Abstract
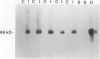
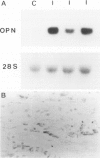
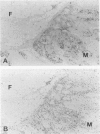
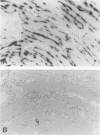
Osteopontin is a secreted glycoprotein implicated in a variety of functions, including cell adhesion and migration. Because these functions may be of general importance in the response of tissue to injury, we examined osteopontin expression after experimental cardiac injury and human myocardial infarction. Rat hearts were injured by transdiaphragmatic freeze-thaw and examined from 1 to 28 days after injury. Osteopontin was absent from normal myocardium by immunocytochemistry, Western blotting, and in situ hybridization. On days 1 and 2 after injury, osteopontin mRNA and protein were expressed at high levels by macrophages infiltrating necrotic myocardium. Double labeling with the macrophage marker ED1, however, demonstrated that only a subset of macrophages expressed osteopontin. Western blot analysis showed a single 66-kd band in injured myocardium that was absent from control tissue. Although macrophages remained abundant in the ensuing granulation response and scar tissue formation, the expression of osteopontin was diminished on day 4 and markedly downregulated at 1 and 4 weeks after injury, with only rare cells expressing the message or protein. In a human heart with an 8-day-old myocardial infarct, there was abundant expression of osteopontin mRNA and protein in macrophages within the necrotic and granulation tissue. Transient expression of osteopontin was also observed in a subset of macrophages infiltrating lung, skin, and skeletal muscle injured during the experiment, indicating the response was not limited to the heart. Thus, synthesis of osteopontin by macrophages appears to be a generalized response in the reaction to tissue injury. Although macrophages persist in these lesions, osteopontin is dramatically downregulated as healing proceeds. These results provide the first evidence that osteopontin may be important in healing after tissue injury, possibly in cellular adhesion, chemotaxis, and/or phagocytosis.
Full text
PDF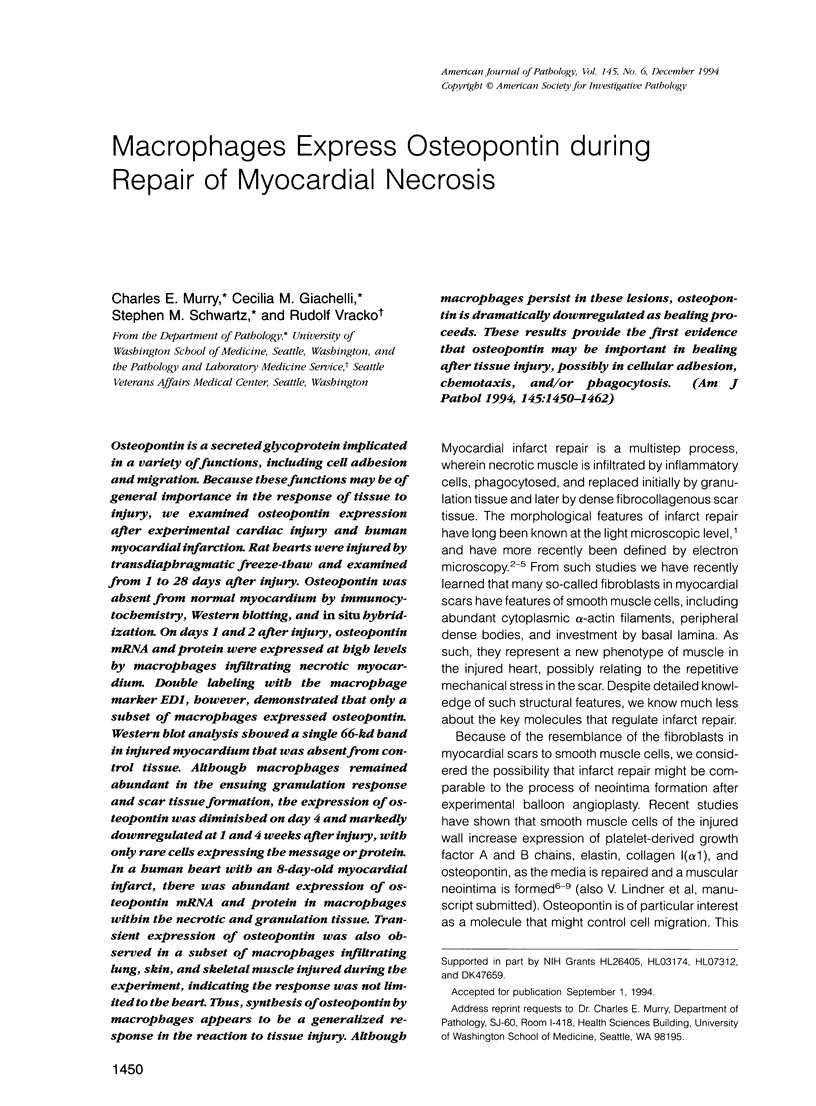




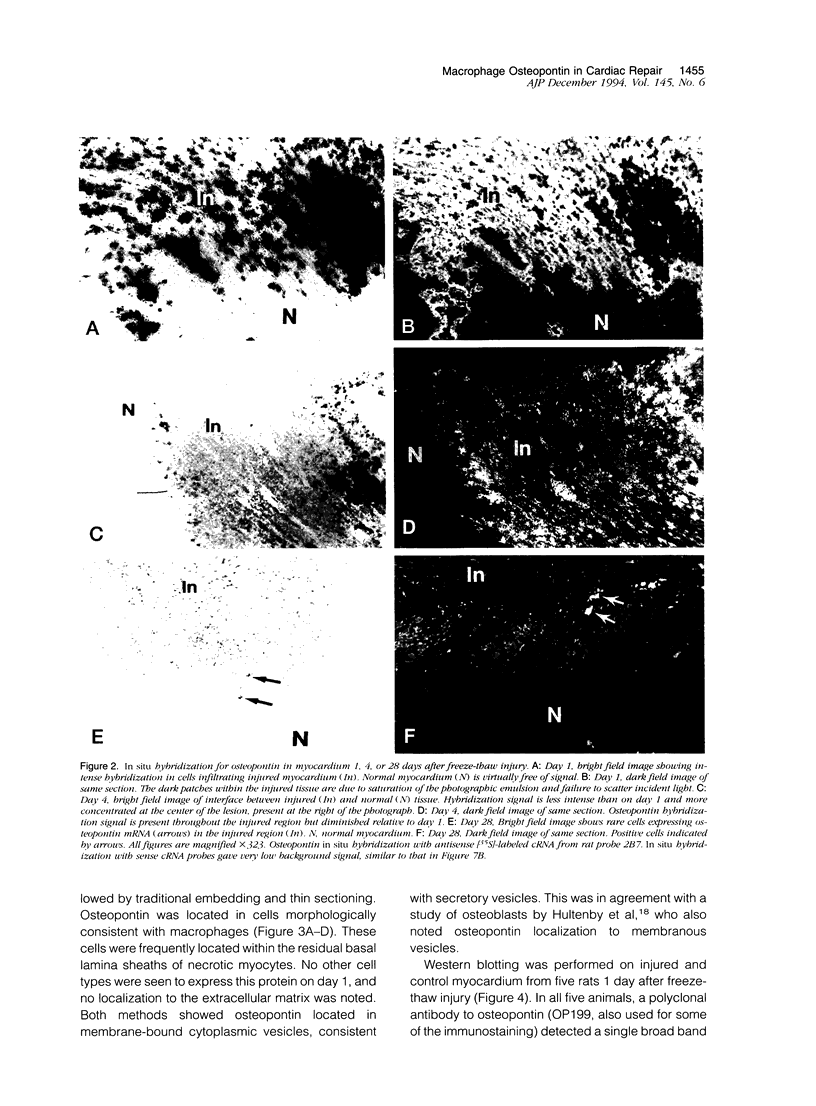
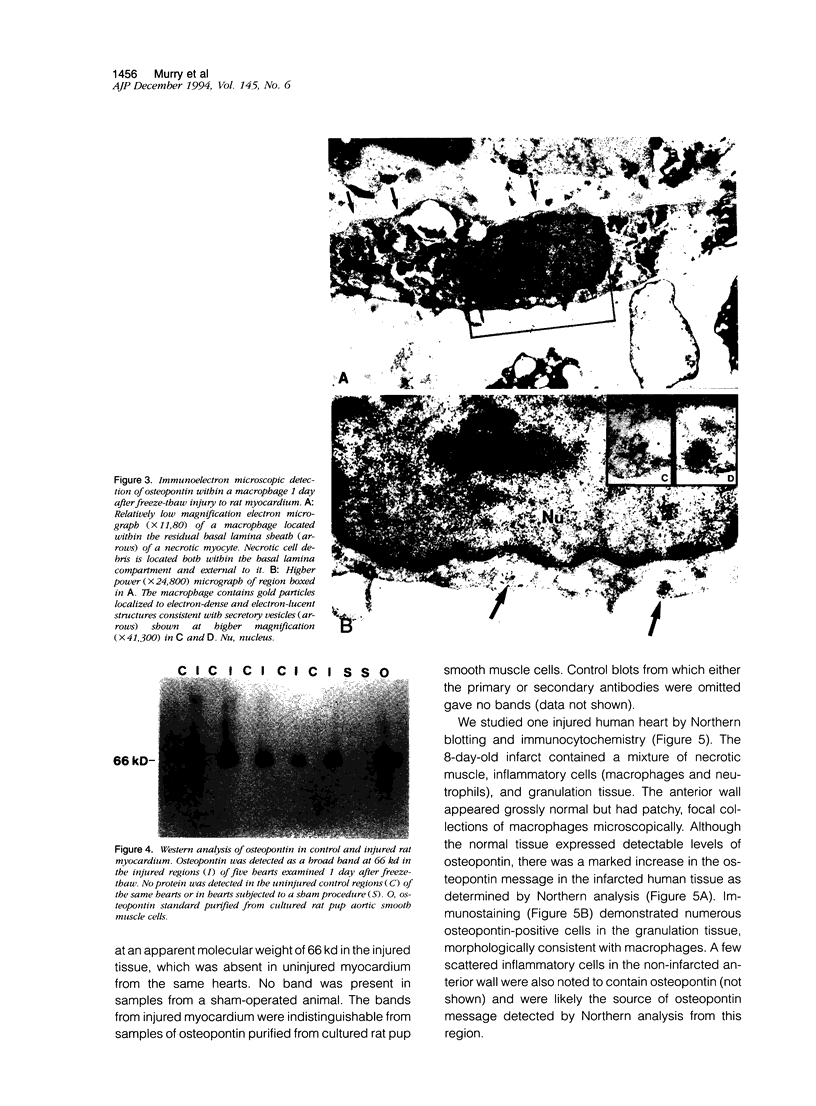
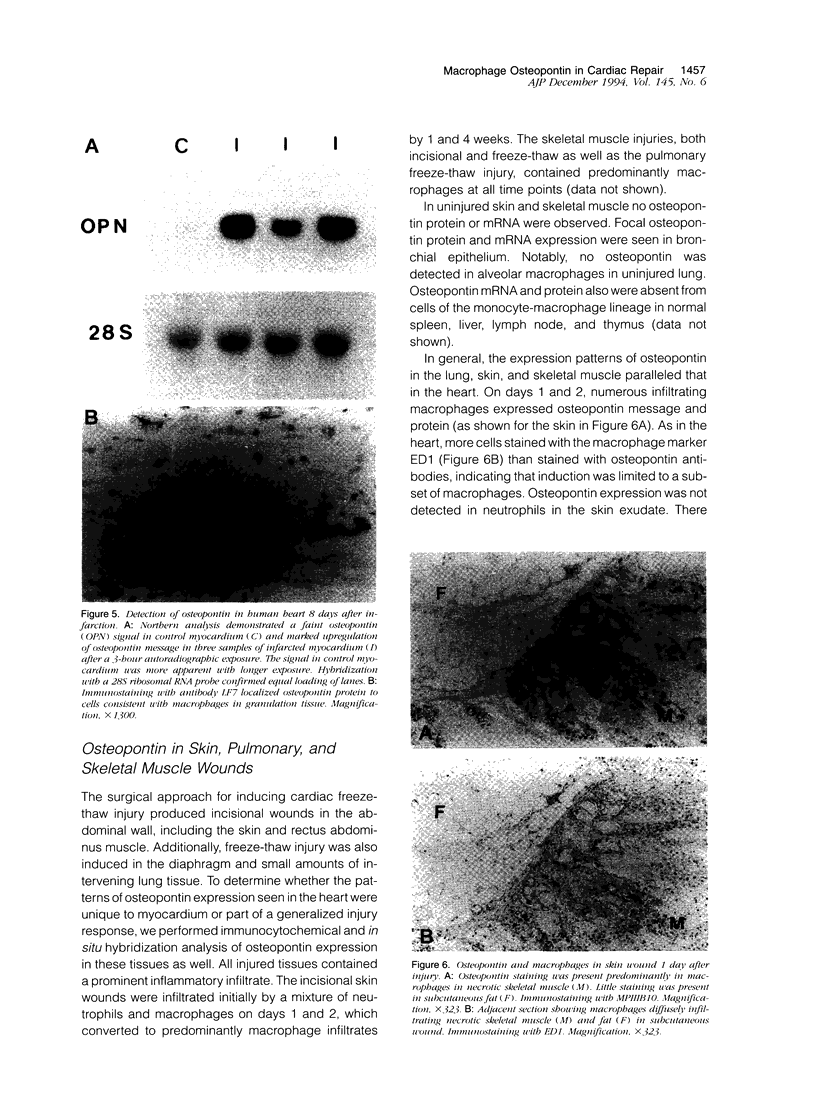





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The activated macrophage and granulomatous inflammation. Curr Top Pathol. 1989;79:151–167. doi: 10.1007/978-3-642-73855-5_7. [DOI] [PubMed] [Google Scholar]
- Alberius P., Johnell O. Repair of intra-membranous bone fractures and defects in rats. Immunolocalization of bone and cartilage proteins and proteoglycans. J Craniomaxillofac Surg. 1991 Jan;19(1):15–20. doi: 10.1016/s1010-5182(05)80266-5. [DOI] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Van de Water L., Papadopoulos-Sergiou A., Perruzzi C. A., Manseau E. J., Dvorak H. F., Senger D. R. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992 Oct;3(10):1169–1180. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. T. The nature and significance of osteopontin. Connect Tissue Res. 1989;23(2-3):123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- Chambers A. F., Behrend E. I., Wilson S. M., Denhardt D. T. Induction of expression of osteopontin (OPN; secreted phosphoprotein) in metastatic, ras-transformed NIH 3T3 cells. Anticancer Res. 1992 Jan-Feb;12(1):43–47. [PubMed] [Google Scholar]
- Chambers A. F., Hota C., Prince C. W. Adhesion of metastatic, ras-transformed NIH 3T3 cells to osteopontin, fibronectin, and laminin. Cancer Res. 1993 Feb 1;53(3):701–706. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Bowden G. T., Chambers A. F., Spearman M. A., Greenberg A. H., Wright J. A., McLeod M., Denhardt D. T. Secreted phosphoprotein mRNA is induced during multi-stage carcinogenesis in mouse skin and correlates with the metastatic potential of murine fibroblasts. Int J Cancer. 1990 Jul 15;46(1):133–137. doi: 10.1002/ijc.2910460124. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Smith J. H., Denhardt D. T. Osteopontin, a transformation-associated cell adhesion phosphoprotein, is induced by 12-O-tetradecanoylphorbol 13-acetate in mouse epidermis. J Biol Chem. 1989 Jun 5;264(16):9682–9689. [PubMed] [Google Scholar]
- Dijkstra C. D., Döpp E. A., Joling P., Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in rat recognized by monoclonal antibodies ED1, ED2 and ED3. Adv Exp Med Biol. 1985;186:409–419. doi: 10.1007/978-1-4613-2463-8_50. [DOI] [PubMed] [Google Scholar]
- Fadok V. A., Savill J. S., Haslett C., Bratton D. L., Doherty D. E., Campbell P. A., Henson P. M. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992 Dec 15;149(12):4029–4035. [PubMed] [Google Scholar]
- Flores M. E., Norgård M., Heinegård D., Reinholt F. P., Andersson G. RGD-directed attachment of isolated rat osteoclasts to osteopontin, bone sialoprotein, and fibronectin. Exp Cell Res. 1992 Aug;201(2):526–530. doi: 10.1016/0014-4827(92)90305-r. [DOI] [PubMed] [Google Scholar]
- Franzén A., Heinegård D. Isolation and characterization of two sialoproteins present only in bone calcified matrix. Biochem J. 1985 Dec 15;232(3):715–724. doi: 10.1042/bj2320715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadeau A. P., Campan M., Millet D., Candresse T., Desgranges C. Osteopontin overexpression is associated with arterial smooth muscle cell proliferation in vitro. Arterioscler Thromb. 1993 Jan;13(1):120–125. doi: 10.1161/01.atv.13.1.120. [DOI] [PubMed] [Google Scholar]
- Giachelli C. M., Bae N., Almeida M., Denhardt D. T., Alpers C. E., Schwartz S. M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993 Oct;92(4):1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachelli C. M., Pichler R., Lombardi D., Denhardt D. T., Alpers C. E., Schwartz S. M., Johnson R. J. Osteopontin expression in angiotensin II-induced tubulointerstitial nephritis. Kidney Int. 1994 Feb;45(2):515–524. doi: 10.1038/ki.1994.67. [DOI] [PubMed] [Google Scholar]
- Giachelli C., Bae N., Lombardi D., Majesky M., Schwartz S. Molecular cloning and characterization of 2B7, a rat mRNA which distinguishes smooth muscle cell phenotypes in vitro and is identical to osteopontin (secreted phosphoprotein I, 2aR). Biochem Biophys Res Commun. 1991 Jun 14;177(2):867–873. doi: 10.1016/0006-291x(91)91870-i. [DOI] [PubMed] [Google Scholar]
- Gordon D., Reidy M. A., Benditt E. P., Schwartz S. M. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H. R., Augustine N. H., Williams P. A., Brown E. J., Bohnsack J. F. Mechanism of fibronectin enhancement of group B streptococcal phagocytosis by human neutrophils and culture-derived macrophages. Infect Immun. 1993 Jun;61(6):2334–2339. doi: 10.1128/iai.61.6.2334-2339.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota S., Imakita M., Kohri K., Ito A., Morii E., Adachi S., Kim H. M., Kitamura Y., Yutani C., Nomura S. Expression of osteopontin messenger RNA by macrophages in atherosclerotic plaques. A possible association with calcification. Am J Pathol. 1993 Oct;143(4):1003–1008. [PMC free article] [PubMed] [Google Scholar]
- Hultenby K., Reinholt F. P., Oldberg A., Heinegård D. Ultrastructural immunolocalization of osteopontin in metaphyseal and cortical bone. Matrix. 1991 Jun;11(3):206–213. doi: 10.1016/s0934-8832(11)80160-5. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Kohri K., Suzuki Y., Yoshida K., Yamamoto K., Amasaki N., Yamate T., Umekawa T., Iguchi M., Sinohara H., Kurita T. Molecular cloning and sequencing of cDNA encoding urinary stone protein, which is identical to osteopontin. Biochem Biophys Res Commun. 1992 Apr 30;184(2):859–864. doi: 10.1016/0006-291x(92)90669-c. [DOI] [PubMed] [Google Scholar]
- Kubota T., Zhang Q., Wrana J. L., Ber R., Aubin J. E., Butler W. T., Sodek J. Multiple forms of SppI (secreted phosphoprotein, osteopontin) synthesized by normal and transformed rat bone cell populations: regulation by TGF-beta. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1453–1459. doi: 10.1016/0006-291x(89)90837-1. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Giachelli C. M., Reidy M. A., Schwartz S. M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res. 1992 Oct;71(4):759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Reidy M. A., Bowen-Pope D. F., Hart C. E., Wilcox J. N., Schwartz S. M. PDGF ligand and receptor gene expression during repair of arterial injury. J Cell Biol. 1990 Nov;111(5 Pt 1):2149–2158. doi: 10.1083/jcb.111.5.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M. P., Prince C. W., Gay S., Austin R. L., Butler W. T. 44-kDal bone phosphoprotein (osteopontin) antigenicity at ectopic sites in newborn rats: kidney and nervous tissues. Cell Tissue Res. 1988 Jan;251(1):23–30. doi: 10.1007/BF00215443. [DOI] [PubMed] [Google Scholar]
- Miyauchi A., Alvarez J., Greenfield E. M., Teti A., Grano M., Colucci S., Zambonin-Zallone A., Ross F. P., Teitelbaum S. L., Cheresh D. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991 Oct 25;266(30):20369–20374. [PubMed] [Google Scholar]
- Miyazaki Y., Setoguchi M., Yoshida S., Higuchi Y., Akizuki S., Yamamoto S. The mouse osteopontin gene. Expression in monocytic lineages and complete nucleotide sequence. J Biol Chem. 1990 Aug 25;265(24):14432–14438. [PubMed] [Google Scholar]
- Noda M., Rodan G. A. Transcriptional regulation of osteopontin production in rat osteoblast-like cells by parathyroid hormone. J Cell Biol. 1989 Feb;108(2):713–718. doi: 10.1083/jcb.108.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Rodan G. A. Type beta transforming growth factor regulates expression of genes encoding bone matrix proteins. Connect Tissue Res. 1989;21(1-4):71–75. doi: 10.3109/03008208909049997. [DOI] [PubMed] [Google Scholar]
- Noda M., Vogel R. L., Craig A. M., Prahl J., DeLuca H. F., Denhardt D. T. Identification of a DNA sequence responsible for binding of the 1,25-dihydroxyvitamin D3 receptor and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9995–9999. doi: 10.1073/pnas.87.24.9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Yoon K., Prince C. W., Butler W. T., Rodan G. A. Transcriptional regulation of osteopontin production in rat osteosarcoma cells by type beta transforming growth factor. J Biol Chem. 1988 Sep 25;263(27):13916–13921. [PubMed] [Google Scholar]
- Nomura S., Wills A. J., Edwards D. R., Heath J. K., Hogan B. L. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988 Feb;106(2):441–450. doi: 10.1083/jcb.106.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien E. R., Alpers C. E., Stewart D. K., Ferguson M., Tran N., Gordon D., Benditt E. P., Hinohara T., Simpson J. B., Schwartz S. M. Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ Res. 1993 Aug;73(2):223–231. doi: 10.1161/01.res.73.2.223. [DOI] [PubMed] [Google Scholar]
- Ohta S., Yamamuro T., Lee K., Okumura H., Kasai R., Hiraki Y., Ikeda T., Iwasaki R., Kikuchi H., Konishi J. Fracture healing induces expression of the proto-oncogene c-fos in vivo. Possible involvement of the Fos protein in osteoblastic differentiation. FEBS Lett. 1991 Jun 17;284(1):42–45. doi: 10.1016/0014-5793(91)80757-t. [DOI] [PubMed] [Google Scholar]
- Patarca R., Freeman G. J., Singh R. P., Wei F. Y., Durfee T., Blattner F., Regnier D. C., Kozak C. A., Mock B. A., Morse H. C., 3rd Structural and functional studies of the early T lymphocyte activation 1 (Eta-1) gene. Definition of a novel T cell-dependent response associated with genetic resistance to bacterial infection. J Exp Med. 1989 Jul 1;170(1):145–161. doi: 10.1084/jem.170.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince C. W., Butler W. T. 1,25-Dihydroxyvitamin D3 regulates the biosynthesis of osteopontin, a bone-derived cell attachment protein, in clonal osteoblast-like osteosarcoma cells. Coll Relat Res. 1987 Sep;7(4):305–313. doi: 10.1016/s0174-173x(87)80036-5. [DOI] [PubMed] [Google Scholar]
- Reinholt F. P., Hultenby K., Oldberg A., Heinegård D. Osteopontin--a possible anchor of osteoclasts to bone. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Wesolowski G., Yoon K., Rodan G. A. Opposing effects of fibroblast growth factor and pertussis toxin on alkaline phosphatase, osteopontin, osteocalcin, and type I collagen mRNA levels in ROS 17/2.8 cells. J Biol Chem. 1989 Nov 25;264(33):19934–19941. [PubMed] [Google Scholar]
- Savill J., Dransfield I., Hogg N., Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990 Jan 11;343(6254):170–173. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- Shiraga H., Min W., VanDusen W. J., Clayman M. D., Miner D., Terrell C. H., Sherbotie J. R., Foreman J. W., Przysiecki C., Neilson E. G. Inhibition of calcium oxalate crystal growth in vitro by uropontin: another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):426–430. doi: 10.1073/pnas.89.1.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Patarca R., Schwartz J., Singh P., Cantor H. Definition of a specific interaction between the early T lymphocyte activation 1 (Eta-1) protein and murine macrophages in vitro and its effect upon macrophages in vivo. J Exp Med. 1990 Jun 1;171(6):1931–1942. doi: 10.1084/jem.171.6.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suva L. J., Seedor J. G., Endo N., Quartuccio H. A., Thompson D. D., Bab I., Rodan G. A. Pattern of gene expression following rat tibial marrow ablation. J Bone Miner Res. 1993 Mar;8(3):379–388. doi: 10.1002/jbmr.5650080315. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Tooney P. A., Agrez M. V., Burns G. F. A re-examination of the molecular basis of cell movement. Immunol Cell Biol. 1993 Apr;71(Pt 2):131–139. doi: 10.1038/icb.1993.14. [DOI] [PubMed] [Google Scholar]
- Vracko R., Cunningham D., Frederickson R. G., Thorning D. Basal lamina of rat myocardium. Its fate after death of cardiac myocytes. Lab Invest. 1988 Jan;58(1):77–87. [PubMed] [Google Scholar]
- Vracko R., Thorning D. Contractile cells in rat myocardial scar tissue. Lab Invest. 1991 Aug;65(2):214–227. [PubMed] [Google Scholar]
- Vracko R., Thorning D., Frederickson R. G., Cunningham D. Myocyte reactions at the borders of injured and healing rat myocardium. Lab Invest. 1988 Jul;59(1):104–114. [PubMed] [Google Scholar]
- Vracko R., Thorning D., Frederickson R. G. Fate of nerve fibers in necrotic, healing, and healed rat myocardium. Lab Invest. 1990 Oct;63(4):490–501. [PubMed] [Google Scholar]
- Vracko R., Thorning D. Freeze-thaw injury of rat heart across an intact diaphragm: a new model for the study of the response of myocardium to injury. Cardiovasc Res. 1985 Feb;19(2):76–84. doi: 10.1093/cvr/19.2.76. [DOI] [PubMed] [Google Scholar]
- Waterhouse P., Parhar R. S., Guo X., Lala P. K., Denhardt D. T. Regulated temporal and spatial expression of the calcium-binding proteins calcyclin and OPN (osteopontin) in mouse tissues during pregnancy. Mol Reprod Dev. 1992 Aug;32(4):315–323. doi: 10.1002/mrd.1080320403. [DOI] [PubMed] [Google Scholar]
- Worcester E. M., Blumenthal S. S., Beshensky A. M., Lewand D. L. The calcium oxalate crystal growth inhibitor protein produced by mouse kidney cortical cells in culture is osteopontin. J Bone Miner Res. 1992 Sep;7(9):1029–1036. doi: 10.1002/jbmr.5650070905. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Kubota T., Zhang Q., Overall C. M., Aubin J. E., Butler W. T., Sodek J. Regulation of transformation-sensitive secreted phosphoprotein (SPPI/osteopontin) expression by transforming growth factor-beta. Comparisons with expression of SPARC (secreted acidic cysteine-rich protein). Biochem J. 1991 Feb 1;273(Pt 3):523–531. doi: 10.1042/bj2730523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K., Buenaga R., Rodan G. A. Tissue specificity and developmental expression of rat osteopontin. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1129–1136. doi: 10.1016/s0006-291x(87)80250-4. [DOI] [PubMed] [Google Scholar]
- Young M. F., Kerr J. M., Termine J. D., Wewer U. M., Wang M. G., McBride O. W., Fisher L. W. cDNA cloning, mRNA distribution and heterogeneity, chromosomal location, and RFLP analysis of human osteopontin (OPN). Genomics. 1990 Aug;7(4):491–502. doi: 10.1016/0888-7543(90)90191-v. [DOI] [PubMed] [Google Scholar]
- Yu S. F., Koerner T. J., Adams D. O. Gene regulation in macrophage activation: differential regulation of genes encoding for tumor necrosis factor, interleukin-1, JE, and KC by interferon-gamma and lipopolysaccharide. J Leukoc Biol. 1990 Nov;48(5):412–419. doi: 10.1002/jlb.48.5.412. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Wrana J. L., Sodek J. Characterization of the promoter region of the porcine opn (osteopontin, secreted phosphoprotein 1) gene. Identification of positive and negative regulatory elements and a 'silent' second promoter. Eur J Biochem. 1992 Jul 15;207(2):649–659. doi: 10.1111/j.1432-1033.1992.tb17092.x. [DOI] [PubMed] [Google Scholar]