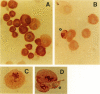
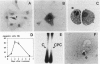
Abstract
We examined the effect of several inhibitors/activators of various protein kinases on the proliferation and apoptosis of nontransformed rat coronary vascular smooth muscle cells (SMC). As expected, all the compounds (calphostin C, KT5720, KT5823, verapamil, W7, and dibutyryl-cAMP) inhibited SMC proliferation, as judged by [3H]thymidine incorporation. Three (calphostin C, verapamil and dibutyryl-cAMP) of the six compounds caused occurrence of the classical apoptotic morphology in SMC. The effect of calphostin C, an inhibitor of protein kinase C, was examined in more detail due to the known involvement of this kinase in regulation of apoptosis in a variety of cell types. In SMC cultures exposed for 1, 2, and 3 days to 0.1 mumol/L calphostin C, 7 +/- 1%, 32 +/- 3%, and 29 +/- 3% of cells underwent apoptosis, respectively, as assessed by cell morphology (control cultures had 1 to 3% of apoptotic cells). The effect of calphostin C was transient in that on day 6 following exposure to this compound the number of apoptotic cells declined to control values. Simultaneous with the induction of apoptotic morphology in SMC, a decline was seen (within 24 hours) in expression of the oncoprotein Bcl-2 in morphologically nonapoptotic SMC. An altered distribution of Bcl-2 was seen in the apoptotic cells. The calphostin C-induced generation of apoptotic cells in SMC cultures and the decline/alteration of Bcl-2 expression were not accompanied by degradation of DNA into nucleosomal fragments. In conclusion, normal, nontransformed rat coronary artery vascular SMC undergo apoptosis when exposed to an inhibitor of protein kinase C (calphostin C), to a calcium channel blocker (verapamil), and to a stimulator of cAMP-dependent protein kinase (dibutyryl-cAMP). The induction of apoptosis by the inhibitor of protein kinase C is accompanied by alterations in the Bcl-2 expression but not by DNA fragmentation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati B., Littlewood T. D., Evan G. I., Land H. The c-Myc protein induces cell cycle progression and apoptosis through dimerization with Max. EMBO J. 1993 Dec 15;12(13):5083–5087. doi: 10.1002/j.1460-2075.1993.tb06202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Evan G. I., Newby A. C. Deregulated expression of the c-myc oncogene abolishes inhibition of proliferation of rat vascular smooth muscle cells by serum reduction, interferon-gamma, heparin, and cyclic nucleotide analogues and induces apoptosis. Circ Res. 1994 Mar;74(3):525–536. doi: 10.1161/01.res.74.3.525. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Ribeiro J. M. Apoptosis and disease. Lancet. 1993 May 15;341(8855):1251–1254. doi: 10.1016/0140-6736(93)91154-e. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Apoptosis. Immunol Today. 1993 Mar;14(3):126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- Diglio C. A., Grammas P., Giacomelli F., Wiener J. Rat heart-derived endothelial and smooth muscle cell cultures: isolation, cloning and characterization. Tissue Cell. 1988;20(4):477–492. doi: 10.1016/0040-8166(88)90051-1. [DOI] [PubMed] [Google Scholar]
- Gadbois D. M., Crissman H. A., Tobey R. A., Bradbury E. M. Multiple kinase arrest points in the G1 phase of nontransformed mammalian cells are absent in transformed cells. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8626–8630. doi: 10.1073/pnas.89.18.8626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntti-Berggren L., Larsson O., Rorsman P., Ammälä C., Bokvist K., Wåhlander K., Nicotera P., Dypbukt J., Orrenius S., Hallberg A. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science. 1993 Jul 2;261(5117):86–90. doi: 10.1126/science.7686306. [DOI] [PubMed] [Google Scholar]
- Kanamori M., Naka M., Asano M., Hidaka H. Effects of N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide and other calmodulin antagonists (calmodulin interacting agents) on calcium-induced contraction of rabbit aortic strips. J Pharmacol Exp Ther. 1981 May;217(2):494–499. [PubMed] [Google Scholar]
- Kase H., Iwahashi K., Nakanishi S., Matsuda Y., Yamada K., Takahashi M., Murakata C., Sato A., Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987 Jan 30;142(2):436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Ando K., Nakano H., Iida T., Ohno H., Morimoto M., Tamaoki T. Calphostins (UCN-1028), novel and specific inhibitors of protein kinase C. I. Fermentation, isolation, physico-chemical properties and biological activities. J Antibiot (Tokyo) 1989 Oct;42(10):1470–1474. doi: 10.7164/antibiotics.42.1470. [DOI] [PubMed] [Google Scholar]
- Leszczynski D., Zhao Y., Yeagley T. J., Foegh M. L. Direct and endothelial cell-mediated effect of cyclosporin A on the proliferation of rat smooth muscle cells in vitro. Am J Pathol. 1993 Jan;142(1):149–155. [PMC free article] [PubMed] [Google Scholar]
- McConkey D. J., Orrenius S., Jondal M. Cellular signalling in programmed cell death (apoptosis). Immunol Today. 1990 Apr;11(4):120–121. doi: 10.1016/0167-5699(90)90048-e. [DOI] [PubMed] [Google Scholar]
- Meredith J. E., Jr, Fazeli B., Schwartz M. A. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993 Sep;4(9):953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Oberhammer F., Wilson J. W., Dive C., Morris I. D., Hickman J. A., Wakeling A. E., Walker P. R., Sikorska M. Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 1993 Sep;12(9):3679–3684. doi: 10.1002/j.1460-2075.1993.tb06042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posternak T., Weimann G. The preparation of acylated derivatives of cyclic nucleotides. Methods Enzymol. 1974;38:399–409. doi: 10.1016/0076-6879(74)38057-3. [DOI] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rösl F. A simple and rapid method for detection of apoptosis in human cells. Nucleic Acids Res. 1992 Oct 11;20(19):5243–5243. doi: 10.1093/nar/20.19.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Fadok V., Henson P., Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993 Mar;14(3):131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]