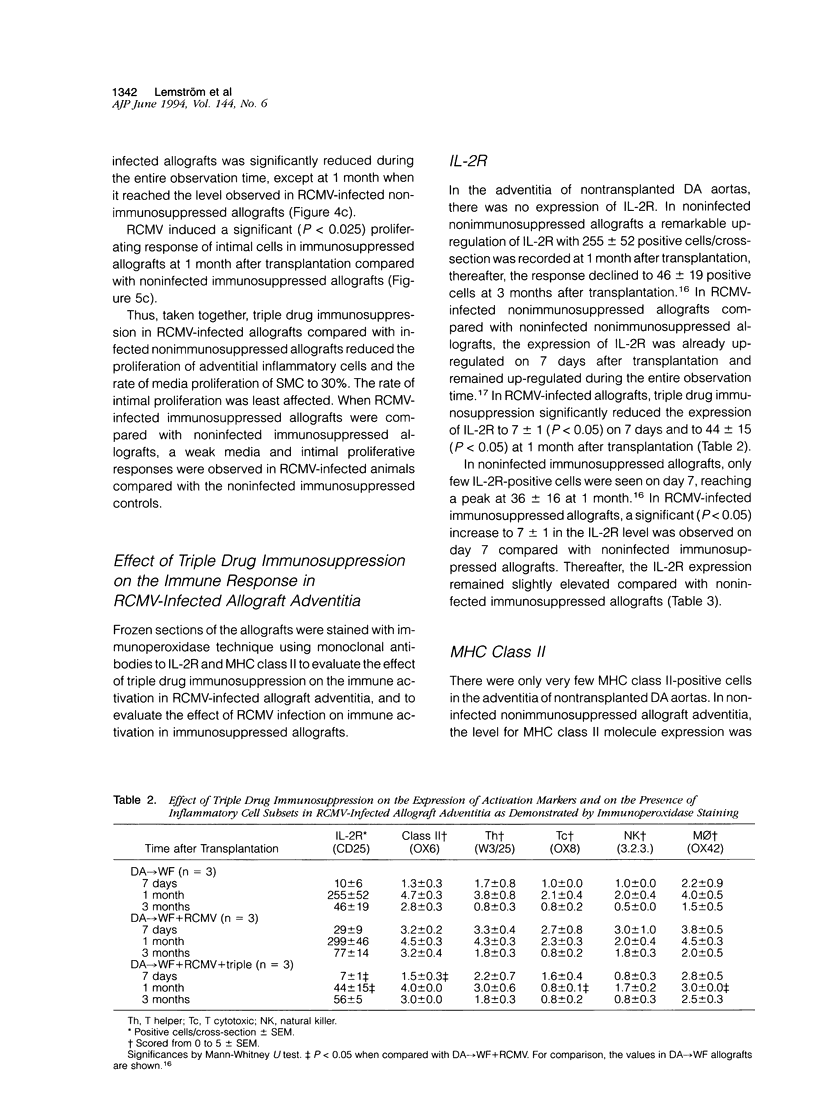
The effect of triple drug immunosuppression (cyclosporine A 10 mg/kg/day+methylprednisolone 0.5 mg/kg/day+azathioprine 2 mg/kg/day) on rat cytomegalovirus (RCMV)-enhanced allograft arteriosclerosis was investigated applying WF (AG-B2, RT1v) recipients of DA (AG-B4, RT1a) aortic allografts. The recipients were inoculated intraperitoneally with 10(5) plaque-forming units of RCMV 1 day after transplantation or left noninfected. The grafts were removed on 7 and 14 days, and at 1, 3, and 6 months after transplantation. The presence of viral infection was demonstrated by plaque assays, cell proliferation by [3H]thymidine autoradiography, and vascular wall alterations by quantitative histology and immunohistochemistry. Triple drug immunosuppression reduced the presence of infectious virus in plaque assays and induced early latency of viral infection. It significantly reduced the peak adventitial inflammatory response (P < 0.05) and reduced and delayed intimal nuclear intensity and intimal thickening (P < 0.05) in RCMV-infected allografts. The proliferative response of smooth muscle cells was reduced by triple drug immunosuppression to 50% of that observed in nonimmunosuppressed RCMV-infected allografts, but still the proliferative peak response was seen at 1 month. Only low level immune activation, ie, the expression of interleukin-2 receptor (P < 0.05) and MHC class II, was observed under triple drug immunosuppression in the adventitia of RCMV-infected allografts, whereas there was no substantial change in the phenotypic distribution of inflammatory cells. In conclusion, although RCMV infection significantly enhances allograft arteriosclerosis also in immunosuppressed allografts, triple drug immunosuppression has no additional detrimental effect but rather a protective one on vascular wall histology. These results further suggest that RCMV-enhanced allograft arteriosclerosis may be an immunopathological condition linked to the host immune response toward the graft and/or the virus rather than a direct virus-induced phenomenon.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhart G. R., Pascoe E. A., Mills A. S., Szentpetery S., Eich D. M., Mohanakumar T., Hastillo A., Thompson J. A., Hess M. L., Lower R. R. Accelerated coronary arteriosclerosis in cardiac transplant recipients. Transplant Rev (Orlando) 1987;1:31–46. doi: 10.1016/s0955-470x(87)80004-6. [DOI] [PubMed] [Google Scholar]
- Beck S., Barrell B. G. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature. 1988 Jan 21;331(6153):269–272. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
- Bruggeman C. A., Debie W. M., Grauls G., Majoor G., van Boven C. P. Infection of laboratory rats with a new cytomegalo-like virus. Arch Virol. 1983;76(3):189–199. doi: 10.1007/BF01311103. [DOI] [PubMed] [Google Scholar]
- Bruggeman C. A., Meijer H., Bosman F., van Boven C. P. Biology of rat cytomegalovirus infection. Intervirology. 1985;24(1):1–9. doi: 10.1159/000149612. [DOI] [PubMed] [Google Scholar]
- Bruggeman C. A., Meijer H., Dormans P. H., Debie W. M., Grauls G. E., van Boven C. P. Isolation of a cytomegalovirus-like agent from wild rats. Arch Virol. 1982;73(3-4):231–241. doi: 10.1007/BF01318077. [DOI] [PubMed] [Google Scholar]
- Dummer J. S., White L. T., Ho M., Griffith B. P., Hardesty R. L., Bahnson H. T. Morbidity of cytomegalovirus infection in recipients of heart or heart-lung transplants who received cyclosporine. J Infect Dis. 1985 Dec;152(6):1182–1191. doi: 10.1093/infdis/152.6.1182. [DOI] [PubMed] [Google Scholar]
- Friedman H. M., Macarak E. J., MacGregor R. R., Wolfe J., Kefalides N. A. Virus infection of endothelial cells. J Infect Dis. 1981 Feb;143(2):266–273. doi: 10.1093/infdis/143.2.266. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Nelson J. A., Walker L., Oldstone M. B. Sequence homology and immunologic cross-reactivity of human cytomegalovirus with HLA-DR beta chain: a means for graft rejection and immunosuppression. J Virol. 1988 Jan;62(1):100–105. doi: 10.1128/jvi.62.1.100-105.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geist L. J., Monick M. M., Stinski M. F., Hunninghake G. W. Cytomegalovirus immediate early genes prevent the inhibitory effect of cyclosporin A on interleukin 2 gene transcription. J Clin Invest. 1992 Nov;90(5):2136–2140. doi: 10.1172/JCI116099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geist L. J., Monick M. M., Stinski M. F., Hunninghake G. W. The immediate early genes of human cytomegalovirus upregulate expression of the interleukin-2 and interleukin-2 receptor genes. Am J Respir Cell Mol Biol. 1991 Sep;5(3):292–296. doi: 10.1165/ajrcmb/5.3.292. [DOI] [PubMed] [Google Scholar]
- Grattan M. T., Moreno-Cabral C. E., Starnes V. A., Oyer P. E., Stinson E. B., Shumway N. E. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989 Jun 23;261(24):3561–3566. [PubMed] [Google Scholar]
- Grundy J. E., Shanley J. D., Griffiths P. D. Is cytomegalovirus interstitial pneumonitis in transplant recipients an immunopathological condition? Lancet. 1987 Oct 31;2(8566):996–999. doi: 10.1016/s0140-6736(87)92560-8. [DOI] [PubMed] [Google Scholar]
- Gui X., Ho M., Camp P. E. Effect of cyclosporin A on murine natural killer cells. Infect Immun. 1982 Jun;36(3):1123–1127. doi: 10.1128/iai.36.3.1123-1127.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P. Warner-Lambert/Parke-Davis Award Lecture. Viral pathogenesis of atherosclerosis. Impact of molecular mimicry and viral genes. Am J Pathol. 1991 Dec;139(6):1195–1211. [PMC free article] [PubMed] [Google Scholar]
- Hosenpud J. D., Shipley G. D., Wagner C. R. Cardiac allograft vasculopathy: current concepts, recent developments, and future directions. J Heart Lung Transplant. 1992 Jan-Feb;11(1 Pt 1):9–23. [PubMed] [Google Scholar]
- Iwamoto G. K., Monick M. M., Clark B. D., Auron P. E., Stinski M. F., Hunninghake G. W. Modulation of interleukin 1 beta gene expression by the immediate early genes of human cytomegalovirus. J Clin Invest. 1990 Jun;85(6):1853–1857. doi: 10.1172/JCI114645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Perfect J. R. Infection and cyclosporine. Rev Infect Dis. 1989 Sep-Oct;11(5):677–690. doi: 10.1093/clinids/11.5.677. [DOI] [PubMed] [Google Scholar]
- Koskinen P. K., Krogerus L. A., Nieminen M. S., Mattila S. P., Häyry P. J., Lautenschlager I. T. Quantitation of cytomegalovirus infection-associated histologic findings in endomyocardial biopsies of heart allografts. J Heart Lung Transplant. 1993 May-Jun;12(3):343–354. [PubMed] [Google Scholar]
- Koskinen P. K. The association of the induction of vascular cell adhesion molecule-1 with cytomegalovirus antigenemia in human heart allografts. Transplantation. 1993 Nov;56(5):1103–1108. doi: 10.1097/00007890-199311000-00011. [DOI] [PubMed] [Google Scholar]
- Lemström K. B., Bruning J. H., Bruggeman C. A., Lautenschlager I. T., Häyry P. J. Cytomegalovirus infection enhances smooth muscle cell proliferation and intimal thickening of rat aortic allografts. J Clin Invest. 1993 Aug;92(2):549–558. doi: 10.1172/JCI116622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebe M., Schüler S., Zais O., Warnecke H., Fleck E., Hetzer R. Role of cytomegalovirus infection in the development of coronary artery disease in the transplanted heart. J Heart Transplant. 1990 Nov-Dec;9(6):707–711. [PubMed] [Google Scholar]
- MacGregor M. P., Lucia H. L., Vine W., FitzGerald P., Bia F. J. Effects of cyclosporine and cortisone on the pathogenesis of primary infection with cytomegalovirus in the guinea pig. J Infect Dis. 1986 Mar;153(3):503–510. doi: 10.1093/infdis/153.3.503. [DOI] [PubMed] [Google Scholar]
- McDonald K., Rector T. S., Braulin E. A., Kubo S. H., Olivari M. T. Association of coronary artery disease in cardiac transplant recipients with cytomegalovirus infection. Am J Cardiol. 1989 Aug 1;64(5):359–362. doi: 10.1016/0002-9149(89)90535-3. [DOI] [PubMed] [Google Scholar]
- Mennander A., Tiisala S., Halttunen J., Yilmaz S., Paavonen T., Häyry P. Chronic rejection in rat aortic allografts. An experimental model for transplant arteriosclerosis. Arterioscler Thromb. 1991 May-Jun;11(3):671–680. doi: 10.1161/01.atv.11.3.671. [DOI] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier R. D., Nelson J. A., Oldstone M. B. Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science. 1985 Nov 29;230(4729):1048–1051. doi: 10.1126/science.2997930. [DOI] [PubMed] [Google Scholar]
- Sharples L. D., Caine N., Mullins P., Scott J. P., Solis E., English T. A., Large S. R., Schofield P. M., Wallwork J. Risk factor analysis for the major hazards following heart transplantation--rejection, infection, and coronary occlusive disease. Transplantation. 1991 Aug;52(2):244–252. doi: 10.1097/00007890-199108000-00012. [DOI] [PubMed] [Google Scholar]
- Smiley M. L., Mar E. C., Huang E. S. Cytomegalovirus infection and viral-induced transformation of human endothelial cells. J Med Virol. 1988 Jun;25(2):213–226. doi: 10.1002/jmv.1890250212. [DOI] [PubMed] [Google Scholar]
- Smith P. D., Saini S. S., Raffeld M., Manischewitz J. F., Wahl S. M. Cytomegalovirus induction of tumor necrosis factor-alpha by human monocytes and mucosal macrophages. J Clin Invest. 1992 Nov;90(5):1642–1648. doi: 10.1172/JCI116035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stals F. S., Bosman F., van Boven C. P., Bruggeman C. A. An animal model for therapeutic intervention studies of CMV infection in the immunocompromised host. Arch Virol. 1990;114(1-2):91–107. doi: 10.1007/BF01311014. [DOI] [PubMed] [Google Scholar]
- Stovin P. G., Sharples L., Hutter J. A., Wallwork J., English T. A. Some prognostic factors for the development of transplant-related coronary artery disease in human cardiac allografts. J Heart Lung Transplant. 1991 Jan-Feb;10(1 Pt 1):38–44. [PubMed] [Google Scholar]
- The Registry of the International Society for Heart and Lung Transplantation: ninth official report--1992. J Heart Lung Transplant. 1992 Jul-Aug;11(4 Pt 1):599–606. [PubMed] [Google Scholar]
- Tumilowicz J. J., Gawlik M. E., Powell B. B., Trentin J. J. Replication of cytomegalovirus in human arterial smooth muscle cells. J Virol. 1985 Dec;56(3):839–845. doi: 10.1128/jvi.56.3.839-845.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustinov J. A., Loginov R. J., Bruggeman C. A., van der Meide P. H., Häyry P. J., Lautenschlager I. T. Cytomegalovirus induces class II expression in rat heart endothelial cells. J Heart Lung Transplant. 1993 Jul-Aug;12(4):644–651. [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970 Nov;135(2):253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]
- Zhang X. Q., Zhang J. T., Ho M. Effects of oral ciclosporin on acute and chronic murine cytomegalovirus infection. Intervirology. 1987;27(3):130–137. doi: 10.1159/000149731. [DOI] [PubMed] [Google Scholar]


