Abstract
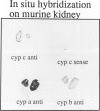
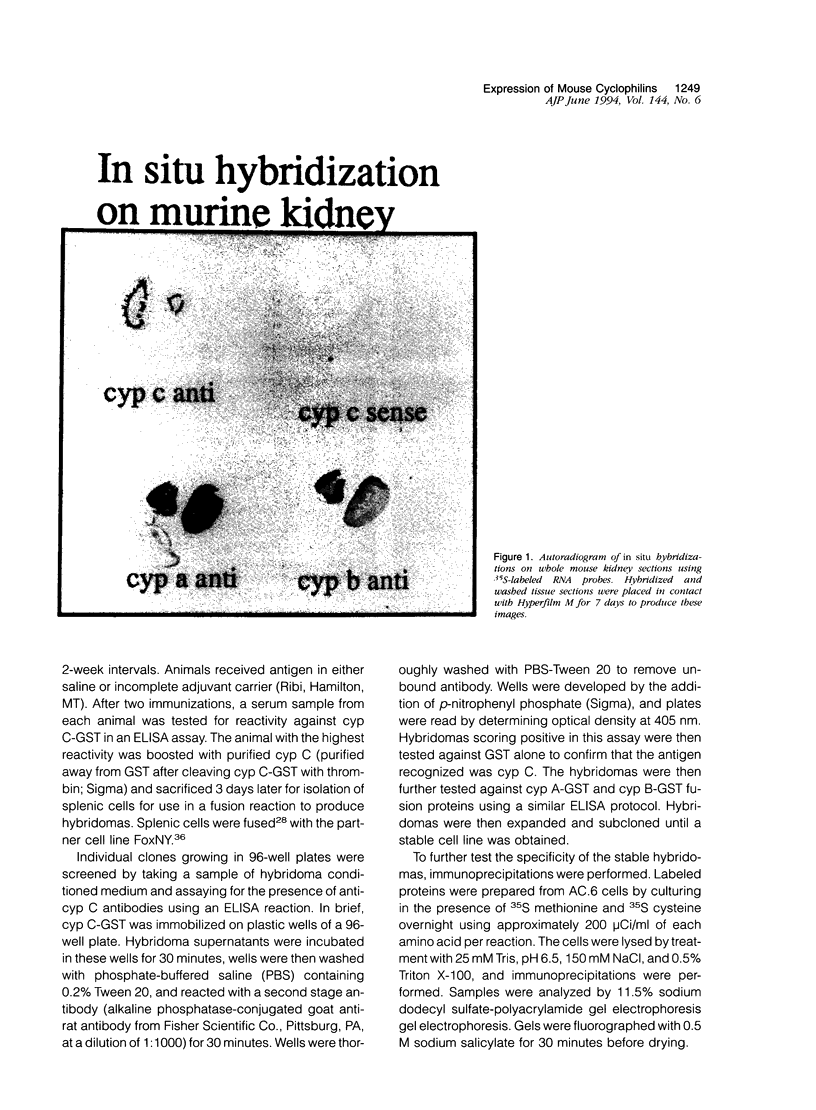
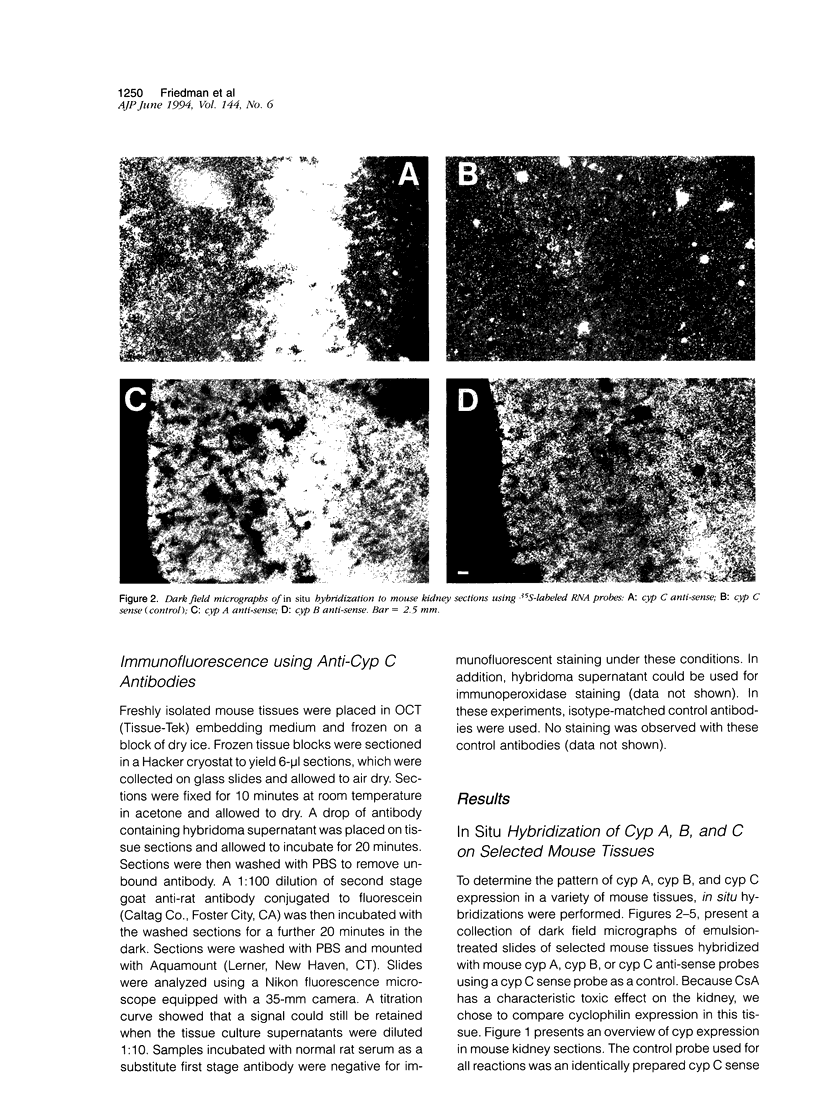
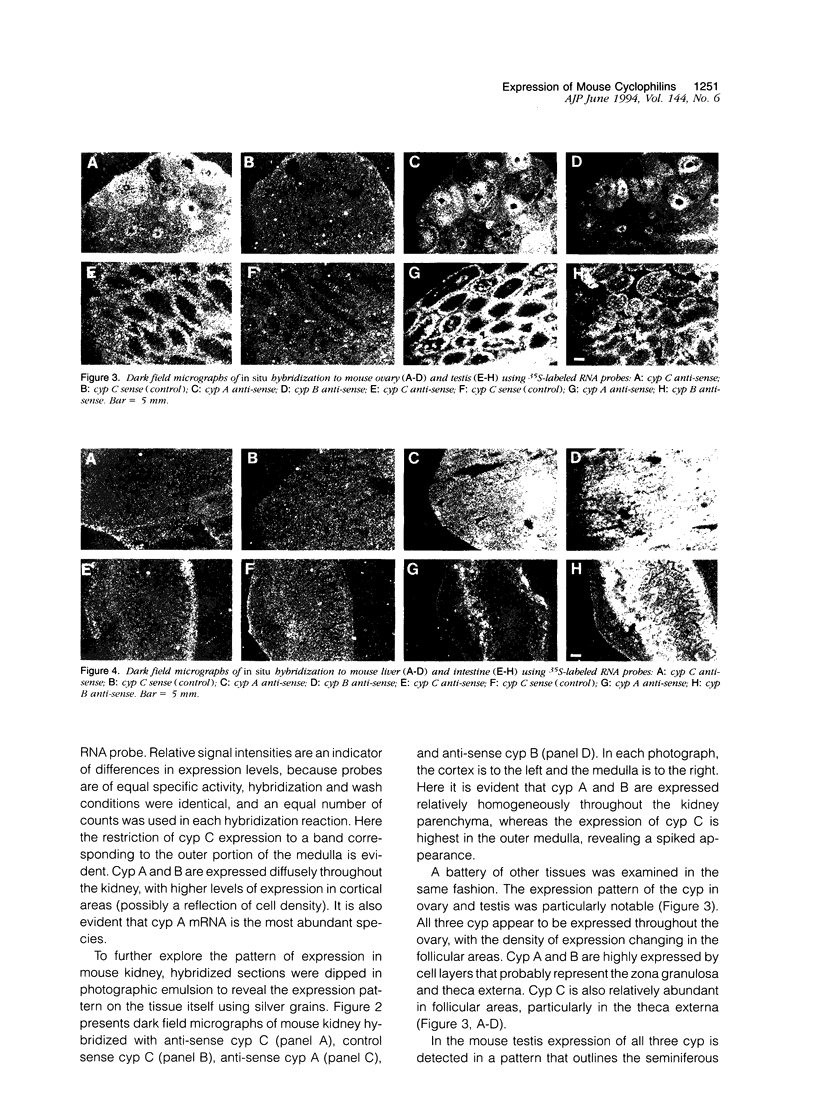
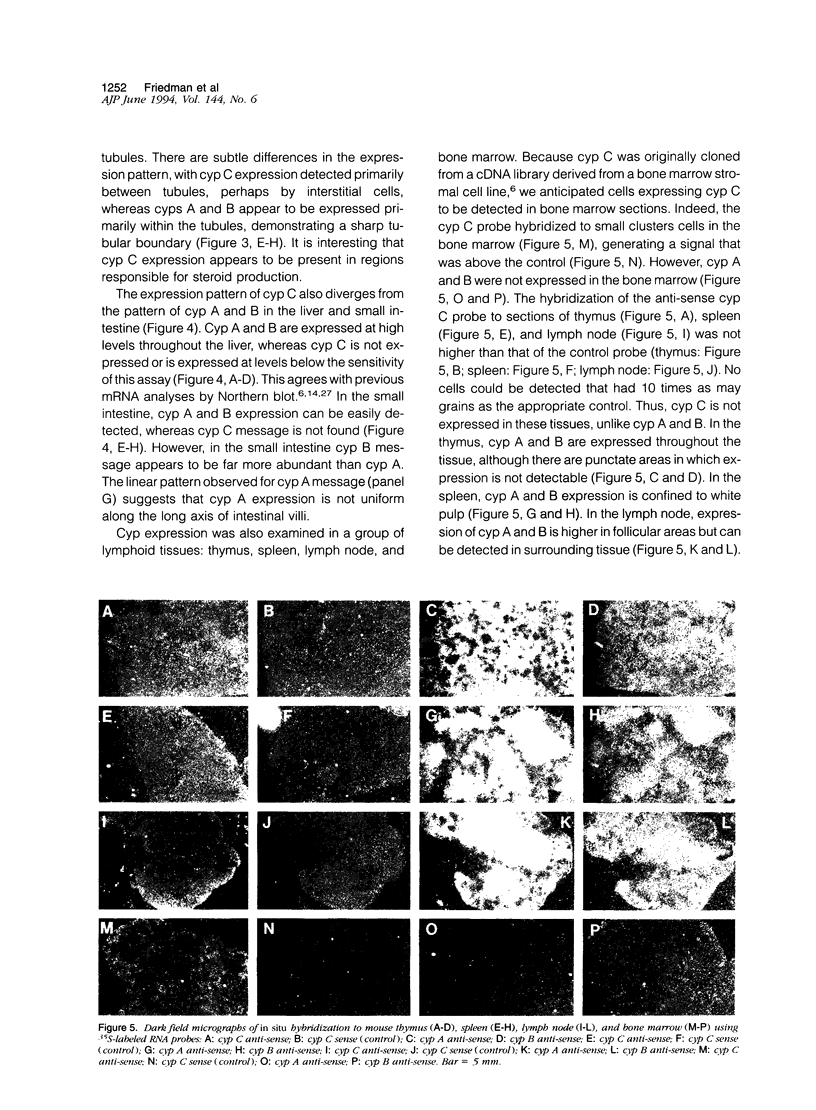
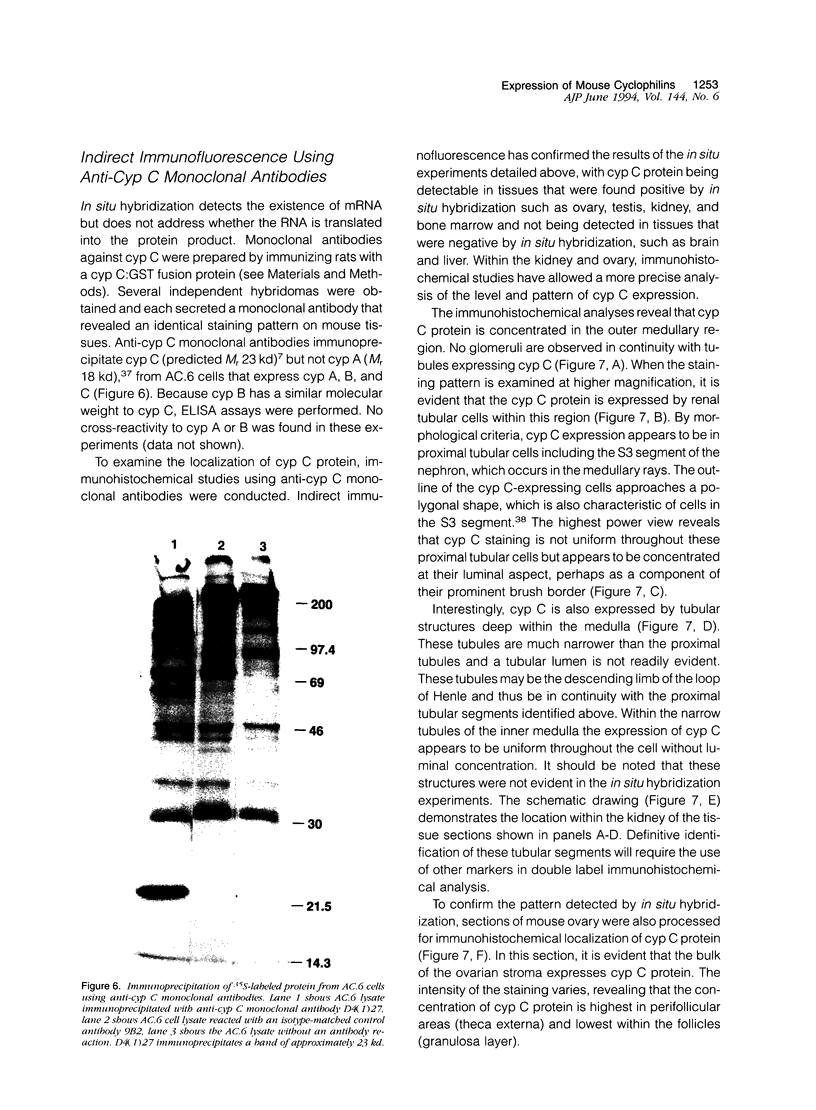
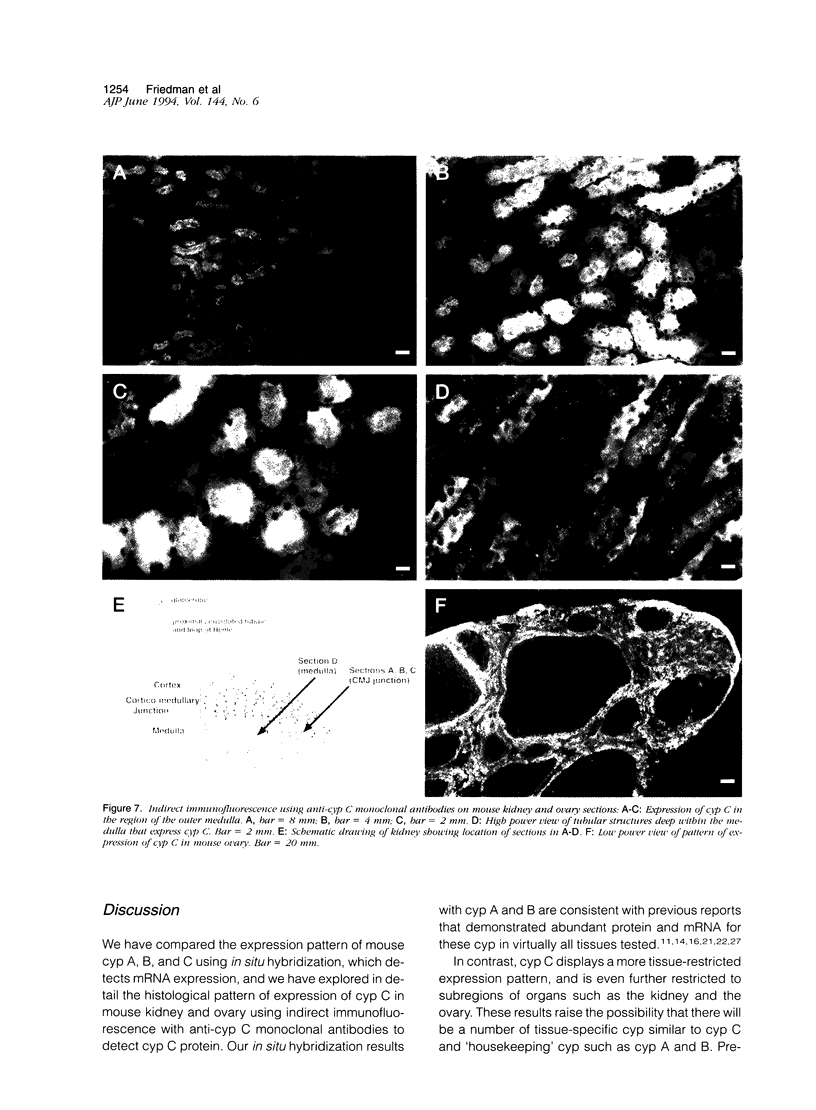
Cyclophilin C (cyp C) is a cyclosporin A (CsA) binding protein originally isolated from a mouse bone marrow stromal cell line. We have compared the expression patterns of the mammalian cyclophilins A, B, and C in mouse tissues using in situ hybridization. These studies reveal that cyp C is expressed in a restricted subset of tissues including mouse ovary, testis, bone marrow, and kidney. Within the kidney, cyp C is highly expressed in a narrow zone in the outer medulla. Using monoclonal antibodies reactive against cyp C, we find that the kidney cells expressing cyp C correspond to the S3 segment of the nephron. The S3 segment has been shown to sustain histopathological damage from high dosages of CsA, raising the possibility that cyp C may be involved in mediating this damage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awazu M., Sugiura M., Inagami T., Ichikawa I., Kon V. Cyclosporine promotes glomerular endothelin binding in vivo. J Am Soc Nephrol. 1991 May;1(11):1253–1258. doi: 10.1681/ASN.V1111253. [DOI] [PubMed] [Google Scholar]
- Bennett W. M., Houghton D. C., Buss W. C. Cyclosporine-induced renal dysfunction: correlations between cellular events and whole kidney function. J Am Soc Nephrol. 1991 May;1(11):1212–1219. doi: 10.1681/ASN.V1111212. [DOI] [PubMed] [Google Scholar]
- Bergsma D. J., Eder C., Gross M., Kersten H., Sylvester D., Appelbaum E., Cusimano D., Livi G. P., McLaughlin M. M., Kasyan K. The cyclophilin multigene family of peptidyl-prolyl isomerases. Characterization of three separate human isoforms. J Biol Chem. 1991 Dec 5;266(34):23204–23214. [PubMed] [Google Scholar]
- Colley N. J., Baker E. K., Stamnes M. A., Zuker C. S. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991 Oct 18;67(2):255–263. doi: 10.1016/0092-8674(91)90177-z. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Foung S. K., Sasaki D. T., Grumet F. C., Engleman E. G. Production of functional human T-T hybridomas in selection medium lacking aminopterin and thymidine. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7484–7488. doi: 10.1073/pnas.79.23.7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Trahey M., Weissman I. Cloning and characterization of cyclophilin C-associated protein: a candidate natural cellular ligand for cyclophilin C. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6815–6819. doi: 10.1073/pnas.90.14.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Weissman I. Two cytoplasmic candidates for immunophilin action are revealed by affinity for a new cyclophilin: one in the presence and one in the absence of CsA. Cell. 1991 Aug 23;66(4):799–806. doi: 10.1016/0092-8674(91)90123-g. [DOI] [PubMed] [Google Scholar]
- Haendler B., Hofer-Warbinek R., Hofer E. Complementary DNA for human T-cell cyclophilin. EMBO J. 1987 Apr;6(4):947–950. doi: 10.1002/j.1460-2075.1987.tb04843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendler B., Keller R., Hiestand P. C., Kocher H. P., Wegmann G., Movva N. R. Yeast cyclophilin: isolation and characterization of the protein, cDNA and gene. Gene. 1989 Nov 15;83(1):39–46. doi: 10.1016/0378-1119(89)90401-0. [DOI] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Galat A., Uehling D. E., Schreiber S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989 Oct 26;341(6244):758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Harrison R. K., Stein R. L. Mechanistic studies of peptidyl prolyl cis-trans isomerase: evidence for catalysis by distortion. Biochemistry. 1990 Feb 20;29(7):1684–1689. doi: 10.1021/bi00459a003. [DOI] [PubMed] [Google Scholar]
- Hasel K. W., Glass J. R., Godbout M., Sutcliffe J. G. An endoplasmic reticulum-specific cyclophilin. Mol Cell Biol. 1991 Jul;11(7):3484–3491. doi: 10.1128/mcb.11.7.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes H. D., Jackson N. M., O'Connor R. P., Hunt D. A., White M. D. Pathogenetic mechanisms of nephrotoxicity: insights into cyclosporine nephrotoxicity. Transplant Proc. 1985 Aug;17(4 Suppl 1):51–62. [PubMed] [Google Scholar]
- Koletsky A. J., Harding M. W., Handschumacher R. E. Cyclophilin: distribution and variant properties in normal and neoplastic tissues. J Immunol. 1986 Aug 1;137(3):1054–1059. [PubMed] [Google Scholar]
- Liu J., Albers M. W., Wandless T. J., Luan S., Alberg D. G., Belshaw P. J., Cohen P., MacKintosh C., Klee C. B., Schreiber S. L. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry. 1992 Apr 28;31(16):3896–3901. doi: 10.1021/bi00131a002. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Liu J., Walsh C. T. Peptidyl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4028–4032. doi: 10.1073/pnas.87.11.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks W. H., Harding M. W., Handschumacher R., Marks C., Lorber M. I. The immunochemical distribution of cyclophilin in normal mammalian tissues. Transplantation. 1991 Aug;52(2):340–345. doi: 10.1097/00007890-199108000-00030. [DOI] [PubMed] [Google Scholar]
- McDonald M. L., Ardito T., Marks W. H., Kashgarian M., Lorber M. I. The effect of cyclosporine administration on the cellular distribution and content of cyclophilin. Transplantation. 1992 Feb;53(2):460–466. doi: 10.1097/00007890-199202010-00037. [DOI] [PubMed] [Google Scholar]
- Myers B. D., Newton L. Cyclosporine-induced chronic nephropathy: an obliterative microvascular renal injury. J Am Soc Nephrol. 1991 Aug;2(2 Suppl 1):S45–S52. doi: 10.1681/ASN.V22s45. [DOI] [PubMed] [Google Scholar]
- Myers B. D., Ross J., Newton L., Luetscher J., Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984 Sep 13;311(11):699–705. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- Perico N., Dadan J., Remuzzi G. Endothelin mediates the renal vasoconstriction induced by cyclosporine in the rat. J Am Soc Nephrol. 1990 Jul;1(1):76–83. [PubMed] [Google Scholar]
- Perico N., Pasini M., Gaspari F., Abbate M., Remuzzi G. Co-participation of thromboxane A2 and leukotriene C4 and D4 in mediating cyclosporine-induced acute renal failure. Transplantation. 1991 Nov;52(5):873–878. doi: 10.1097/00007890-199111000-00023. [DOI] [PubMed] [Google Scholar]
- Price E. R., Zydowsky L. D., Jin M. J., Baker C. H., McKeon F. D., Walsh C. T. Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Shortridge R. D., Larrivee D. C., Ono T., Ozaki M., Pak W. L. Drosophila ninaA gene encodes an eye-specific cyclophilin (cyclosporine A binding protein). Proc Natl Acad Sci U S A. 1989 Jul;86(14):5390–5394. doi: 10.1073/pnas.86.14.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991 Jan 18;251(4991):283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Schönbrunner E. R., Mayer S., Tropschug M., Fischer G., Takahashi N., Schmid F. X. Catalysis of protein folding by cyclophilins from different species. J Biol Chem. 1991 Feb 25;266(6):3630–3635. [PubMed] [Google Scholar]
- Seethalakshmi L., Menon M., Pallias J. D., Khauli R. B., Diamond D. A. Cyclosporine: its harmful effects on testicular function and male fertility. Transplant Proc. 1989 Feb;21(1 Pt 1):928–930. [PubMed] [Google Scholar]
- Shieh B. H., Stamnes M. A., Seavello S., Harris G. L., Zuker C. S. The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature. 1989 Mar 2;338(6210):67–70. doi: 10.1038/338067a0. [DOI] [PubMed] [Google Scholar]
- Stamnes M. A., Shieh B. H., Chuman L., Harris G. L., Zuker C. S. The cyclophilin homolog ninaA is a tissue-specific integral membrane protein required for the proper synthesis of a subset of Drosophila rhodopsins. Cell. 1991 Apr 19;65(2):219–227. doi: 10.1016/0092-8674(91)90156-s. [DOI] [PubMed] [Google Scholar]
- Swanson S. K., Born T., Zydowsky L. D., Cho H., Chang H. Y., Walsh C. T., Rusnak F. Cyclosporin-mediated inhibition of bovine calcineurin by cyclophilins A and B. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3741–3745. doi: 10.1073/pnas.89.9.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart R. T., Samloff I. M. Stable antibody-producing murine hybridomas. Science. 1983 Mar 11;219(4589):1228–1230. doi: 10.1126/science.6402815. [DOI] [PubMed] [Google Scholar]
- Theodor L., Peleg D., Meyuhas O. P31, a mammalian housekeeping protein encoded by a multigene family containing a high proportion of pseudogenes. Biochim Biophys Acta. 1985 Nov 13;826(2-3):137–146. doi: 10.1016/0167-4781(85)90119-8. [DOI] [PubMed] [Google Scholar]
- Tisher C. C., Bulger R. E., Trump B. F. Human renal ultrastructure. I. Proximal tubule of healthy individuals. Lab Invest. 1966 Aug;15(8):1357–1394. [PubMed] [Google Scholar]
- Tropschug M., Nicholson D. W., Hartl F. U., Köhler H., Pfanner N., Wachter E., Neupert W. Cyclosporin A-binding protein (cyclophilin) of Neurospora crassa. One gene codes for both the cytosolic and mitochondrial forms. J Biol Chem. 1988 Oct 5;263(28):14433–14440. [PubMed] [Google Scholar]
- Weinberg J. M. Issues in the pathophysiology of nephrotoxic renal tubular cell injury pertinent to understanding cyclosporine nephrotoxicity. Transplant Proc. 1985 Aug;17(4 Suppl 1):81–90. [PubMed] [Google Scholar]
- Wolf G., Killen P. D., Neilson E. G. Cyclosporin A stimulates transcription and procollagen secretion in tubulointerstitial fibroblasts and proximal tubular cells. J Am Soc Nephrol. 1990 Dec;1(6):918–922. doi: 10.1681/ASN.V16918. [DOI] [PubMed] [Google Scholar]