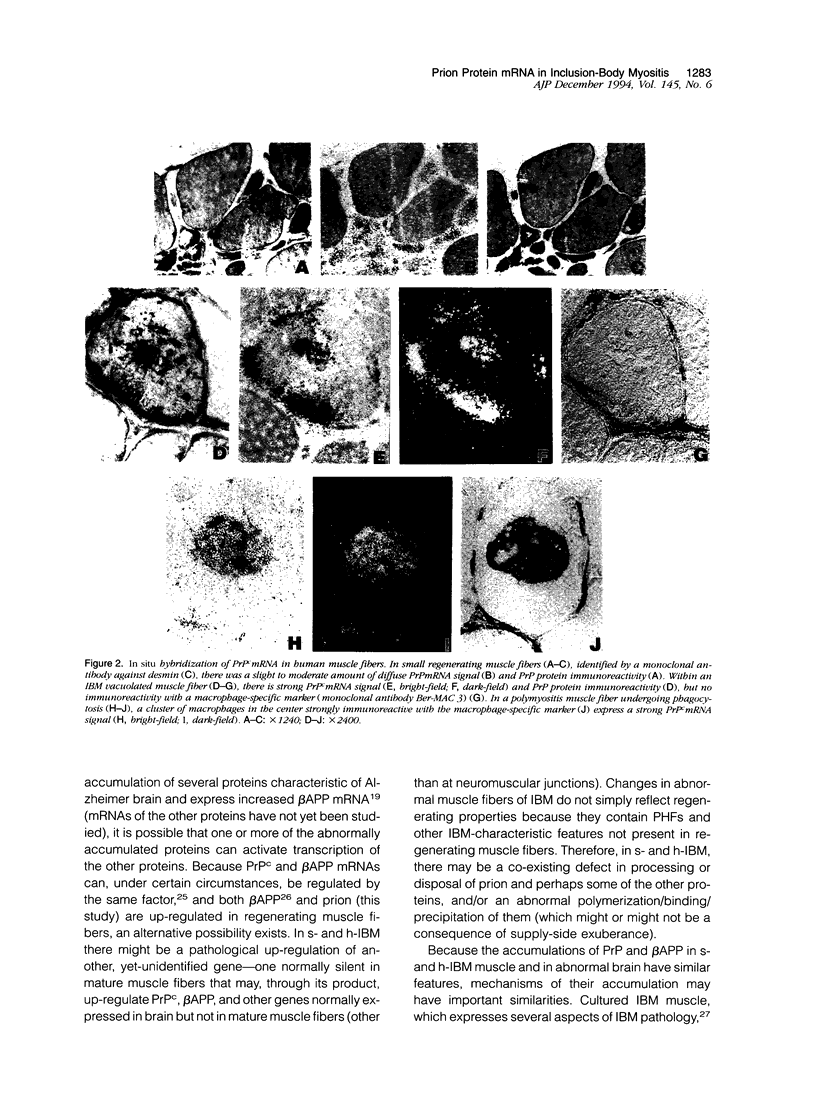
Abstract
Sporadic inclusion-body myositis is the most common progressive muscle disease of older patients. The muscle biopsy demonstrates mononuclear cell inflammation and vacuolated muscle fibers containing paired helical filaments and 6 to 10-nm fibrils, both resembling those of Alzheimer brain, and Congo-red positivity. Hereditary inclusion-body myopathy designates patients cytopathologically similar but without inflammation. In both muscle diseases, prion, and several proteins characteristic of Alzheimer brain--eg, beta-amyloid protein and hyperphosphorylated tau (which normally are expressed mainly in neurons), and apolipoprotein E--are abnormally accumulated in vacuolated muscle fibers, by unknown mechanisms. We now demonstrate in both muscle diseases that prion mRNA is strongly expressed in the vacuolated muscle fibers, which suggests that their accumulated prion protein results, at least partly, from increased gene expression. This, to our knowledge, is the first demonstration of abnormally increased prion mRNA in human disease. Another novel finding is the increased prion mRNA in human muscle macrophages, and both increased prion protein and prion mRNA in regenerating muscle fibers. The latter indicates that prion may play a role in human muscle development.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askanas V., Alvarez R. B., Engel W. K. beta-Amyloid precursor epitopes in muscle fibers of inclusion body myositis. Ann Neurol. 1993 Oct;34(4):551–560. doi: 10.1002/ana.410340408. [DOI] [PubMed] [Google Scholar]
- Askanas V., Bilak M., Engel W. K., Alvarez R. B., Tomé F., Leclerc A. Prion protein is abnormally accumulated in inclusion-body myositis. Neuroreport. 1993 Oct 25;5(1):25–28. doi: 10.1097/00001756-199310000-00006. [DOI] [PubMed] [Google Scholar]
- Askanas V., Bilak M., Engel W. K., Leclerc A., Tomé F. Prion protein is strongly immunolocalized at the postsynaptic domain of human normal neuromuscular junctions. Neurosci Lett. 1993 Sep 3;159(1-2):111–114. doi: 10.1016/0304-3940(93)90811-x. [DOI] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Alvarez R. B. Light and electron microscopic localization of beta-amyloid protein in muscle biopsies of patients with inclusion-body myositis. Am J Pathol. 1992 Jul;141(1):31–36. [PMC free article] [PubMed] [Google Scholar]
- Askanas V., Engel W. K., Bilak M., Alvarez R. B., Selkoe D. J. Twisted tubulofilaments of inclusion body myositis muscle resemble paired helical filaments of Alzheimer brain and contain hyperphosphorylated tau. Am J Pathol. 1994 Jan;144(1):177–187. [PMC free article] [PubMed] [Google Scholar]
- Askanas V., Mirabella M., Engel W. K., Alvarez R. B., Weisgraber K. H. Apolipoprotein E immunoreactive deposits in inclusion-body muscle diseases. Lancet. 1994 Feb 5;343(8893):364–365. doi: 10.1016/s0140-6736(94)91208-4. [DOI] [PubMed] [Google Scholar]
- Askanas V., Serdaroglu P., Engel W. K., Alvarez R. B. Immunolocalization of ubiquitin in muscle biopsies of patients with inclusion body myositis and oculopharyngeal muscular dystrophy. Neurosci Lett. 1991 Sep 2;130(1):73–76. doi: 10.1016/0304-3940(91)90230-q. [DOI] [PubMed] [Google Scholar]
- Bendheim P. E., Barry R. A., DeArmond S. J., Stites D. P., Prusiner S. B. Antibodies to a scrapie prion protein. Nature. 1984 Aug 2;310(5976):418–421. doi: 10.1038/310418a0. [DOI] [PubMed] [Google Scholar]
- Bendheim P. E., Potempska A., Kascsak R. J., Bolton D. C. Purification and partial characterization of the normal cellular homologue of the scrapie agent protein. J Infect Dis. 1988 Dec;158(6):1198–1208. doi: 10.1093/infdis/158.6.1198. [DOI] [PubMed] [Google Scholar]
- Cashman N. R., Loertscher R., Nalbantoglu J., Shaw I., Kascsak R. J., Bolton D. C., Bendheim P. E. Cellular isoform of the scrapie agent protein participates in lymphocyte activation. Cell. 1990 Apr 6;61(1):185–192. doi: 10.1016/0092-8674(90)90225-4. [DOI] [PubMed] [Google Scholar]
- DeArmond S. J., Prusiner S. B. The neurochemistry of prion diseases. J Neurochem. 1993 Nov;61(5):1589–1601. doi: 10.1111/j.1471-4159.1993.tb09792.x. [DOI] [PubMed] [Google Scholar]
- ENGEL W. K., CUNNINGHAM G. G. RAPID EXAMINATION OF MUSCLE TISSUE. AN IMPROVED TRICHROME METHOD FOR FRESH-FROZEN BIOPSY SECTIONS. Neurology. 1963 Nov;13:919–923. doi: 10.1212/wnl.13.11.919. [DOI] [PubMed] [Google Scholar]
- Engel W. K. Muscle biopsies in neuromuscular diseases. Pediatr Clin North Am. 1967 Nov;14(4):963–995. doi: 10.1016/s0031-3955(16)32067-3. [DOI] [PubMed] [Google Scholar]
- Kretzschmar H. A., Stowring L. E., Westaway D., Stubblebine W. H., Prusiner S. B., Dearmond S. J. Molecular cloning of a human prion protein cDNA. DNA. 1986 Aug;5(4):315–324. doi: 10.1089/dna.1986.5.315. [DOI] [PubMed] [Google Scholar]
- Mendell J. R., Sahenk Z., Gales T., Paul L. Amyloid filaments in inclusion body myositis. Novel findings provide insight into nature of filaments. Arch Neurol. 1991 Dec;48(12):1229–1234. doi: 10.1001/archneur.1991.00530240033013. [DOI] [PubMed] [Google Scholar]
- Mobley W. C., Neve R. L., Prusiner S. B., McKinley M. P. Nerve growth factor increases mRNA levels for the prion protein and the beta-amyloid protein precursor in developing hamster brain. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9811–9815. doi: 10.1073/pnas.85.24.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Hsiao K. K. Human prion diseases. Ann Neurol. 1994 Apr;35(4):385–395. doi: 10.1002/ana.410350404. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Puckett C., Concannon P., Casey C., Hood L. Genomic structure of the human prion protein gene. Am J Hum Genet. 1991 Aug;49(2):320–329. [PMC free article] [PubMed] [Google Scholar]
- Sarkozi E., Askanas V., Johnson S. A., Engel W. K., Alvarez R. B. beta-Amyloid precursor protein mRNA is increased in inclusion-body myositis muscle. Neuroreport. 1993 Jun;4(6):815–818. doi: 10.1097/00001756-199306000-00055. [DOI] [PubMed] [Google Scholar]
- Watanabe R., Duchen L. W. Cerebral amyloid in human prion disease. Neuropathol Appl Neurobiol. 1993 Jun;19(3):253–260. doi: 10.1111/j.1365-2990.1993.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Westaway D., DeArmond S. J., Cayetano-Canlas J., Groth D., Foster D., Yang S. L., Torchia M., Carlson G. A., Prusiner S. B. Degeneration of skeletal muscle, peripheral nerves, and the central nervous system in transgenic mice overexpressing wild-type prion proteins. Cell. 1994 Jan 14;76(1):117–129. doi: 10.1016/0092-8674(94)90177-5. [DOI] [PubMed] [Google Scholar]