Abstract
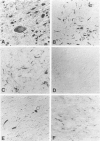
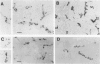
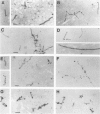
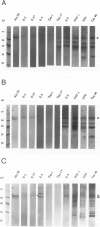
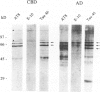
Corticobasal degeneration (CBD) is a neurodegenerative disorder associated with extensive cytoskeletal abnormalities. These include tau-positive neuropil threads and grains, ballooned or swollen neurons, neurofibrillary tangles, and glial inclusions. Given the presence of tau-positive structures in CBD, we investigated whether abnormalities in tau proteins associated with CBD were similar to those in Alzheimer's disease (AD). Fractions of abnormal tau proteins were isolated as Sarkosyl-insoluble pellets. By electron microscopic examination, the fraction from CBD contained twisted filaments that differed from paired helical filaments of AD. In CBD, filaments were shorter in length, rarely longer than 400 nm, 10 to 20% wider in the maximum and minimum widths (26 to 28 nm and 13 to 14 nm, respectively), and the periodic twist (169 to 202 nm) was twice as long as that in AD. Immunogold labeling with a panel of tau-reactive antibodies (Alz 50, Tau 14, AH-1, E-11, PHF-1, and Tau 46) showed no apparent differences in the pattern of tau immunoreactivity between filaments of CBD and AD. Western blots revealed that polypeptides of abnormal tau were present in both fractions; however, only two polypeptides (68 and 64 kd) were present in CBD as compared with three (68, 64, and 60 kd) in AD. Both of these polypeptides were reactive with additional antibodies (E-9, Tau-1 after dephosphorylation, AT8, and NP8). Only one polypeptide (68 kd) bound an antibody to adult-specific tau sequence encoded by exon 2, but neither was reactive with antibodies to adult-specific sequences encoded by exons 3 and 10. The results suggest that abnormalities in the number and heterogeneity of isoforms of tau may be one of the factors contributing to ultrastructural differences in pathological filaments of CBD and AD.
Full text
PDF



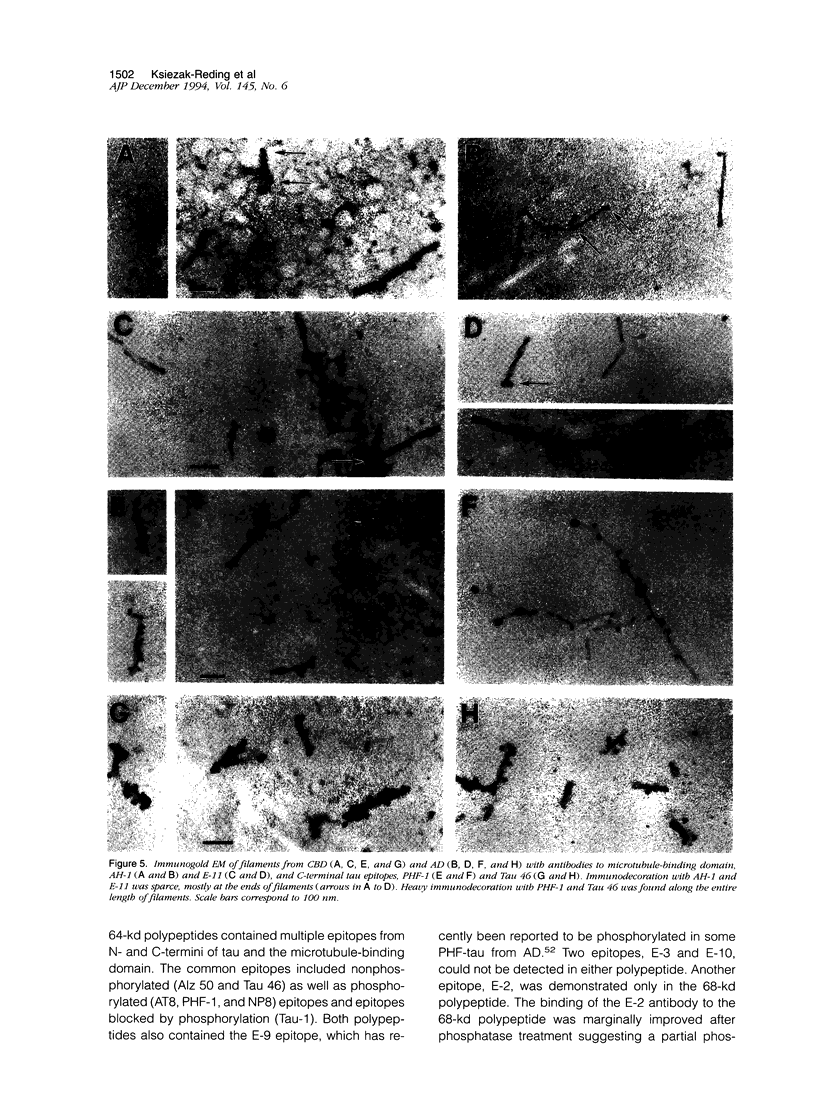
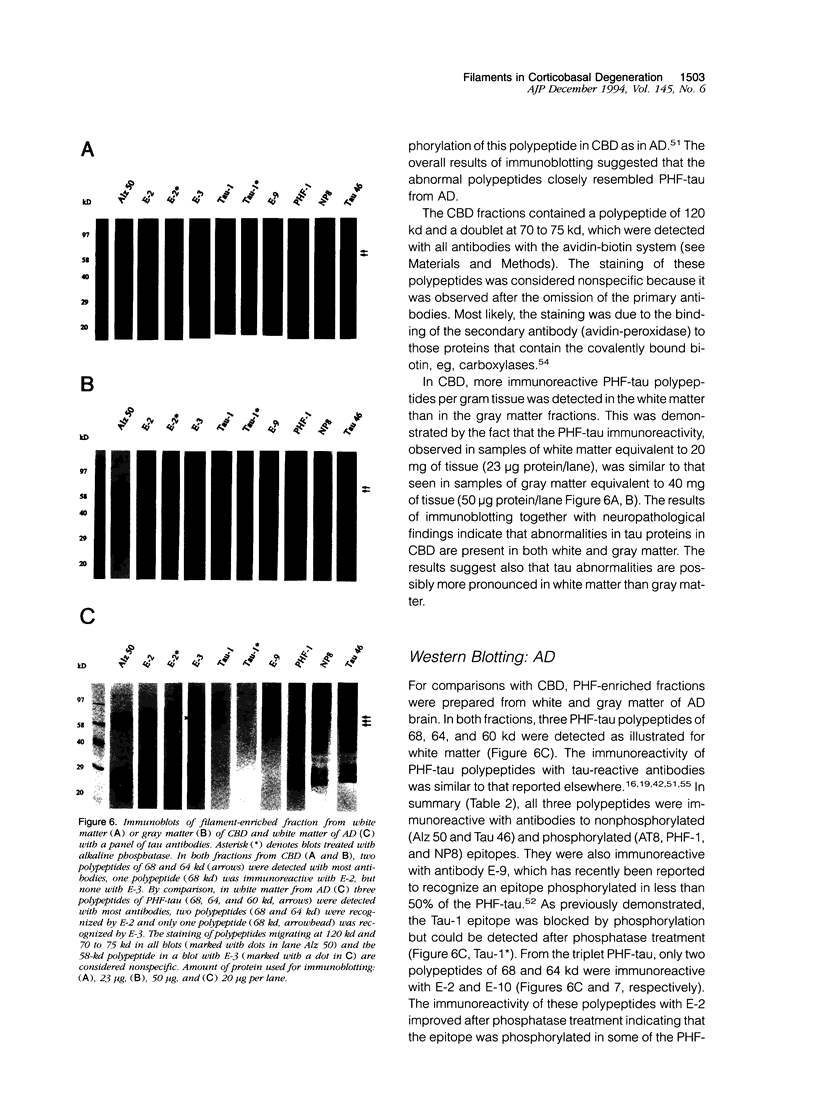
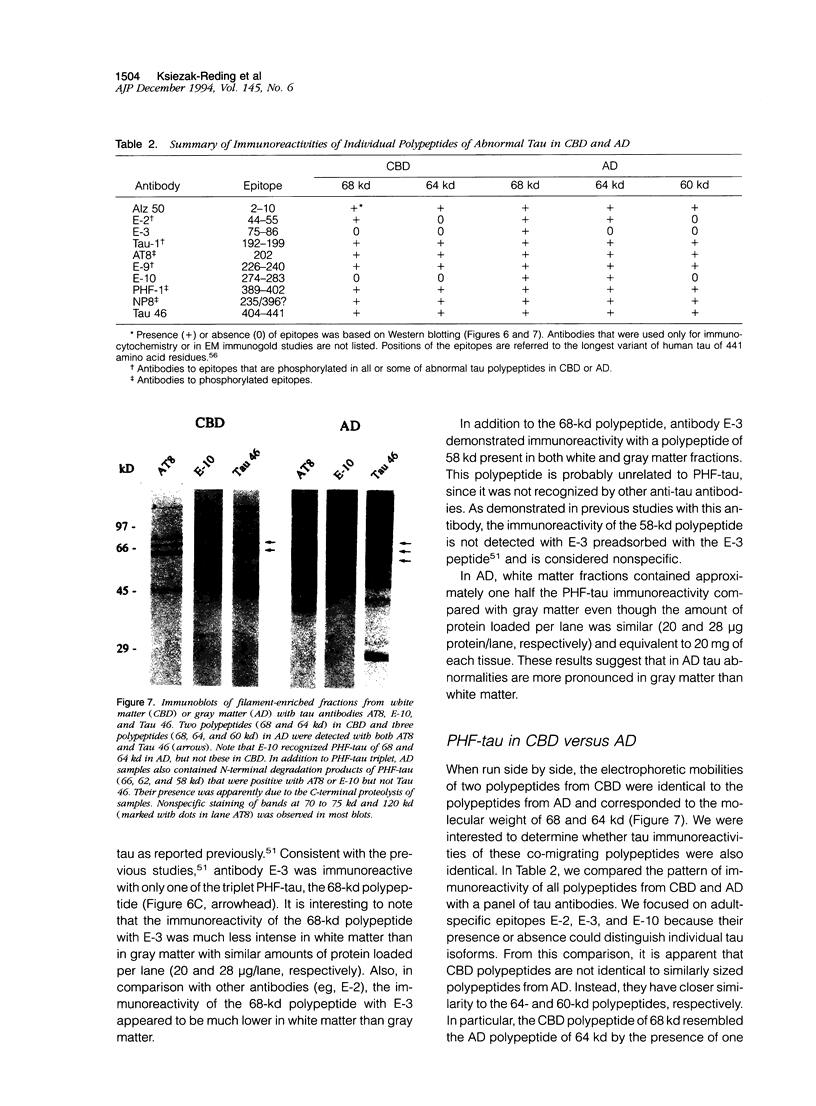




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Argyrophilic grains: characteristic pathology of cerebral cortex in cases of adult onset dementia without Alzheimer changes. Neurosci Lett. 1987 Apr 23;76(1):124–127. doi: 10.1016/0304-3940(87)90204-7. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E., Grundke-Iqbal I., Iqbal K. Occurrence of neuropil threads in the senile human brain and in Alzheimer's disease: a third location of paired helical filaments outside of neurofibrillary tangles and neuritic plaques. Neurosci Lett. 1986 Apr 24;65(3):351–355. doi: 10.1016/0304-3940(86)90288-0. [DOI] [PubMed] [Google Scholar]
- Butler M., Shelanski M. L. Microheterogeneity of microtubule-associated tau proteins is due to differences in phosphorylation. J Neurochem. 1986 Nov;47(5):1517–1522. doi: 10.1111/j.1471-4159.1986.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Chandler C. S., Ballard F. J. Distribution and degradation of biotin-containing carboxylases in human cell lines. Biochem J. 1985 Dec 1;232(2):385–393. doi: 10.1042/bj2320385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977 Oct 25;116(2):227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Olesen O. F., Smith M. J., Jakes R., Goedert M. Assembly of Alzheimer-like filaments from full-length tau protein. FEBS Lett. 1994 Jan 10;337(2):135–138. doi: 10.1016/0014-5793(94)80260-2. [DOI] [PubMed] [Google Scholar]
- Crowther R. A. Structural aspects of pathology in Alzheimer's disease. Biochim Biophys Acta. 1990 Nov 14;1096(1):1–9. doi: 10.1016/0925-4439(90)90004-9. [DOI] [PubMed] [Google Scholar]
- Delacourte A., Flament S., Dibe E. M., Hublau P., Sablonnière B., Hémon B., Shérrer V., Défossez A. Pathological proteins Tau 64 and 69 are specifically expressed in the somatodendritic domain of the degenerating cortical neurons during Alzheimer's disease. Demonstration with a panel of antibodies against Tau proteins. Acta Neuropathol. 1990;80(2):111–117. doi: 10.1007/BF00308912. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Crystal H., Mattiace L. A., Kress Y., Schwagerl A., Ksiezak-Reding H., Davies P., Yen S. H. Diffuse Lewy body disease: light and electron microscopic immunocytochemistry of senile plaques. Acta Neuropathol. 1989;78(6):572–584. doi: 10.1007/BF00691284. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Kress Y., Crowe A., Yen S. H. Monoclonal antibodies to Alzheimer neurofibrillary tangles. 2. Demonstration of a common antigenic determinant between ANT and neurofibrillary degeneration in progressive supranuclear palsy. Am J Pathol. 1985 Aug;120(2):292–303. [PMC free article] [PubMed] [Google Scholar]
- Dickson D. W., Ksiezak-Reding H., Liu W. K., Davies P., Crowe A., Yen S. H. Immunocytochemistry of neurofibrillary tangles with antibodies to subregions of tau protein: identification of hidden and cleaved tau epitopes and a new phosphorylation site. Acta Neuropathol. 1992;84(6):596–605. doi: 10.1007/BF00227736. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Yen S. H., Suzuki K. I., Davies P., Garcia J. H., Hirano A. Ballooned neurons in select neurodegenerative diseases contain phosphorylated neurofilament epitopes. Acta Neuropathol. 1986;71(3-4):216–223. doi: 10.1007/BF00688042. [DOI] [PubMed] [Google Scholar]
- Flament S., Delacourte A., Verny M., Hauw J. J., Javoy-Agid F. Abnormal Tau proteins in progressive supranuclear palsy. Similarities and differences with the neurofibrillary degeneration of the Alzheimer type. Acta Neuropathol. 1991;81(6):591–596. doi: 10.1007/BF00296367. [DOI] [PubMed] [Google Scholar]
- Gibb W. R., Luthert P. J., Marsden C. D. Corticobasal degeneration. Brain. 1989 Oct;112(Pt 5):1171–1192. doi: 10.1093/brain/112.5.1171. [DOI] [PubMed] [Google Scholar]
- Goedert M., Jakes R., Crowther R. A., Six J., Lübke U., Vandermeeren M., Cras P., Trojanowski J. Q., Lee V. M. The abnormal phosphorylation of tau protein at Ser-202 in Alzheimer disease recapitulates phosphorylation during development. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5066–5070. doi: 10.1073/pnas.90.11.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Jakes R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 1990 Dec;9(13):4225–4230. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R. Localization of the Alz-50 epitope in recombinant human microtubule-associated protein tau. Neurosci Lett. 1991 May 27;126(2):149–154. doi: 10.1016/0304-3940(91)90541-z. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Jakes R., Rutherford D., Crowther R. A. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989 Oct;3(4):519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Goedert M., Spillantini M. G., Potier M. C., Ulrich J., Crowther R. A. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 1989 Feb;8(2):393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P. A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5827–5831. doi: 10.1073/pnas.87.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. G., Davies P., Schein J. D., Binder L. I. Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau. J Biol Chem. 1992 Jan 5;267(1):564–569. [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmler A. Structure of the bovine tau gene: alternatively spliced transcripts generate a protein family. Mol Cell Biol. 1989 Apr;9(4):1389–1396. doi: 10.1128/mcb.9.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T., Nakamura Y. Distribution and ultrastructure of Alzheimer's neurofibrillary tangles in postencephalitic Parkinsonism of Economo type. Acta Neuropathol. 1981;55(1):59–62. doi: 10.1007/BF00691532. [DOI] [PubMed] [Google Scholar]
- KIDD M. ALZHEIMER'S DISEASE--AN ELECTRON MICROSCOPICAL STUDY. Brain. 1964 Jun;87:307–320. doi: 10.1093/brain/87.2.307. [DOI] [PubMed] [Google Scholar]
- Kenessey A., Yen S. H. The extent of phosphorylation of fetal tau is comparable to that of PHF-tau from Alzheimer paired helical filaments. Brain Res. 1993 Nov 26;629(1):40–46. doi: 10.1016/0006-8993(93)90478-6. [DOI] [PubMed] [Google Scholar]
- Khachaturian Z. S. Diagnosis of Alzheimer's disease. Arch Neurol. 1985 Nov;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Bakalis S., Neve R. L. Developmentally regulated expression of specific tau sequences. Neuron. 1989 Apr;2(4):1389–1397. doi: 10.1016/0896-6273(89)90077-9. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Orecchio L. D., Binder L., Trojanowski J. Q., Lee V. M., Lee G. Epitopes that span the tau molecule are shared with paired helical filaments. Neuron. 1988 Nov;1(9):817–825. doi: 10.1016/0896-6273(88)90129-8. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Binder L. I., Yen S. H. Alzheimer disease proteins (A68) share epitopes with tau but show distinct biochemical properties. J Neurosci Res. 1990 Mar;25(3):420–430. doi: 10.1002/jnr.490250320. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Binder L. I., Yen S. H. Immunochemical and biochemical characterization of tau proteins in normal and Alzheimer's disease brains with Alz 50 and Tau-1. J Biol Chem. 1988 Jun 15;263(17):7948–7953. [PubMed] [Google Scholar]
- Ksiezak-Reding H., Chien C. H., Lee V. M., Yen S. H. Mapping of the Alz 50 epitope in microtubule-associated proteins tau. J Neurosci Res. 1990 Mar;25(3):412–419. doi: 10.1002/jnr.490250319. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Dickson D. W., Davies P., Yen S. H. Recognition of tau epitopes by anti-neurofilament antibodies that bind to Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1987 May;84(10):3410–3414. doi: 10.1073/pnas.84.10.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Liu W. K., Yen S. H. Phosphate analysis and dephosphorylation of modified tau associated with paired helical filaments. Brain Res. 1992 Dec 4;597(2):209–219. doi: 10.1016/0006-8993(92)91476-u. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Morgan K., Dickson D. W. Tau immunoreactivity and SDS solubility of two populations of paired helical filaments that differ in morphology. Brain Res. 1994 Jun 27;649(1-2):185–196. doi: 10.1016/0006-8993(94)91063-4. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Yen S. H. Structural stability of paired helical filaments requires microtubule-binding domains of tau: a model for self-association. Neuron. 1991 May;6(5):717–728. doi: 10.1016/0896-6273(91)90169-z. [DOI] [PubMed] [Google Scholar]
- Köpke E., Tung Y. C., Shaikh S., Alonso A. C., Iqbal K., Grundke-Iqbal I. Microtubule-associated protein tau. Abnormal phosphorylation of a non-paired helical filament pool in Alzheimer disease. J Biol Chem. 1993 Nov 15;268(32):24374–24384. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang E., Szendrei G. I., Lee V. M., Otvos L., Jr Immunological and conformation characterization of a phosphorylated immunodominant epitope on the paired helical filaments found in Alzheimer's disease. Biochem Biophys Res Commun. 1992 Sep 16;187(2):783–790. doi: 10.1016/0006-291x(92)91264-q. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Balin B. J., Otvos L., Jr, Trojanowski J. Q. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991 Feb 8;251(4994):675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Otvos L., Jr, Carden M. J., Hollosi M., Dietzschold B., Lazzarini R. A. Identification of the major multiphosphorylation site in mammalian neurofilaments. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1998–2002. doi: 10.1073/pnas.85.6.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. The purification of tau protein and the occurrence of two phosphorylation states of tau in brain. J Biol Chem. 1984 Oct 10;259(19):12241–12245. [PubMed] [Google Scholar]
- Liu W. K., Dickson D. W., Yen S. H. Amino acid residues 226-240 of tau, which encompass the first Lys-Ser-Pro site of tau, are partially phosphorylated in Alzheimer paired helical filament-tau. J Neurochem. 1994 Mar;62(3):1055–1061. doi: 10.1046/j.1471-4159.1994.62031055.x. [DOI] [PubMed] [Google Scholar]
- Liu W. K., Dickson D. W., Yen S. H. Heterogeneity of tau proteins in Alzheimer's disease. Evidence for increased expression of an isoform and preferential distribution of a phosphorylated isoform in neurites. Am J Pathol. 1993 Feb;142(2):387–394. [PMC free article] [PubMed] [Google Scholar]
- Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Titani K., Ihara Y. Ubiquitin is conjugated with amino-terminally processed tau in paired helical filaments. Neuron. 1993 Jun;10(6):1151–1160. doi: 10.1016/0896-6273(93)90063-w. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Harris P., Kosik K. S., Kurnit D. M., Donlon T. A. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 1986 Dec;387(3):271–280. doi: 10.1016/0169-328x(86)90033-1. [DOI] [PubMed] [Google Scholar]
- Papasozomenos S. C., Binder L. I. Phosphorylation determines two distinct species of Tau in the central nervous system. Cell Motil Cytoskeleton. 1987;8(3):210–226. doi: 10.1002/cm.970080303. [DOI] [PubMed] [Google Scholar]
- Paulus W., Selim M. Corticonigral degeneration with neuronal achromasia and basal neurofibrillary tangles. Acta Neuropathol. 1990;81(1):89–94. doi: 10.1007/BF00662643. [DOI] [PubMed] [Google Scholar]
- Powell H. C., London G. W., Lampert P. W. Neurofibrillary tangles in progressive supranuclear palsy. Electron microscopic observations. J Neuropathol Exp Neurol. 1974 Jan;33(1):98–106. doi: 10.1097/00005072-197401000-00007. [DOI] [PubMed] [Google Scholar]
- Rebeiz J. J., Kolodny E. H., Richardson E. P., Jr Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol. 1968 Jan;18(1):20–33. doi: 10.1001/archneur.1968.00470310034003. [DOI] [PubMed] [Google Scholar]
- Tabaton M., Mandybur T. I., Perry G., Onorato M., Autilio-Gambetti L., Gambetti P. The widespread alteration of neurites in Alzheimer's disease may be unrelated to amyloid deposition. Ann Neurol. 1989 Dec;26(6):771–778. doi: 10.1002/ana.410260614. [DOI] [PubMed] [Google Scholar]
- Tabaton M., Whitehouse P. J., Perry G., Davies P., Autilio-Gambetti L., Gambetti P. Alz 50 recognizes abnormal filaments in Alzheimer's disease and progressive supranuclear palsy. Ann Neurol. 1988 Sep;24(3):407–413. doi: 10.1002/ana.410240309. [DOI] [PubMed] [Google Scholar]
- Takauchi S., Hosomi M., Marasigan S., Sato M., Hayashi S., Miyoshi K. An ultrastructural study of Pick bodies. Acta Neuropathol. 1984;64(4):344–348. doi: 10.1007/BF00690400. [DOI] [PubMed] [Google Scholar]
- Takauchi S., Mizuhara T., Miyoshi K. Unusual paired helical filaments in progressive supranuclear palsy. Acta Neuropathol. 1983;59(3):225–228. doi: 10.1007/BF00703207. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Oyanagi K., Makifuchi T., Ikuta F., Homma A., Homma Y., Horikawa Y., Tokiguchi S. Corticobasal degeneration: etiopathological significance of the cytoskeletal alterations. Acta Neuropathol. 1994;87(6):545–553. doi: 10.1007/BF00293314. [DOI] [PubMed] [Google Scholar]
- Wiche G., Oberkanins C., Himmler A. Molecular structure and function of microtubule-associated proteins. Int Rev Cytol. 1991;124:217–273. doi: 10.1016/s0074-7696(08)61528-4. [DOI] [PubMed] [Google Scholar]
- Wischik C. M., Novak M., Edwards P. C., Klug A., Tichelaar W., Crowther R. A. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., McGeer P. L., McGeer E. G. Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett. 1992 Jan 20;135(1):99–102. doi: 10.1016/0304-3940(92)90145-w. [DOI] [PubMed] [Google Scholar]