Abstract
Previous morphological immunoenzymatic studies with organelle-specific antibodies have disclosed an apparent fragmentation of the Golgi apparatus in large numbers of motor neurons in 12 cases of sporadic, non-Guamanian amyotrophic lateral sclerosis (ALS) in three cases of other types of motor neuron disease and in one case of a mitochondrial myopathy with cytochrome c oxidase deficiency. Motor neurons with fragmented Golgi apparatus were moderately atrophic; in these cells, discrete immunostained elements of the organelle were twice as many as in normal neurons, and the size of each Golgi element and the percentage of the cytoplasmic area occupied by the Golgi apparatus were reduced (Am J Pathol 1992, 140: 731-737). In this report we have confirmed the fragmentation of the organelle of motor neurons in the spinal cord in six sporadic cases of Guamanian ALS. In four of the six cases the clinical course was 1 to 2 years. The percentages of motor neurons with fragmented Golgi apparatus varied from 38 to 92. Motor neurons from three additional cases of Guamanian ALS of clinical duration from 5 to 7 years did not show fragmentation of the Golgi apparatus. In two cases of Guamanian ALS and in one non-Guamanian ALS, all neurons with ubiquitin-positive skein-like or granular inclusions believed to be pathognomonic for ALS had fragmented Golgi apparatus. To examine whether the fragmentation of the Golgi apparatus results from reactions to either neuronal deafferentation or to lesions of proximal axons, we conducted two experimental studies. In the first study, we examined in cats the Golgi apparatus of deafferented neurons of the dorsal lateral geniculate nucleus. In the second study, we examined the Golgi apparatus of motor neurons in the spinal cord of rats with proximal axonopathy induced by beta,beta'-iminodipropionitrile. In these two experiments, the neuronal Golgi apparatus studied by immunoenzymatic techniques and morphometry, was not fragmented. Taken together, the results of these studies strongly suggest that the fragmentation of the Golgi apparatus of motor neurons in ALS represents an important and perhaps early change of the organelle that may be involved in the pathogenesis of ALS. The fragmentation of the Golgi apparatus of motor neurons is a fairly specific and easily recognizable marker of ALS and may be used together with other criteria for comparisons between the human disease and proposed animal models of the disorder.
Full text
PDF



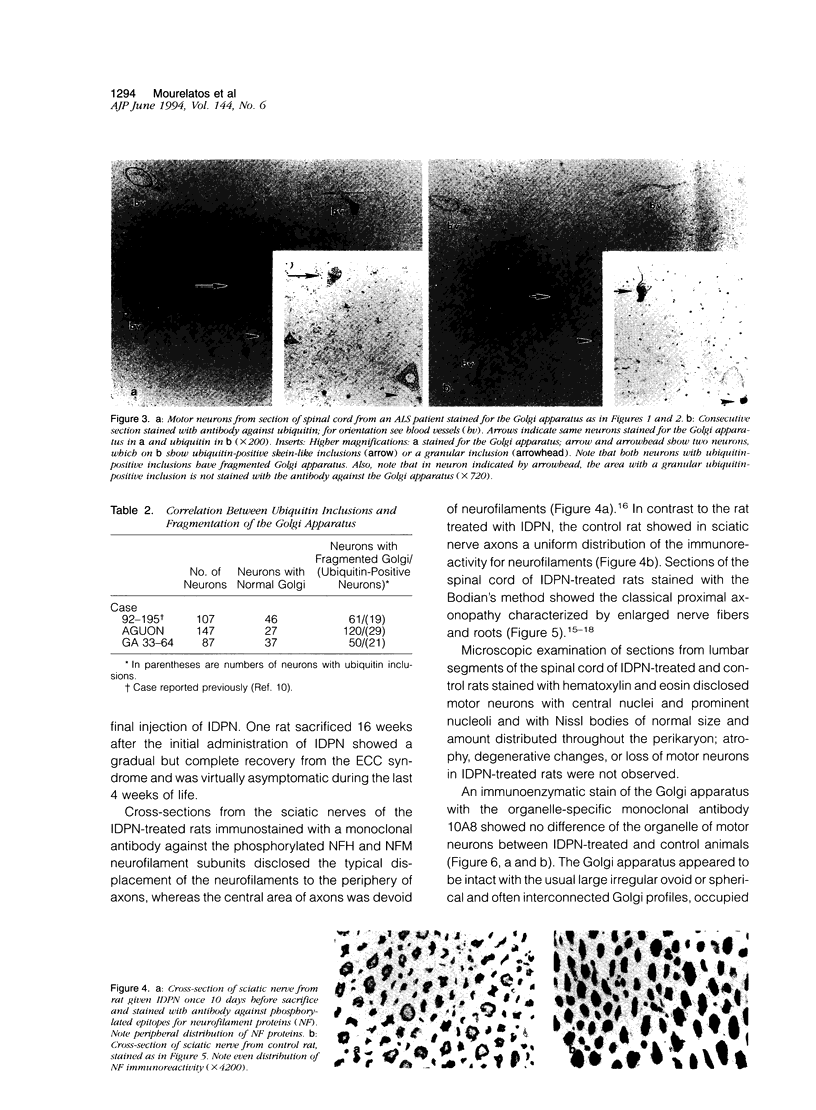


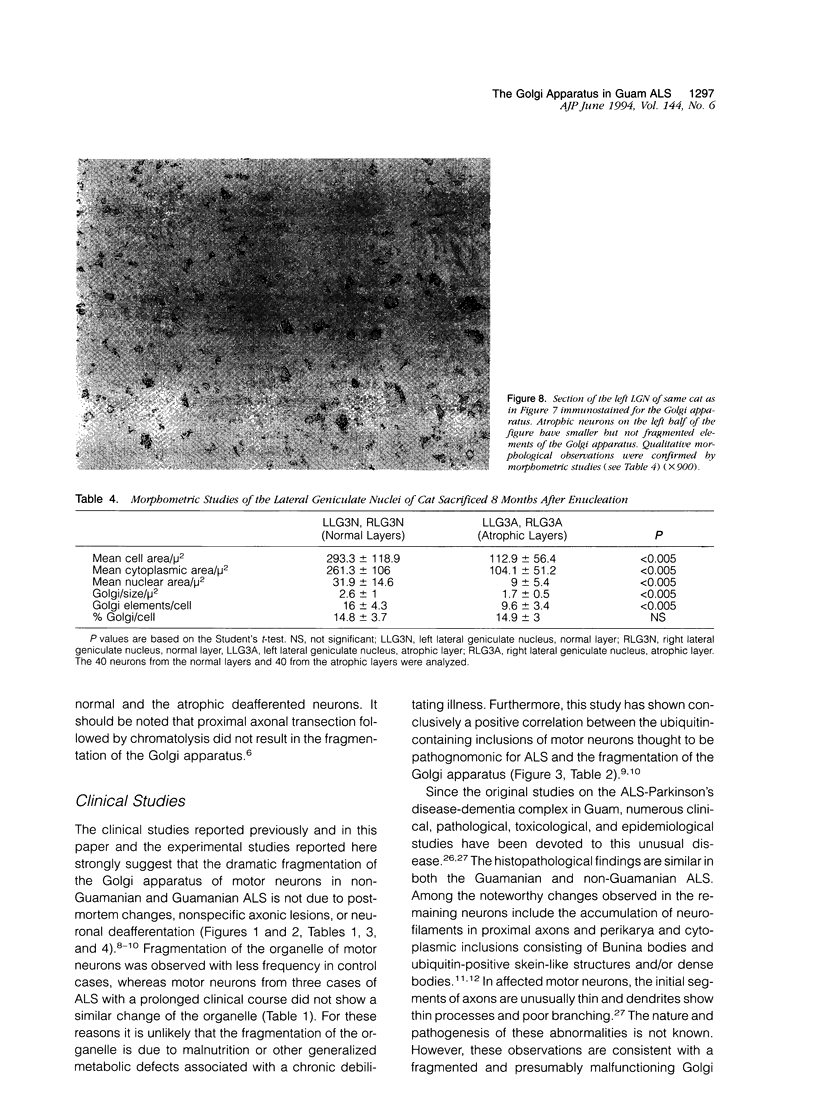



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHOU S. M., HARTMANN H. A. AXONAL LESIONS AND WALTZING SYNDROME AFTER IDPN ADMINISTRATION IN RATS. WITH A CONCEPT--"AXOSTASIS". Acta Neuropathol. 1964 May 5;3:428–450. doi: 10.1007/BF00688453. [DOI] [PubMed] [Google Scholar]
- Campadelli G., Brandimarti R., Di Lazzaro C., Ward P. L., Roizman B., Torrisi M. R. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2798–2802. doi: 10.1073/pnas.90.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croul S. E., Mezitis S. G., Gonatas N. K. An anti-organelle antibody in pathology. The chromatolytic reaction studied with a monoclonal antibody against the Golgi apparatus. Am J Pathol. 1988 Nov;133(2):355–362. [PMC free article] [PubMed] [Google Scholar]
- Croul S., Mezitis S. G., Stieber A., Chen Y. J., Gonatas J. O., Goud B., Gonatas N. K. Immunocytochemical visualization of the Golgi apparatus in several species, including human, and tissues with an antiserum against MG-160, a sialoglycoprotein of rat Golgi apparatus. J Histochem Cytochem. 1990 Jul;38(7):957–963. doi: 10.1177/38.7.2355176. [DOI] [PubMed] [Google Scholar]
- Côté F., Collard J. F., Julien J. P. Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Cell. 1993 Apr 9;73(1):35–46. doi: 10.1016/0092-8674(93)90158-m. [DOI] [PubMed] [Google Scholar]
- Deng H. X., Hentati A., Tainer J. A., Iqbal Z., Cayabyab A., Hung W. Y., Getzoff E. D., Hu P., Herzfeldt B., Roos R. P. Amyotrophic lateral sclerosis and structural defects in Cu,Zn superoxide dismutase. Science. 1993 Aug 20;261(5124):1047–1051. doi: 10.1126/science.8351519. [DOI] [PubMed] [Google Scholar]
- Denlinger R. H., Anthony D. C., Amarnath V., Graham D. G. Comparison of location, severity, and dose response of proximal axonal lesions induced by 3,3'-iminodipropionitrile and deuterium substituted analogs. J Neuropathol Exp Neurol. 1992 Nov;51(6):569–576. doi: 10.1097/00005072-199211000-00001. [DOI] [PubMed] [Google Scholar]
- Duden R., Griffiths G., Frank R., Argos P., Kreis T. E. Beta-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to beta-adaptin. Cell. 1991 Feb 8;64(3):649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Gonatas J. O., Mezitis S. G., Stieber A., Fleischer B., Gonatas N. K. MG-160. A novel sialoglycoprotein of the medial cisternae of the Golgi apparatus [published eeratum appears in J Biol Chem 1989 Mar 5;264(7):4264]. J Biol Chem. 1989 Jan 5;264(1):646–653. [PubMed] [Google Scholar]
- Gonatas N. K. Presidential address. The role of neuronal golgi apparatus in a centripetal membrane vesicular traffic. J Neuropathol Exp Neurol. 1982 Jan;41(1):6–17. doi: 10.1097/00005072-198201000-00002. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Stieber A., Mourelatos Z., Chen Y., Gonatas J. O., Appel S. H., Hays A. P., Hickey W. F., Hauw J. J. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis. Am J Pathol. 1992 Mar;140(3):731–737. [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gregor P., Reeves R. H., Jabs E. W., Yang X., Dackowski W., Rochelle J. M., Brown R. H., Jr, Haines J. L., O'Hara B. F., Uhl G. R. Chromosomal localization of glutamate receptor genes: relationship to familial amyotrophic lateral sclerosis and other neurological disorders of mice and humans. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3053–3057. doi: 10.1073/pnas.90.7.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. W., Hoffman P. N., Clark A. W., Carroll P. T., Price D. L. Slow axonal transport of neurofilament proteins: impairment of beta,beta'-iminodipropionitrile administration. Science. 1978 Nov 10;202(4368):633–635. doi: 10.1126/science.81524. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. Quantitative studies of transneuronal atrophy in the dorsal lateral geniculate nucleus of cats and kittens. J Comp Neurol. 1973 Jun 15;149(4):423–438. doi: 10.1002/cne.901490403. [DOI] [PubMed] [Google Scholar]
- Hammerschlag R., Stone G. C., Bolen F. A., Lindsey J. D., Ellisman M. H. Evidence that all newly synthesized proteins destined for fast axonal transport pass through the Golgi apparatus. J Cell Biol. 1982 Jun;93(3):568–575. doi: 10.1083/jcb.93.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Hirano A., Donnenfeld H. A Golgi study of the large anterior horn cells of the lumbar cords in normal spinal cords and in amyotrophic lateral sclerosis. Acta Neuropathol. 1987;75(1):34–40. doi: 10.1007/BF00686790. [DOI] [PubMed] [Google Scholar]
- Kreis T. E. Role of microtubules in the organisation of the Golgi apparatus. Cell Motil Cytoskeleton. 1990;15(2):67–70. doi: 10.1002/cm.970150202. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W., Trojanowski J. Q. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987 Nov;7(11):3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S., Goto S., Kusaka H., Imai T., Murakami N., Hashizume Y., Okazaki H., Hirano A. Ubiquitin-positive inclusion in anterior horn cells in subgroups of motor neuron diseases: a comparative study of adult-onset amyotrophic lateral sclerosis, juvenile amyotrophic lateral sclerosis and Werdnig-Hoffmann disease. J Neurol Sci. 1993 Apr;115(2):208–213. doi: 10.1016/0022-510x(93)90226-o. [DOI] [PubMed] [Google Scholar]
- McNamara J. O., Fridovich I. Human genetics. Did radicals strike Lou Gehrig? Nature. 1993 Mar 4;362(6415):20–21. doi: 10.1038/362020a0. [DOI] [PubMed] [Google Scholar]
- Mourelatos Z., Adler H., Hirano A., Donnenfeld H., Gonatas J. O., Gonatas N. K. Fragmentation of the Golgi apparatus of motor neurons in amyotrophic lateral sclerosis revealed by organelle-specific antibodies. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4393–4395. doi: 10.1073/pnas.87.11.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourelatos Z., Yachnis A., Rorke L., Mikol J., Gonatas N. K. The Golgi apparatus of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 1993 Jun;33(6):608–615. doi: 10.1002/ana.410330609. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S. C., Autilio-Gambetti L., Gambetti P. Reorganization of axoplasmic organelles following beta, beta'-iminodipropionitrile administration. J Cell Biol. 1981 Dec;91(3 Pt 1):866–871. doi: 10.1083/jcb.91.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. HISTOCHEMICAL AND ULTRASTRUCTURAL STUDIES ON HELA CELL CULTURES EXPOSED TO SPINDLE INHIBITORS WITH SPECIAL REFERENCE TO THE INTERPHASE CELL. J Histochem Cytochem. 1964 Sep;12:704–711. doi: 10.1177/12.9.704. [DOI] [PubMed] [Google Scholar]
- Rowland L. P. Ten central themes in a decade of ALS research. Adv Neurol. 1991;56:3–23. [PubMed] [Google Scholar]
- Schmidt M. L., Carden M. J., Lee V. M., Trojanowski J. Q. Phosphate dependent and independent neurofilament epitopes in the axonal swellings of patients with motor neuron disease and controls. Lab Invest. 1987 Mar;56(3):282–294. [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Siddique T., Figlewicz D. A., Pericak-Vance M. A., Haines J. L., Rouleau G., Jeffers A. J., Sapp P., Hung W. Y., Bebout J., McKenna-Yasek D. Linkage of a gene causing familial amyotrophic lateral sclerosis to chromosome 21 and evidence of genetic-locus heterogeneity. N Engl J Med. 1991 May 16;324(20):1381–1384. doi: 10.1056/NEJM199105163242001. [DOI] [PubMed] [Google Scholar]
- Stone J., Hansen S. M. The projection of the cat's retina on the lateral geniculate nucleus. J Comp Neurol. 1966 Apr;126(4):601–624. [PubMed] [Google Scholar]
- Stone R. Guam: deadly disease dying out. Science. 1993 Jul 23;261(5120):424–426. doi: 10.1126/science.8332906. [DOI] [PubMed] [Google Scholar]
- Weiner O. H., Murphy J., Griffiths G., Schleicher M., Noegel A. A. The actin-binding protein comitin (p24) is a component of the Golgi apparatus. J Cell Biol. 1993 Oct;123(1):23–34. doi: 10.1083/jcb.123.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Cork L. C., Griffin J. W., Cleveland D. W. Increased expression of neurofilament subunit NF-L produces morphological alterations that resemble the pathology of human motor neuron disease. Cell. 1993 Apr 9;73(1):23–33. doi: 10.1016/0092-8674(93)90157-l. [DOI] [PubMed] [Google Scholar]