Abstract
Mammalian circadian rhythms are generated by a master clock located in the suprachiasmatic nuclei and entrained by light-activated signaling pathways. In hamsters, the mechanism responsible for light-induced phase advances involves the activation of guanylyl cyclase, cGMP and its related kinase (PKG). It is not completely known whether interference with this pathway affects entrainment of the clock, including adaptation to changing light schedules. Here we report that cGMP-specific phosphodiesterase 5 is present in the hamster suprachiasmatic nuclei, and administration of the inhibitor sildenafil (3.5 mg/kg, i.p.) enhances circadian responses to light and decreases the amount of time necessary for reentrainment after phase advances of the light–dark cycle. These results suggest that sildenafil may be useful for treatment of circadian adaptation to environmental changes, including transmeridian eastbound flight schedules.
Keywords: cGMP phosphodiesterase, resynchronization, suprachiasmatic nuclei, phase advance
In mammals, the mechanism for the generation and entrainment of circadian rhythms resides in the hypothalamic suprachiasmatic nuclei (SCN), and the principal signal that adjusts this biological clock with environmental timing is the light–dark (LD) cycle (1, 2). Light reaches the SCN through the retinohypothalamic tract and causes the release of neurotransmitters (glutamate and PACAP), which initiates a signal transduction cascade in SCN neurons that ultimately results in a phase shift of the circadian system (3–9). Exposure to light pulses at night synchronizes the clock by inducing phase delays during the early night and phase advances during the late subjective night (i.e., when under constant conditions the animal behaves as if it were the night) (10). Both responses involve an increase of intracellular Ca2+ levels (11) and the activation of several components like phosphatases and kinases, including the mitogen-activated protein kinase family (12–14) and the Ca2+/calmodulin-dependent kinase II (15). These pathways ultimately rely on the activation of transcription factors such as CREB and ELK-1 (16, 17) and clock genes (18).
In contrast to the effects of light on circadian rhythms at night, animals are most responsive to nonphotic stimuli, such as injection of saline, forced activity in novel environments, or exposure to a dark pulse during the day (19). The cAMP/PKA pathway has traditionally been involved in nonphotic resetting in the daytime domain (20, 21). Application of cAMP analogs in vitro induces prominent phase advances in the subjective day but not in the subjective night (22). Although there is also evidence that suggests a role for cAMP/PKA at night, this role appears to be to promote the effects of light/GLU in early night but to oppose them in late night (23).
In hamsters, responses to light during the subjective night are mediated through a common signaling pathway involving glutamate, Ca2+, Ca2+/calmodulin-dependent kinase II, and neuronal NO synthase, which couple photic stimulation to the transcriptional activation of clock genes (15, 24–27). However, downstream of NO these pathways bifurcate, leading to different events that occur only during the early or the late night. During the late night, the activation of the guanylyl cyclase–cGMP–cGMP-dependent protein kinase (PKG) pathway is known to be involved in phase advances but not phase delays (28–32). Therefore, the accessibility of specific signaling pathways is fundamental for regulation of circadian timing.
cGMP levels in the hamster SCN exhibit daily and circadian variations with maximum values during the day. This variation appears to be related to temporal changes in cGMP-phosphodiesterase (PDE) activity and not to guanylyl cyclase activity (31). During the night, cGMP levels are increased significantly after light pulses at circadian time (CT) 18 (late night) but are unaffected by the same photic stimulus at CT 14 (early night), confirming its role in mediating phase advances but not delays. Moreover, PKG inhibition blocks light-induced phase advances but not delays (30, 31).
cGMP-specific PDE inhibitors, which prevent the hydrolysis of cGMP, allow the accumulation of this nucleotide in the cells. Sildenafil, which is present in the commercial agent Viagra, used for the treatment of erectile dysfunction, specifically inhibits the breakdown of cellular cGMP by PDE5 (33) and thereby prolongs and enhances the effects of the NO/cGMP pathway. Because cGMP levels seem to be of paramount importance in phase-advancing mechanisms, we have studied the effects of sildenafil, a well known PDE5 inhibitor, on circadian behavior, under the hypothesis that an increase of cGMP levels in the SCN would enhance photic responses. We examined the effects of sildenafil both on the resynchronization rate after a 6-h change of the LD cycle and on the response to single light pulses during the subjective night.
Results
RT-PCR analysis was used to confirm the presence of PDE5 in the hamster SCN. Strong expression of the PDE5 isoform was evident [supporting information (SI) Fig. 5A]. PDE9 was also present in the SCN, with lower expression than PDE5 (SI Fig. 5B).
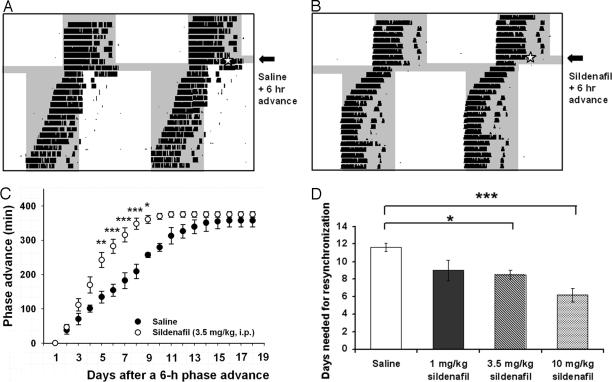
To study the effect of sildenafil on locomotor activity rhythms, hamsters were injected with this compound before an abrupt advance of 6 h of the LD cycle. When the LD cycle was shifted, the circadian rhythm of running-wheel activity was gradually resynchronized to the new LD cycle. An i.p. injection of 3.5 mg/kg at zeitgeber time (ZT) 18 (with ZT 12 defined as the time of lights off) of sildenafil on the day of the environmental change significantly accelerated entrainment to the new cycle (Fig. 1 A and B). Thus, sildenafil-treated groups took significantly less time to resynchronize to the new LD cycle as compared with the vehicle-treated group (Fig. 1 C and D; 12 ± 2 days for saline and 8 ± 1 days for 3.5 mg/kg sildenafil, mean ± SD for six animals per group; P < 0.05, ANOVA followed by Tukey's test). A lower dose, 1 mg/kg sildenafil, failed to accelerate resynchronization (9 ± 3 days; P > 0.05 vs. control), whereas a dose of 10 mg/kg sildenafil was even more effective on reentrainment rate (6 ± 2 days, P < 0.001 vs. control). As shown in Fig. 1D, 10 mg/kg sildenafil decreased reentrainment time by 50%, whereas 3.5 mg/kg and 1 mg/kg decreased this time by 33% and 25%, respectively. However, we used the intermediate dose for the rest of the experiments because at that dose animals did not manifest the effects of sildenafil-induced penile erections. Indeed, a dose–response study in rats showed that 5 mg/kg per day i.p. was the optimal erectogenic dose of sildenafil (34).
Fig. 1.
Effects of sildenafil on circadian reentrainment. Double-plotted actograms of hamster wheel-running activity showing reentrainment to a 6-h advance of the LD cycle after the injection of vehicle (A) and sildenafil (B) (3.5 mg/kg, i.p.) at ZT 18 on the day of the cycle change. Periods of darkness are shaded in gray. (C) Summary of phase advances (minutes) on each day after the change in the LD cycle (n = 6 animals per group, means ± SEM). Open and filled circles indicate sildenafil and saline, respectively. ∗∗∗, P < 0.001; ∗∗, P < 0.01; ∗, P < 0.05 (Student's t test). (D) Dose–response curve for sildenafil effects (mean ± SEM, n = 6). ∗∗∗, P < 0.001; ∗, P < 0.05 (ANOVA followed by Tukey's test).
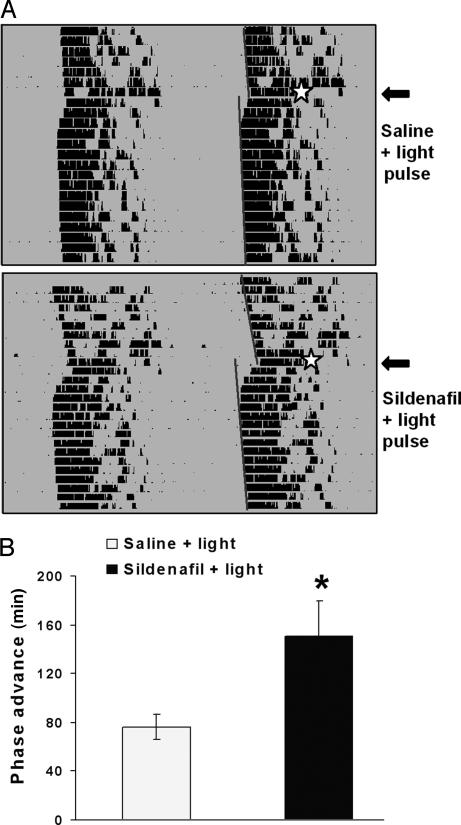
Reentrainment can be considered to be the effect of transient, pulsatile effects of light (usually called nonparametric) as well as tonic, parametric effects of the light cycle (35). We tested the effect of the PDE5 inhibitor on the well known nonparametric effects of light, which are defined by phase shifts induced by short light pulses at different times of the day. Sildenafil elicited an increase in light-induced phase advances of activity rhythms when injected 45 min (but not 15 or 90 min) before a light pulse at CT 18 (with CT 12 defined as the time of locomotor activity onset). A 15-min light pulse (50 lux) at CT 18 after vehicle injection induced an average phase advance of 76 ± 23 min, which was increased significantly by a sildenafil injection 45 min before the light stimulation (150.4 ± 64.8 min; P < 0.05, ANOVA followed by Dunnett's test) (Fig. 2), whereas an injection 90 min before the light pulse elicited a phase advance of 123 ± 27 min, which was not significantly different from controls (mean ± SD from five to six animals per group). Sildenafil alone did not modify activity rhythms. Another cGMP PDE inhibitor, zaprinast (3.5 mg/kg, i.p.), had a similar effect as sildenafil on light-induced phase advances when tested at CT 18 (data not shown).
Fig. 2.
Effects of sildenafil on light-induced phase advances. (A) Double-plotted actograms of hamster wheel-running activity showing vehicle or sildenafil (3.5 mg/kg, i.p.) injection 45 min before a light pulse at CT 18. Light stimulation is indicated by a star. Activity onsets are indicated by straight lines drawn over the actograms. (B) Quantification of phase advances (mean ± SEM, n = 5). ∗, P < 0.05 (Student's t test).
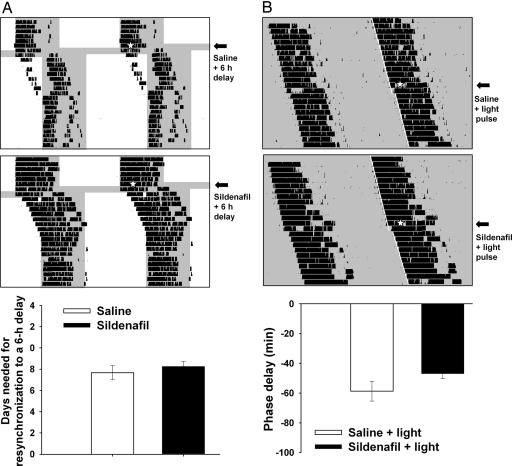
In addition, sildenafil did not affect either reentrainment rates after a delay in the LD cycle (Fig. 3A) or light-induced phase delays of the circadian locomotor activity rhythm after a light pulse at CT 14 (Fig. 3B).
Fig. 3.
Effects of sildenafil on phase delays. (A) Effect on reentrainment of wheel-running activity rhythm following a 6-h phase delay of the LD cycle. Double-plotted actograms of hamster wheel-running activity showing reentrainment to a 6-h delay of the LD cycle after the injection of vehicle (Top) and sildenafil (3.5 mg/kg, i.p.; Middle). Injections of either saline or sildenafil were given at ZT 14 on the day of the cycle change (white star). (Bottom) Mean ± SEM (n = 4 animals per group) of days needed for reentrainment. (B) Effect of sildenafil on light-induced phase delays after light pulses at CT 14. Representative actograms show vehicle (Top) or sildenafil (Middle) injections. (Bottom) Mean ± SEM (n = 4 animals per group).
To confirm inhibition of PDE by sildenafil, cGMP levels were measured in the hamster SCN by using a direct cGMP enzyme immunoassay kit (Assay Designs, Ann Arbor, MI). The amount of cGMP at CT 18 was 0.29 ± 0.15 pmol cGMP per milligram of protein (control values). Administration of 3.5 mg/kg sildenafil induced a 2-fold increase in SCN cGMP levels 45 min after injection, reaching values comparable to cerebellar cGMP levels, whereas saline injections had no effect (sildenafil, 0.63 ± 0.08 pmol cGMP per milligram of protein; saline, 0.37 ± 0.11 pmol cGMP per milligram of protein; cerebellum, 0.70 ± 0.15; values are given as mean ± SD; P < 0.05, sildenafil vs. control and sildenafil vs. saline, ANOVA followed by Student–Newman–Keuls multiple-comparisons test; n = 4). Sildenafil injections 90 min before light pulses did not increase cGMP levels in the SCN with respect to controls or saline-treated animals (sildenafil, 0.37 ± 0.20 pmol cGMP per milligram of protein; saline, 0.26 ± 0.16 pmol cGMP per milligram of protein; n = 4).
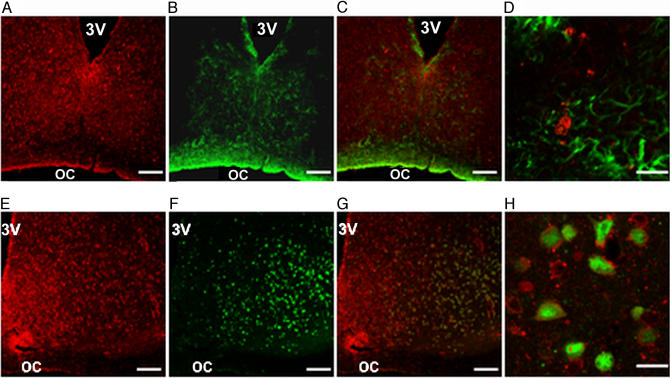
We also aimed to determine cGMP localization in the SCN. Distribution of this nucleotide was studied by immunohistochemistry on 40-μm coronal brain sections. cGMP was present in the whole SCN, although more cGMP-like immunoreactivity labeling was observed in the ventral portion of the SCN. Labeling of cGMP was prominent in the cytoplasm. Confocal microscopy showed colocalization of cGMP with the neuron-specific nuclear protein (NeuN) and lack of colocalization with the glial fibrillary acidic protein (GFAP), indicating neuronal localization of cGMP in the SCN (Fig. 4).
Fig. 4.
Neuronal localization of cGMP in the SCN. Combination of single confocal images for cGMP (red fluorescence) and NeuN or GFAP (green fluorescence) staining. (A) cGMP. (B) GFAP. (C) Double-labeling of cGMP-GFAP. (D) Higher magnification of C shows no colocalization between cGMP and GFAP. (E) cGMP. (F) Neuron-specific nuclear protein, NeuN. (G) Double-labeling of cGMP-NeuN. (H) Higher magnification of G shows cells with both cGMP (cytoplasmic) and NeuN (nuclear), suggesting neuronal expression of cGMP in the SCN. (Scale bars: 100 μm in A–C and E–G and 20 μm in D and H.)
Discussion
In the present study we have examined the effects of a selective PDE5 inhibitor, sildenafil, on the ability of hamsters to adapt to a 6-h phase change in the LD cycle. Our results demonstrate that sildenafil administration significantly accelerates reentrainment to advancing cycles. Sildenafil also increases the response for single light pulses at CT 18, when light induces phase advances. These effects of sildenafil are mediated by a 2-fold increase in SCN cGMP levels 45 min after injection, higher than that previously published with light alone (31). There is a clear phase specificity for this pathway, because sildenafil had no effect on reentrainment after a phase delay of the LD cycle or on light-induced phase delays under constant dark conditions. Because cGMP levels vary in the SCN under both LD and constant dark conditions (31), the phase dependency of sildenafil administration could be related to this endogenous variation, because cGMP increases would represent differential values with respect to basal levels for the cyclic nucleotide. In summary, our results offer strong support for a differential pathway responsible for circadian delay or advance mechanisms (because sildenafil lacked any effects in the early night), as well as a corroboration of a cGMP role in photic entrainment and a specific role for PDE5 in this process.
The NO–cGMP–PKG pathway is involved in circadian phase advances in response to light (28–32). A better knowledge of the transduction pathway might provide pharmacological tools for the treatment of circadian disorders, such as phase delay or advance of the human sleep–wake cycle, jet-lag, and shift work-related disturbances. Although our results do not provide a direct link between the NO–cGMP signal transduction pathway and the circadian molecular clockwork, we have preliminary evidence that indicates that PKG activity inhibition affects light-induced period expression (P.V.A., H. de la Iglesia, and D.A.G., unpublished observations). In this sense, a profound understanding of neurochemical pathways in the circadian clock might also lead to the better-known molecular feedback loops of clock genes and their products.
cGMP effects are mediated by three main mechanisms: activation of PKG, activation of cyclic nucleotide-gated ion channels (CNGCs), and extracellular effects (36). Because there is no description of CNGCs or extracellular release of cGMP in the SCN, it is unlikely that these mechanisms underlie the effect of cGMP on photic entrainment. We and others have determined a clear role of PKG in light-induced phase shifts, suggesting that this is a likely mechanism to explain cGMP-mediated sildenafil effects (20, 31).
Reentrainment can be considered to be the effect of transient, nonparametric effects of light (i.e., after brief light pulse stimulation) as well as tonic, parametric effects of the light cycle (including a complete photoperiod). We also tested the effect of the cGMP inhibitor on phase shifts induced by short light pulses at different times of the day. Although sildenafil induced an increase in light-induced phase advances, the posttreatment circadian period did not change with respect to previous conditions. However, because the slope of the reentrainment curve after sildenafil administration is significantly increased with respect to controls, it is likely that under tonic conditions the compound elicits a change in the speed of the oscillator (at least in terms of the adaptation to changing LD cycles). However, the precise mechanism of action for sildenafil effects on the circadian clock remains to be established.
Sildenafil alone did not induce phase shifts, in accordance with the fact that cGMP analogs do not induce photic-like phase shifts in vivo (30). Indeed, there is some kind of in vivo brake for this pathway, because cGMP analogs do induce phase advances when administered in vitro during the subjective night (29). A putative explanation for this phenomenon is that light induces the expression of a PKG substrate, without which sildenafil-induced increases in cGMP levels (and therefore, PKG activity) will not be able to affect entrainment. Among other candidates identified by phosphorylation gel analysis, we recently found the expression of DARPP-32 in the SCN (P.V.A. and D.A.G., unpublished observations), which in other brain tissues is a PKG substrate (37) and has recently been shown to affect photic entrainment in mice (38).
It is also interesting to state that a single sildenafil administration is able to exert behavioral changes regarding the circadian response to light. Indeed, PDE inhibitions result not only in an increase of cGMP levels but also a concomitant increase of PKG activity (including autophosphorylation events), which in turn further affects PDE function (39).
Shift work and chronic jet-lag reduce mental acuity and increase the risk of a number of medical problems. Current treatments include chronotheraphy, exposure to bright light, and melatonin/ramelteon administration (40–42), which offer varying degrees of effectiveness. Our results indicate that interference with the cGMP-related pathway might be a useful therapeutical tool, especially for jet-lag symptoms due to eastbound flights. Indeed, a recent work states the deleterious effects of phase advances but not delays of the light cycle in aged mice (43). A potential jet-lag treatment for advancing cycles could also be important for the safety of counterclockwise rotating shift work and the potential long-term health consequences for airline crews regularly crossing time zones. Sildenafil could also be useful in other circadian disorders that involve poor synchronization with the environment, including delayed sleep-phase syndrome and adaptation to changing light schedules. Administration of this compound could therefore be useful to improve entrainment under natural light conditions; moreover, it could be used for increasing photic responses when administering light therapies.
Other experimental and clinical jet-lag treatments include the use of chronobiotics such as melatonin (38, 39). In most animal models of jet-lag several administrations of the drug are needed for a significant reentrainment (in comparison with the single-dose effect of sildenafil, with a similar acceleration rate) (44, 45). In experimental animals nonphotic stimuli such as availability of novel running wheels also accelerates resynchronization (46); however, although there are indications of nonphotic, activity-related stimulation in humans, our pharmacological approach seems to be more readily available for clinical testing.
The use of sildenafil is particularly appealing because this drug has been thoroughly studied in terms of its pharmacological effectiveness and safety (34, 47). At least 21 genes encode PDE proteins in mammals, each containing several distinct transcriptional units, bringing the number of PDE proteins to >50. To date, 11 PDE families have been characterized based on amino acid sequences, substrate specificities, endogenous and exogenous regulators, and pharmacological properties: PDE1 (Ca2+-CaM-stimulated), PDE2 (cGMP-stimulated), PDE3 (cGMP-inhibited), PDE4 (cAMP-specific), PDE5 (cGMP-specific), PDE6 (photoreceptor), PDE7 (cAMP high affinity), PDE8 (cAMP high affinity), PDE9 (cGMP high affinity), PDE10 (dual substrate), and PDE11 (dual substrate). Thus, PDE families specific for cGMP are PDE5, PDE6, and PDE9 (47). PDE6 primary localization is in photoreceptive cells, where it plays a central role in the retinal phototransduction cascade. We have looked for cGMP-specific PDEs in the SCN, excluding PDE6 because of its retinal localization. Interestingly, not only PDE5 but also PDE9 was expressed in this tissue, with the former being a more specific target for sildenafil.
It could be argued that the retina or the intergeniculate leaflet, also involved in entrainment (48), might be alternative targets for the systemic administration of the compound. However, because we found a specific increase of cGMP in the SCN after sildenafil administration (as well as a significant expression of PDE5 in these nuclei), it seems likely that at least one of the targets for this inhibitor is in the SCN.
Although there are some common side effects that are associated with sildenafil therapy, the dose needed for reentrainment in humans could be lower than the one used for treatment of erectile dysfunction. Transient aberrations in vision are thought to be caused by inhibition of the retinal PDE6 family; however, its inhibition by sildenafil is 10-fold weaker than that for PDE5 (33). Moreover, no consistent pattern has emerged to suggest any long-term effect of sildenafil on the retina or other structures of the eye or on the ocular circulation (49).
In summary, here we show that direct inhibition of PDE5 in the SCN affects photic entrainment in a clear phase-specific manner, indicating a potential benefit for circadian disorders that require a modulation of light signaling to the clock.
Materials and Methods
Animals.
Male adult (3–4 months old) Syrian hamsters (Mesocricetus auratus) were raised in our colony and maintained in a 14:10-h LD cycle (lights on at 0600 hours), with food and water ad libitum and room temperature set at 20 ± 2°C. All animal procedures were performed in strict accordance with National Institutes of Health rules for animal care and maintenance.
Activity Rhythm Recording.
For the resynchronization experiment, animals were transferred to individual cages equipped with a running wheel (17-cm diameter) and with light intensity averaging 200 lux at cage level. Running-wheel activity was continuously recorded for each animal by using a digital system that registers wheel revolutions and stored at 5-min intervals for further analysis. Animals were initially maintained under a 14:10-h LD cycle (lights on at 1900 hours) for at least 10 days. Then, hamsters were subjected to an abrupt 6-h advance in the phase of the LD cycle. On the day of the phase shift, i.p. injection of sildenafil or vehicle was given at ZT 18, defining ZT 12 as the time of lights off. Time for reentrainment to the new LD cycle was defined as the time it took for each animal to adjust its activity onset with the new cycle (onset at the new time of lights off ± 15 min). Daily onsets of activity were determined following the criteria from Edelstein et al. (50). Briefly, activity onset was defined as the 10-min bin that contained at least 80 wheel revolutions, followed by another bin of at least another 80 wheel revolutions within 40 min. The effect of 1, 3.5, or 10 mg/kg sildenafil [extracted from commercial preparations according to Francis et al. (51)] was tested at ZT 14 or 18 in comparison to vehicle administration (sterile saline).
For light pulse experiments, hamsters were placed in constant dark (DD) conditions and exposed to a 15-min white light pulse of 50 lux at either CT 14 or 18 (with CT 12 defined as the onset of wheel running activity in DD). Phase shifts were calculated by fitting a line (by three observers masked to the experimental procedure) through activity onsets 5 days prior and between 5 and 15 days after light exposure and then comparing these two lines on the day of the light pulse. Hamsters received an i.p. injection of either drug or vehicle 45 min before light stimulation.
RT-PCR.
Total RNA from hamster SCN, kidney, spleen, and heart was isolated in accordance to standard procedures (TRIzol; Life Technologies, Gaithersburg, MD). cDNA was synthesized from 3 μg of RNA by using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). Specific oligonucleotide primers were as follows: PDE5, 5′-CAGGAAATGGTGGGACCTTC (forward) and 5′-AAGGCTTCCAGGAACTGCTC (reverse); PDE9, 5′-AGAACTTATCCAGGGCGTGC (forward) and 5′-TGTCACACAGAAGCAGTGCC (reverse). GAPDH was used as an internal control: 5′-CTGCACCACCACCTGCTTAG (forward) and 5′-CTTTCTCCAGGCGACATGTG (reverse). PCR was performed under the following conditions: denaturing at 94°C for 10 s, annealing at 62°C (PDE5) or 60°C (PDE9) for 15 s, and primer extension at 72°C for 60 s in 35 cycles. PCR products were separated by electrophoresis on a 1% agarose gel and stained with SYBR Green (Molecular Probes, Eugene, OR). A 619-bp fragment for PDE5, a 511-bp fragment for PDE9, and a 309-bp fragment for GAPDH were amplified.
Determination of cGMP Levels.
cGMP content was determined by using a direct enzyme immunoassay kit (Correlate-EIA 900-014; Assay Designs, Ann Arbor, MI). Hamsters received an injection of sildenafil (3.5 mg/kg, i.p.) or vehicle (sterile saline solution) at CT 18 and were killed by decapitation 45–90 min later. The SCN were quickly punched out and immediately frozen in liquid nitrogen to avoid endogenous cGMP degradation. Tissue was grounded to a fine powder under liquid nitrogen and then homogenized in 0.1 M HCl. The overnight acetylated format of the kit was used. Endogenous levels of cGMP fitted on the kit sensitivity and were comparable to those previously published by RIA methods (29). To precisely compare the effect of sildenafil on cGMP levels, we avoided using other PDE inhibitors like IBMX.
Immunohistochemistry.
Animals were deeply anesthetized (ketamine:xylazine, 150:10 mg/kg, i.p.) and perfused intracardially with 0.1 M PBS followed by fixative solution (4% paraformaldehyde in 0.1 M phosphate buffer). After perfusion, brains were dissected and postfixed overnight at 4°C in the same solution. Brains were then transferred into 30% sucrose–paraformaldehyde solution for 48 h. Coronal sections (40 μm) were cut with a freezing microtome and collected in 0.1 M phosphate buffer. Sections were washed with 0.4% Triton X-100 in 0.01 M PBS (PBS-T). Nonspecific binding sites were blocked with 0.1% BSA and 2% normal horse serum (NHS) in PBS-T for 1 h at room temperature. Sections were incubated with cGMP primary antibody 1:4,000 (sheep anti-formaldehyde-fixed cGMP, diluted in 0.4% PBST with 2% NHS, a generous gift from J. De Vente, Maastricht University, Maastricht, The Netherlands) for 48 h at 4°C (52). Biotinylated anti-sheep IgG (1:2,000; Vector Laboratories) was used as a secondary antibody for 2 h at room temperature, followed by rhodamine–avidin incubation (1:500; Vector Laboratories) for 2 h at room temperature.
For colocalization studies, sections were further incubated overnight at 4°C with GFAP (rabbit anti-GFAP, 1:1,000; Dako, Glostrup, Denmark) or with neuron-specific nuclear protein (NeuN, mouse anti-NeuN, 1:50; Chemicon, Temecula, CA) antibodies (in 0.4% PBST with 2% NHS). Labeling was visualized by using fluorescein anti-rabbit or anti-mouse antibodies (1:500; Vector Laboratories, Burlingame, CA).
Confocal laser scanning microscopy (Olympus FV-300 microscope) was performed at 488 nm and 543 nm to reveal fluorescein and rhodamine, respectively. The two channels were scanned separately and merged by using Olympus software. Each optical section (0.4 μm) was averaged four times.
Supplementary Material
Acknowledgments
We thank Drs. M. Harrington, R. Rosenstein, and H. de la Iglesia for critical reading of the manuscript. This work was supported by Agencia Nacional de Promoción Científica y Tecnológica, Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina, Comision de Investigaciones Cientificas de la Provincia de Buenos Aires, and Universidad Nacional de Quilmes.
Abbreviations
- CT
circadian time
- LD
light–dark
- PDE
phosphodiesterase
- SCN
suprachiasmatic nuclei
- ZT
zeitgeber time
- PKG
cGMP-dependent protein kinase
- GFAP
glial fibrillary acidic protein.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703388104/DC1.
References
- 1.Pittendrigh CS, Daan A. J Comp Physiol. 1976;106:291–331. [Google Scholar]
- 2.Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind's Clock. New York: Oxford Univ Press; 1991. [Google Scholar]
- 3.Ebling FJ. Prog Neurobiol. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- 4.Hannibal J. Cell Tissue Res. 2002;309:73–88. doi: 10.1007/s00441-002-0574-3. [DOI] [PubMed] [Google Scholar]
- 5.Dziema H, Obrietan K. J Neurophysiol. 2002;88:1374–1386. doi: 10.1152/jn.2002.88.3.1374. [DOI] [PubMed] [Google Scholar]
- 6.Golombek DA, Ferreyra GA, Agostino PV, Murad AD, Rubio MF, Pizzio GA, Katz ME, Marpegan L, Bekinschtein TA. Front Biosci. 2003;8:56–70. doi: 10.2741/1038. [DOI] [PubMed] [Google Scholar]
- 7.Meijer JH, Schwartz WJ. J Biol Rhythms. 2003;18:235–249. doi: 10.1177/0748730403018003006. [DOI] [PubMed] [Google Scholar]
- 8.Golombek DA, Agostino PV, Plano SA, Ferreyra GA. Neurochem Int. 2004;45:929–936. doi: 10.1016/j.neuint.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 9.Morin LP, Allen CL. Brain Res Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Pittendrigh CS. In: Handbook of Behavioral Neurobiology: Biological Rhythms. Aschoff J, editor. New York: Plenum; 1981. pp. 95–124. [Google Scholar]
- 11.Colwell CS. Eur J Neurosci. 2001;13:1420–1428. doi: 10.1046/j.0953-816x.2001.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butcher GQ, Lee B, Obrietan K. J Neurophysiol. 2003;90:3854–3863. doi: 10.1152/jn.00524.2003. [DOI] [PubMed] [Google Scholar]
- 13.Obrietan K, Impey S, Storm DR. Nat Neurosci. 1998;1:693–700. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- 14.Pizzio GA, Hainich EC, Ferreyra GA, Coso OA, Golombek DA. NeuroReport. 2003;14:1417–1419. doi: 10.1097/00001756-200308060-00002. [DOI] [PubMed] [Google Scholar]
- 15.Agostino PV, Ferreyra GA, Murad AD, Watanabe Y, Golombek DA. Neurochem Int. 2004;44:617–625. doi: 10.1016/j.neuint.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Tischkau SA, Mitchell JW, Tyan SH, Buchanan GF, Gillette MU. J Biol Chem. 2003;278:718–723. doi: 10.1074/jbc.M209241200. [DOI] [PubMed] [Google Scholar]
- 17.Coogan AN, Piggins HD. J Neurosci. 2003;23:3085–3093. doi: 10.1523/JNEUROSCI.23-07-03085.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lowrey PL, Takahashi JS. Annu Rev Genet. 2000;34:533–562. doi: 10.1146/annurev.genet.34.1.533. [DOI] [PubMed] [Google Scholar]
- 19.Mrosovsky N. Biol Rev. 1996;71:343–372. doi: 10.1111/j.1469-185x.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 20.Gillette MU, Mitchell JW. Cell Tissue Res. 2002;309:99–107. doi: 10.1007/s00441-002-0576-1. [DOI] [PubMed] [Google Scholar]
- 21.Hannibal J, Ding JM, Chen D, Fahrenkrug J, Larsen PJ, Gillette MU, Mikkelsen JD. J Neurosci. 1997;17:2637–2644. doi: 10.1523/JNEUROSCI.17-07-02637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prosser RA, Gillette MU. J Neurosci. 1989;9:1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tischkau SA, Gallman EA, Buchanan GF, Gillette MU. J Neurosci. 2000;20:7830–7837. doi: 10.1523/JNEUROSCI.20-20-07830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golombek DA, Ralph MR. Neurosci Lett. 1995;192:101–104. doi: 10.1016/0304-3940(95)99209-n. [DOI] [PubMed] [Google Scholar]
- 25.Melo L, Golombek DA, Ralph MR. J Biol Rhythms. 1997;12:319–326. doi: 10.1177/074873049701200404. [DOI] [PubMed] [Google Scholar]
- 26.Ferreyra GA, Cammarota MP, Golombek DA. Brain Res. 1998;797:190–196. doi: 10.1016/s0006-8993(98)00376-x. [DOI] [PubMed] [Google Scholar]
- 27.Ding JM, Faiman LE, Hurts WJ, Kuriashkina LR, Gillette MU. J Neurosci. 1997;17:667–675. doi: 10.1523/JNEUROSCI.17-02-00667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber ET, Gannon RL, Rea MA. Neurosci Lett. 1995;197:227–230. doi: 10.1016/0304-3940(95)11961-u. [DOI] [PubMed] [Google Scholar]
- 29.Prosser RA, McArthur AJ, Gillette MU. Proc Natl Acad Sci USA. 1989;86:6812–6815. doi: 10.1073/pnas.86.17.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathur A, Golombek DA, Ralph MR. Am J Physiol. 1996;270:R1031–R1036. doi: 10.1152/ajpregu.1996.270.5.R1031. [DOI] [PubMed] [Google Scholar]
- 31.Ferreyra GA, Golombek DA. Am J Physiol. 2001;280:R1348–R1355. doi: 10.1152/ajpregu.2001.280.5.R1348. [DOI] [PubMed] [Google Scholar]
- 32.Tischkau SA, Weber ET, Abbott SM, Mitchell JW, Gillette MU. J Neurosci. 2003;23:7543–7550. doi: 10.1523/JNEUROSCI.23-20-07543.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corbin JD, Francis SH. J Biol Chem. 1999;274:13729–13732. doi: 10.1074/jbc.274.20.13729. [DOI] [PubMed] [Google Scholar]
- 34.De Young L, Yu D, Freeman D, Brock GB. Int J Impot Res. 2003;15:347–354. doi: 10.1038/sj.ijir.3901026. [DOI] [PubMed] [Google Scholar]
- 35.Johnson CH, Elliott JA, Foster R. Chronobiol Int. 2003;20:741–774. doi: 10.1081/cbi-120024211. [DOI] [PubMed] [Google Scholar]
- 36.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 37.Nishi A, Watanabe Y, Higashi H, Tanaka M, Nairn AC, Greengard P. Proc Natl Acad Sci USA. 2005;102:1199–1204. doi: 10.1073/pnas.0409138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan L, Bobula JM, Svenningsson P, Greengard P, Silver R. J Neurosci. 2006;26:9434–9438. doi: 10.1523/JNEUROSCI.2538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Francis SH, Zoraghi R, Kotera J, Ke H, Bessay EP, Blount MA, Corbin JD. In: Cyclic Nucleotide Phosphodiesterases in Health and Disease. Beavo JA, Francis SH, Houslay M, editors. Boca Raton, FL: CRC Press; 2006. pp. 131–165. [Google Scholar]
- 40.Zisapel N. CNS Drugs. 2001;15:311–328. doi: 10.2165/00023210-200115040-00005. [DOI] [PubMed] [Google Scholar]
- 41.Revell VL, Eastman CI. J Biol Rhythm. 2005;20:353–365. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirai K, Kita M, Ohta H, Nishikawa H, Fujiwara Y, Ohkawa S, Miyamoto M. J Biol Rhythms. 2005;20:27–37. doi: 10.1177/0748730404269890. [DOI] [PubMed] [Google Scholar]
- 43.Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Curr Biol. 2006;16:R914–R916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oxenkrug GF, Requintina PJ. CNS Spectr. 2003;8:139–148. doi: 10.1017/s109285290001837x. [DOI] [PubMed] [Google Scholar]
- 45.Golombek DA, Cardinali DP. Chronobiol Int. 1993;10:435–441. doi: 10.1080/07420529309059719. [DOI] [PubMed] [Google Scholar]
- 46.Mrosovsly N, Salmon PA. Nature. 1987;330:372–373. doi: 10.1038/330372a0. [DOI] [PubMed] [Google Scholar]
- 47.Bender AT, Beavo JA. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 48.Harrington ME. Neurosci Biobehav Rev. 1997;21:705–727. doi: 10.1016/s0149-7634(96)00019-x. [DOI] [PubMed] [Google Scholar]
- 49.Laties A, Sharlip I. J Sex Med. 2006;3:12–27. doi: 10.1111/j.1743-6109.2005.00194.x. [DOI] [PubMed] [Google Scholar]
- 50.Edelstein K, de la Iglesia HO, Schwartz WJ, Mrosovsky N. Neuroscience. 2003;118:253–261. doi: 10.1016/s0306-4522(02)00908-9. [DOI] [PubMed] [Google Scholar]
- 51.Francis SH, Sekhar KR, Rouse AB, Grimes KA, Corbin JD. Int J Impot Res. 2003;15:369–372. doi: 10.1038/sj.ijir.3901040. [DOI] [PubMed] [Google Scholar]
- 52.De Vente J, Assan E, Gambaryan S, Markerink-van Ittersum M, Axer H, Gallatz K, Lohmann SM, Palkovits M. Neuroscience. 2001;108:27–49. doi: 10.1016/s0306-4522(01)00401-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.