Abstract
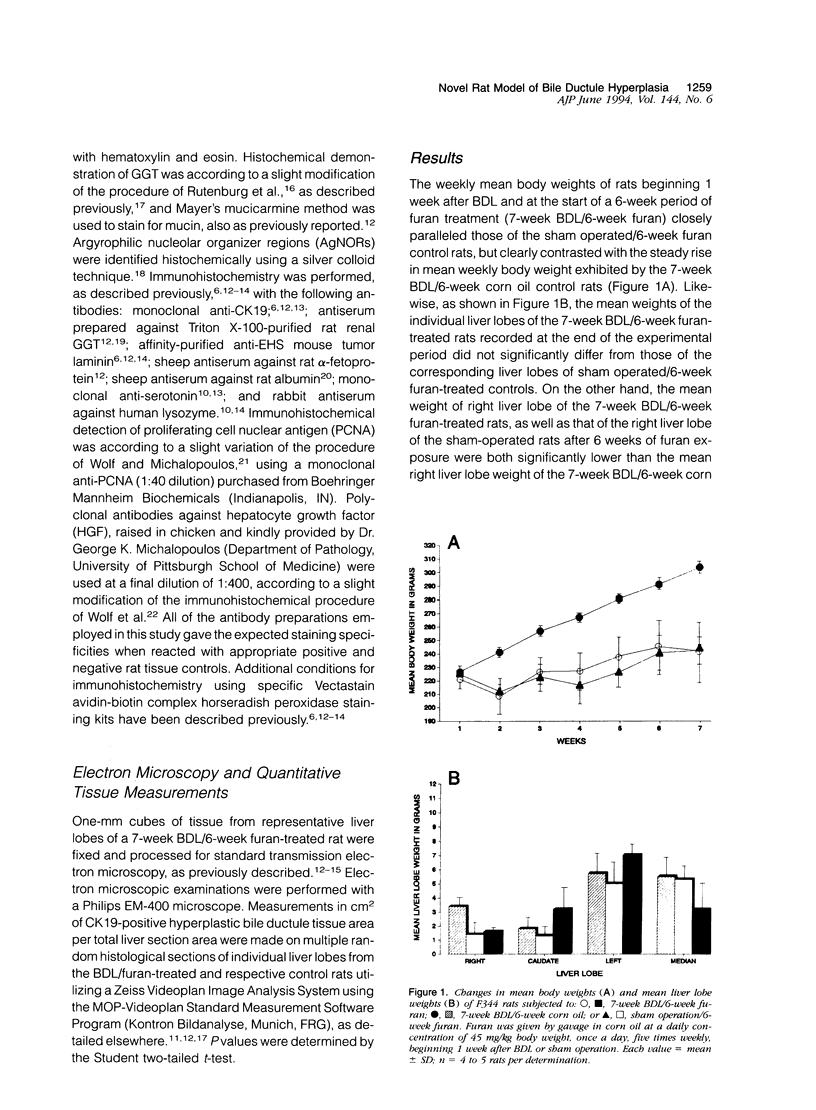
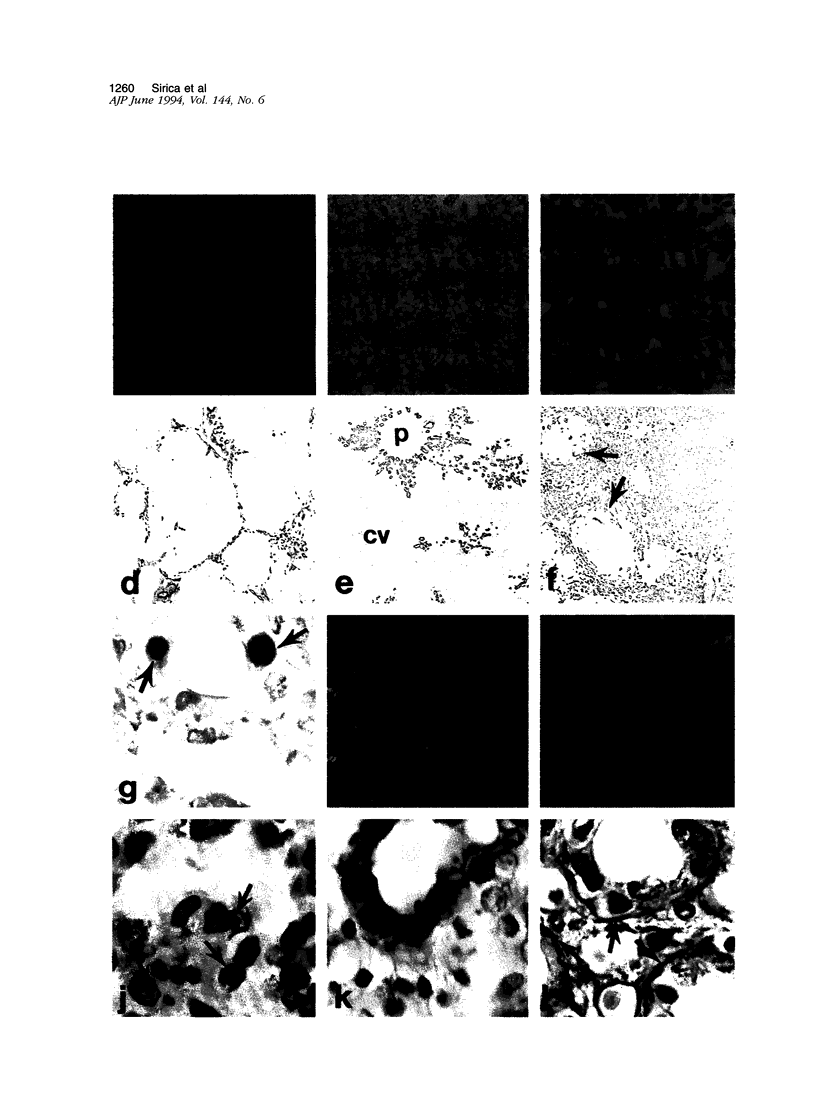
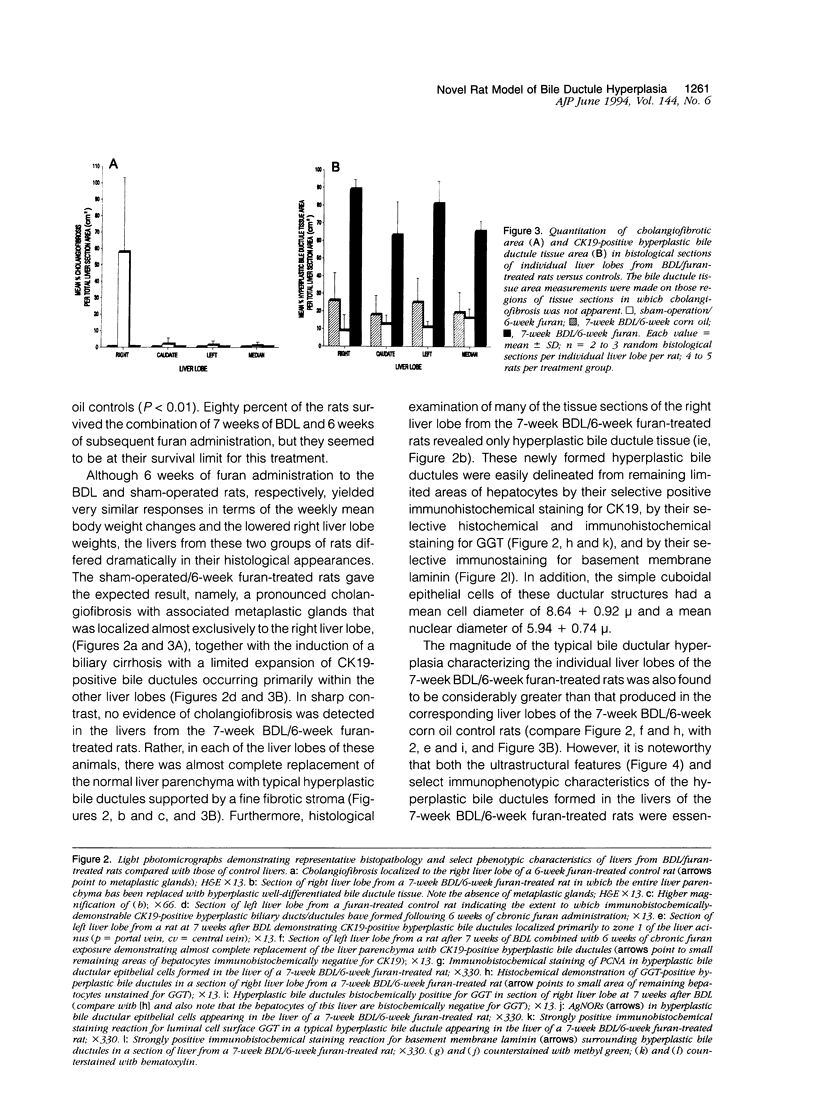
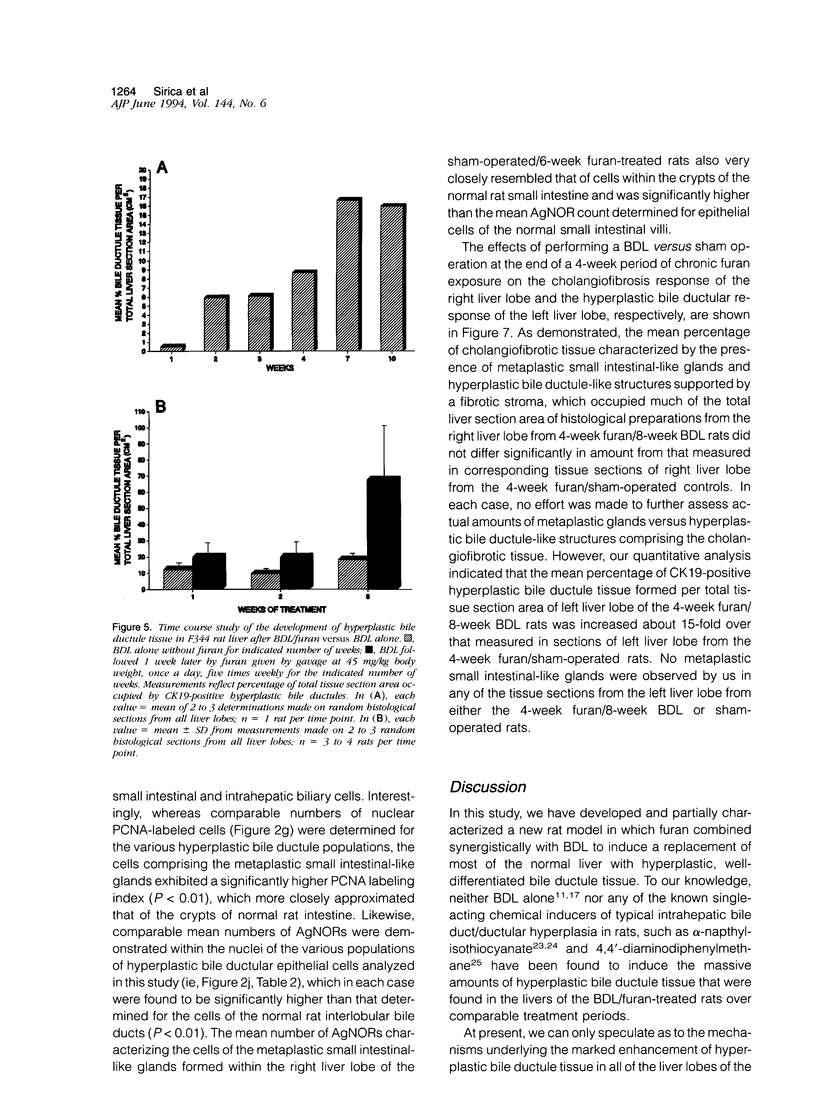
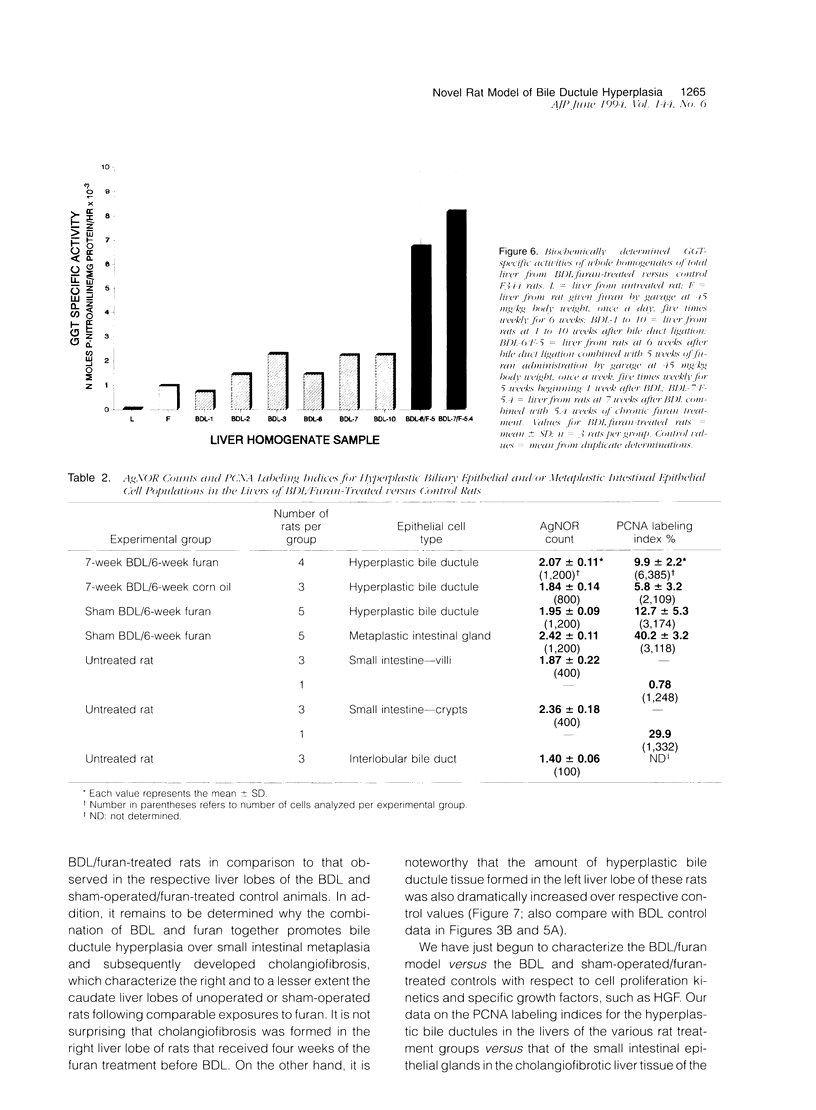
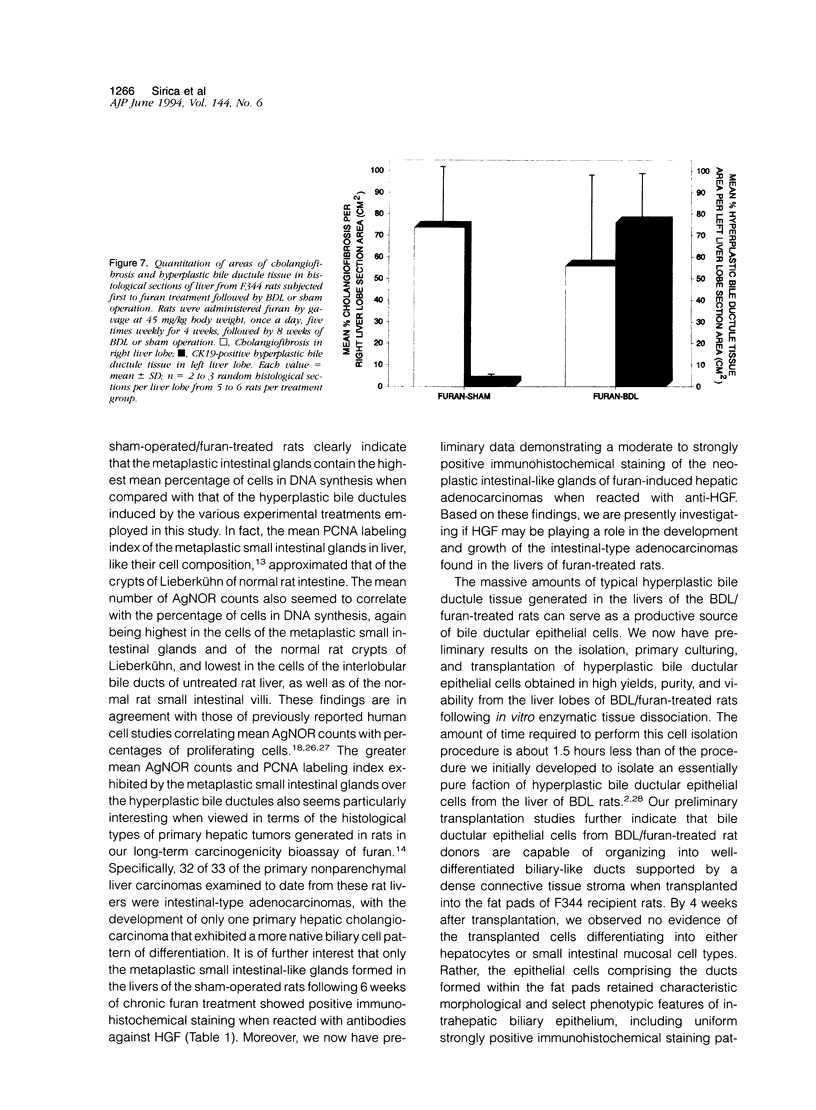
A novel rat model was developed in which furan combined in a unique synergistic manner with bile duct ligation to induce replacement of most of liver with well-differentiated hyperplastic bile ductules. Multiple tissue sections of liver from Fischer 344 male rats first subjected to a bile duct ligation and 1 week later given furan by gavage at 45 mg/kg body weight, once a day, five times weekly for 5 to 6 weeks, exhibited a mean percent of bile ductule tissue per total liver section area of 72.6 +/- 16.3% compared to control values of 20.0 +/- 4.2% for bile duct-ligated rats that received corn oil by gavage instead of furan and 11.9 +/- 3.1% for rats that were given a sham operation followed by furan. This dramatic difference was also reflected by the very high mean gamma-glutamyl transpeptidase specific activity of liver homogenates from the bile duct-ligated/furan-treated rats, which was approximately 8 x 10(3) nmoles p-nitroaniline/mg protein/hour versus values of approximately 2 x 10(3) for bile duct-ligated/corn oil control, approximately 1 x 10(3) for sham-operated/furan-treated control, and 44.9 for untreated rat. The data presented support a potentially powerful experimental model for investigating bile ductular cell functions, differentiation, and proliferation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crocker J., Nar P. Nucleolar organizer regions in lymphomas. J Pathol. 1987 Feb;151(2):111–118. doi: 10.1002/path.1711510203. [DOI] [PubMed] [Google Scholar]
- Elmore L. W., Sirica A. E. "Intestinal-type" of adenocarcinoma preferentially induced in right/caudate liver lobes of rats treated with furan. Cancer Res. 1993 Jan 15;53(2):254–259. [PubMed] [Google Scholar]
- Elmore L. W., Sirica A. E. Phenotypic characterization of metaplastic intestinal glands and ductular hepatocytes in cholangiofibrotic lesions rapidly induced in the caudate liver lobe of rats treated with furan. Cancer Res. 1991 Oct 15;51(20):5752–5759. [PubMed] [Google Scholar]
- Elmore L. W., Sirica A. E. Sequential appearance of intestinal mucosal cell types in the right and caudate liver lobes of furan-treated rats. Hepatology. 1992 Nov;16(5):1220–1226. [PubMed] [Google Scholar]
- Evarts R. P., Nagy P., Nakatsukasa H., Marsden E., Thorgeirsson S. S. In vivo differentiation of rat liver oval cells into hepatocytes. Cancer Res. 1989 Mar 15;49(6):1541–1547. [PubMed] [Google Scholar]
- Gall J. A., Bhathal P. S. Origin and involution of hyperplastic bile ductules following total biliary obstruction. Liver. 1990 Apr;10(2):106–115. doi: 10.1111/j.1600-0676.1990.tb00443.x. [DOI] [PubMed] [Google Scholar]
- LOPEZ M., MAZZANTI L. Experimental investigations on alpha-naphthyl-iso-thiocyanate as a hyperplastic agent of the biliary ducts in the rat. J Pathol Bacteriol. 1955 Jan-Apr;69(1-2):243–250. doi: 10.1002/path.1700690132. [DOI] [PubMed] [Google Scholar]
- Lemire J. M., Shiojiri N., Fausto N. Oval cell proliferation and the origin of small hepatocytes in liver injury induced by D-galactosamine. Am J Pathol. 1991 Sep;139(3):535–552. [PMC free article] [PubMed] [Google Scholar]
- Mathis G. A., Walls S. A., D'Amico P., Gengo T. F., Sirica A. E. Enzyme profile of rat bile ductular epithelial cells in reference to the resistance phenotype in hepatocarcinogenesis. Hepatology. 1989 Mar;9(3):477–485. doi: 10.1002/hep.1840090323. [DOI] [PubMed] [Google Scholar]
- Mathis G. A., Walls S. A., Sirica A. E. Biochemical characteristics of hyperplastic rat bile ductular epithelial cells cultured "on top" and "inside" different extracellular matrix substitutes. Cancer Res. 1988 Nov 1;48(21):6145–6153. [PubMed] [Google Scholar]
- Mathis G. A., Wyss P. A., Schuetz E. G., Hughey R. P., Sirica A. E. Expression of multiple proteins structurally related to gamma-glutamyl transpeptidase in non-neoplastic adult rat hepatocytes in vivo and in culture. J Cell Physiol. 1991 Feb;146(2):234–241. doi: 10.1002/jcp.1041460207. [DOI] [PubMed] [Google Scholar]
- Nonomura A., Matsubara F., Mizukami Y., Izumi R., Nakanuma Y., Kurumaya H., Watanabe K., Takayanagi N. Demonstration of nucleolar organizer regions in intrahepatic bile duct carcinoma by the silver-staining technique. Liver. 1990 Oct;10(5):269–277. doi: 10.1111/j.1600-0676.1990.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- STEINER J. W., CARRUTHERS J. S. Electron microscopy of hyperplastic ductular cells in alpha-naphthyl isothiocyanateinduced cirrhosis. Lab Invest. 1963 Apr;12:471–498. [PubMed] [Google Scholar]
- Sirica A. E., Cihla H. P. Isolation and partial characterizations of oval and hyperplastic bile ductular cell-enriched populations from the livers of carcinogen and noncarcinogen-treated rats. Cancer Res. 1984 Aug;44(8):3454–3466. [PubMed] [Google Scholar]
- Sirica A. E., Elmore L. W., Sano N. Characterization of rat hyperplastic bile ductular epithelial cells in culture and in vivo. Dig Dis Sci. 1991 Apr;36(4):494–501. doi: 10.1007/BF01298882. [DOI] [PubMed] [Google Scholar]
- Sirica A. E., Mathis G. A., Sano N., Elmore L. W. Isolation, culture, and transplantation of intrahepatic biliary epithelial cells and oval cells. Pathobiology. 1990;58(1):44–64. doi: 10.1159/000163564. [DOI] [PubMed] [Google Scholar]
- Sirica A. E., Sattler C. A., Cihla H. P. Characterization of a primary bile ductular cell culture from the livers of rats during extrahepatic cholestasis. Am J Pathol. 1985 Jul;120(1):67–78. [PMC free article] [PubMed] [Google Scholar]
- Sirica A. E., Williams T. W. Appearance of ductular hepatocytes in rat liver after bile duct ligation and subsequent zone 3 necrosis by carbon tetrachloride. Am J Pathol. 1992 Jan;140(1):129–136. [PMC free article] [PubMed] [Google Scholar]
- Slott P. A., Liu M. H., Tavoloni N. Origin, pattern, and mechanism of bile duct proliferation following biliary obstruction in the rat. Gastroenterology. 1990 Aug;99(2):466–477. doi: 10.1016/0016-5085(90)91030-a. [DOI] [PubMed] [Google Scholar]
- Tatematsu M., Kaku T., Medline A., Farber E. Intestinal metaplasia as a common option of oval cells in relation to cholangiofibrosis in liver of rats exposed to 2-acetylaminofluorene. Lab Invest. 1985 Apr;52(4):354–362. [PubMed] [Google Scholar]
- Tavoloni N. The intrahepatic biliary epithelium: an area of growing interest in hepatology. Semin Liver Dis. 1987 Nov;7(4):280–292. doi: 10.1055/s-2008-1040583. [DOI] [PubMed] [Google Scholar]
- Terada T., Nakanuma Y., Ohta T., Nagakawa T. Histological features and interphase nucleolar organizer regions in hyperplastic, dysplastic and neoplastic epithelium of intrahepatic bile ducts in hepatolithiasis. Histopathology. 1992 Sep;21(3):233–240. doi: 10.1111/j.1365-2559.1992.tb00381.x. [DOI] [PubMed] [Google Scholar]
- Wolf H. K., Michalopoulos G. K. Hepatocyte regeneration in acute fulminant and nonfulminant hepatitis: a study of proliferating cell nuclear antigen expression. Hepatology. 1992 Apr;15(4):707–713. doi: 10.1002/hep.1840150426. [DOI] [PubMed] [Google Scholar]
- Wolf H. K., Zarnegar R., Michalopoulos G. K. Localization of hepatocyte growth factor in human and rat tissues: an immunohistochemical study. Hepatology. 1991 Sep;14(3):488–494. [PubMed] [Google Scholar]