Abstract
Historical data provide a baseline against which to judge the significance of recent ecological shifts and guide conservation strategies, especially for species decimated by pre-20th century harvesting. Northern fur seals (NFS; Callorhinus ursinus) are a common pinniped species in archaeological sites from southern California to the Aleutian Islands, yet today they breed almost exclusively on offshore islands at high latitudes. Harvest profiles from archaeological sites contain many unweaned pups, confirming the presence of temperate-latitude breeding colonies in California, the Pacific Northwest, and the eastern Aleutian Islands. Isotopic results suggest that prehistoric NFS fed offshore across their entire range, that California populations were distinct from populations to the north, and that populations breeding at temperate latitudes in the past used a different reproductive strategy than modern populations. The extinction of temperate-latitude breeding populations was asynchronous geographically. In southern California, the Pacific Northwest, and the eastern Aleutians, NFS remained abundant in the archaeological record up to the historical period ≈200 years B.P.; thus their regional collapse is plausibly attributed to historical hunting or some other anthropogenic ecosystem disturbance. In contrast, NFS populations in central and northern California collapsed at ≈800 years B.P., long before European contact. The relative roles of human hunting versus climatic factors in explaining this ecological shift are unclear, as more paleoclimate information is needed from the coastal zone.
Keywords: Callorhinus ursinus, historic ecology, stable isotopes, zooarchaeology, ancient DNA
Humans, including prehistoric indigenous groups, play a major role in shaping their environment, such that some of the ecosystems we are familiar with today operated differently in the past (1). Paleoecological data illuminate the natural history of species on ecologically and evolutionarily relevant timescales, providing a means of evaluating the significance of current ecological trends that is vital to the success of long-term conservation strategies (2). This perspective is especially important for species that have suffered recent declines in population size because of human disturbance. Here we use archaeometric, isotopic, genetic, and chronologic data to reveal prehistoric shifts in the ecology of northern fur seals (NFS; Callorhinus ursinus) and then briefly explore the factors driving these changes.
Post-Columbian explorers encountered just two NFS breeding populations along the entire margin of the northeast Pacific Ocean (Fig. 1), a small one on the Farallon Islands (≈38°N) off San Francisco Bay (3) and a much larger one on the Pribilof Islands (≈57°N) in the eastern Bering Sea (4). In striking contrast, NFS fossils are substantial components of archaeological sites from southern California to the eastern Aleutian Islands (5–8) (Fig. 1). The latter finding is puzzling because the modern pattern of offshore foraging, primarily high-latitude island breeding, and a short lactation period (4) should have made NFS largely unavailable to human hunters at temperate latitudes. Even if indigenous people had the maritime technologies needed to exploit offshore/pelagic prey, regular offshore hunting of NFS, which are relatively small pinnipeds that occur today at low densities (9), would be a suboptimal foraging strategy. There are two potential explanations for the common occurrence of NFS in archaeological sites. (i) They may be the remains of individuals, largely from high-latitude breeding colonies, that accidentally stranded and were scavenged by humans. This explanation requires no major changes in NFS breeding or migratory behavior, although it might require a larger source population at high latitudes to explain the higher stranding frequency in the past. (ii) NFS may have come from nearby haul-outs or breeding colonies (5–8). This explanation requires a marked increase in the number and/or size of NFS breeding colonies at temperate latitudes. Under the second scenario, NFS would have been especially susceptible to human predation if they congregated at high densities close to shore or if they weaned their pups at an older age than modern populations. We evaluate these possible explanations using information obtained from NFS remains in archaeological sites from the western Aleutian Islands to southern California (Fig. 1).
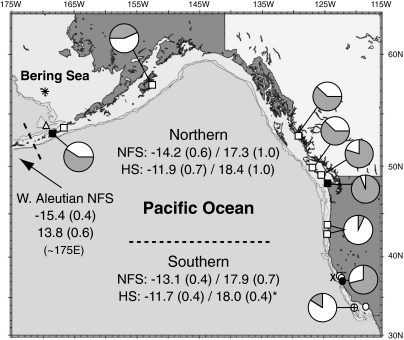
Fig. 1.
Mean bone collagen isotope values of ancient pinnipeds as well as abundance estimates from selected archaeological sites. Pie diagrams show the relative abundance of NFS remains (shaded) versus other pinniped remains based on the number of identifiable specimens. Mean δ13C/δ15N values (SD) for adult female NFS and HS are reported beneath each labeled group of NFS or HS; the asterisk denotes previously published HS data from the southern region (19). A site-by-site compilation of pinniped isotope values is available as SI Table 1. NFS cluster into northeastern Pacific (squares), California (circles), and western Aleutian populations based on significant differences in isotopic values. Filled symbols denote sites with harvest profile data (see Fig. 2). Locations of islands mentioned in text are shown: ✷, Pribilof Islands, eastern Bering Sea; ▵, Bogoslof Island, eastern Aleutians; X, Farallon Islands off San Francisco Bay; ⊕, SMI off southern California.
Currently, NFS in the eastern North Pacific are in decline for unknown reasons. Population estimates are at a historic low for the Pribilof Islands stock in the eastern Bering Sea (9), where ≈65% of the global population breeds (4). Adult females, after spending autumn through late spring at sea, return to the Pribilof Islands to breed in late June. Most pups are born between July 3rd and July 11th and are weaned in early November (4), at which time young-of-the-year (YOY) and adult females migrate as far south as California during the winter months (10). Adult males from the Pribilofs population remain in the Gulf of Alaska throughout the winter (11). Recently, migrants from the Pribilofs have established breeding colonies on San Miguel Island (SMI) off southern California (12) and Bogoslof Island in the eastern Aleutians (13) (Fig. 1). The SMI and Bogoslof populations breed on a schedule similar to the Pribilof population; modern NFS wean their pups at ≈4 months of age across their entire range. Recent reports also indicate that NFS are breeding on the Farallon Islands off central California (3), but this small colony has not yet been formally studied.
Results and Discussion
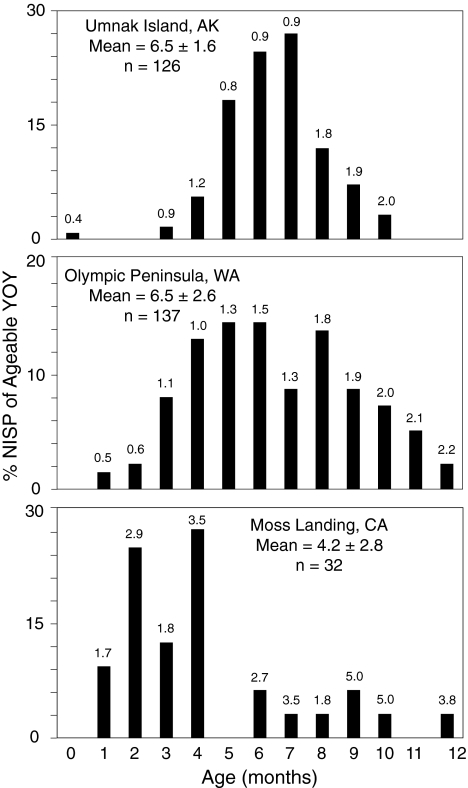
Definitive evidence for numerous prehistoric breeding colonies along the northeast Pacific margin comes from harvest (mortality) profiles from archaeological sites, which reveal the age/sex classes exploited by ancient humans. Assuming ancient NFS populations nursed at least as long as the modern population, YOY equaling 4 months of age must be preweaned pups and, by extension, must signal the presence of a colony near the site of recovery. Coupling relative abundance estimates (Fig. 1) and harvest profiles (Fig. 2) for prehistoric NFS provides three firm conclusions. First, pups of 4 months of age represent a substantial portion of the distribution at most sites, establishing that NFS had breeding colonies in California, the Pacific Northwest, and the eastern Aleutian Islands. Second, the high abundance of NFS relative to other pinnipeds throughout much of this distribution strongly suggests direct predation by humans on nearby colonies, not scavenging of strandings. Finally, strong representation of 5- to 12-month-old individuals in the profiles from the eastern Aleutians (Fig. 2A) and the Olympic Peninsula (Fig. 2B) indicates that immature NFS were available to humans year-round in these regions, not just during a short, 4-month breeding season as would be the case with modern NFS.
Fig. 2.
NFS YOY harvest profiles for selected archaeological assemblages, representing the percentage of identifiable specimens (where NISP is number of identifiable specimens) versus age (in months). The mean age in months (SD) and the number of specimens for each distribution are noted. Numbers above each bar represent the median SD of age error estimates (in months) for all skeletal elements assigned to a particular age class.
Stable isotope data provide insights into the foraging ecology and maternal strategies of ancient NFS populations [supporting information (SI) Text]. Natural variations in stable carbon isotope (δ13C) values provide information on foraging location (14, 15) because food web δ13C values are higher in nearshore versus open-ocean ecosystems (16, 17). In all sites where they co-occur (Fig. 1), prehistoric adult female NFS have significantly lower δ13C values than harbor seals (HS, Phoca vitulina; ref. 18), which are nonmigratory and forage close to shore (19). Consistent 13C-depletion in prehistoric NFS relative to nearshore-foraging HS indicates that NFS were foraging in deep, offshore waters over their entire range. Thus, the apparent availability of NFS to prehistoric human hunters was not because they foraged close to shore. Furthermore, both carbon and nitrogen (δ15N) isotope values can also provide information on foraging latitude because phytoplankton δ13C and δ15N values are higher in temperate than in high-latitude ecosystems, and both values decline steeply from east to west across the Bering Sea and Aleutian Islands (16, 20, 21). Isotopically, prehistoric adult female NFS cluster into three geographically defined groups: a southern group (California) with high isotope values, a northern group (eastern Aleutian/Gulf of Alaska/Pacific Northwest) with intermediate values, and a western Aleutian group with very low isotope values (Fig. 1). Isotopic distinctions among seals from different regions confirm that prehistoric NFS from California were not immigrants from northern waters but instead were year-round residents.
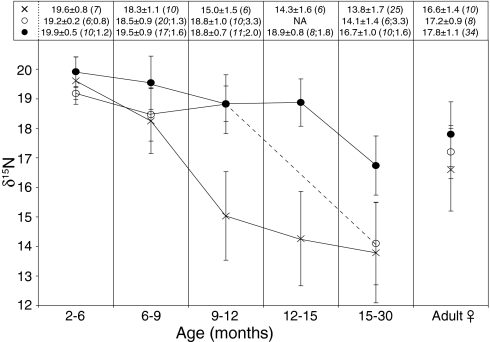
δ15N values have also been used to characterize the duration of nursing and approximate age at weaning for many mammals, including otariids (eared seals; refs. 14 and 22). In theory, lactating mothers catabolize their own tissues to produce milk for their offspring; thus nursing offspring appear to be feeding one trophic level higher than their mother (i.e., a δ15N increase of ≈3–5‰). To determine why 5- to 12-month-old YOY are common in some sites, we measured δ15N values of modern and prehistoric NFS between 2 and 30 months of age. Fig. 3 shows δ15N results for three NFS bone ontogenetic series, including one from the (modern) Pribilof Islands and two from northeast Pacific archaeological sites. Careful consideration of bone collagen turnover rates is required when analyzing bone ontogenetic series because turnover is approximately correlated with growth rates and, by extension, age. For the modern Pribilof series, there is a robust trend in δ15N values, which shows a gradual and significant decline in values from individuals 2–6 to 9–12 months of age. If modern NFS from the Pribilof Islands begin to ingest solid food shortly after weaning at ≈4 months of age and we assume that recently weaned animals consume solid prey types similar to those consumed by juveniles 12–15 months of age, it takes ≈8 months for the δ15N signal associated with nursing to be completely diluted by bone collagen turnover (22). δ15N results from the archaeological series show a very different pattern (Fig. 3). There is no difference in δ15N values between the archaeological series and the modern Pribilof series for the 2- to 6-month age class. This pattern is expected because modern Pribilof adult female δ15N values (15) are statistically indistinguishable from values of Umnak Island and Ozette adult females (Fig. 3). However, high δ15N values persist to the 9- to 12-month age class at Umnak Island and to the 12- to 15-month age class at Ozette.
Fig. 3.
Bone collagen δ15N values for one modern and two Holocene NFS ontogenetic series (2–30 months of age): ×, modern Pribilof Islands (Bering Sea); ○, prehistoric Umnak Island (eastern Aleutians, Alaska); ●, prehistoric Ozette (Olympic Peninsula, Washington). Mean δ15N values ± SD for age groups are presented in the table above the figure. Numbers in parentheses are sample size (shown with italics) followed by the median SD of age error estimates (in months) for all skeletal elements assigned to a particular age class. The dashed line indicates no data for the 12- to 15-month age class for the prehistoric Umnak Island site.
There are four potential explanations for why high δ15N values persist to older ages in prehistoric YOY. The first is that ancient NFS were weaned at the same age but grew much faster than modern NFS from the Pribilofs, resulting in systematic overestimation of age-at-death for archaeological specimens. A comparison of age-specific bone growth series reveals that prehistoric NFS males from Ozette (Washington) grew slower, not faster, than modern Pribilof males (SI Fig. 5 and SI Text), refuting this hypothesis. Because starvation may cause δ15N values of body tissues to increase (23), a second possibility is that all of the archaeological YOY we sampled were starved individuals. This is an unlikely scenario, especially in sites where YOY are abundant and ample archaeological evidence suggests NFS were actively hunted (5–8). A third possibility is that, upon weaning at ≈4 months, pups immediately began to feed one trophic level higher than adult females, then switched to feed at a lower trophic level than adult females at ≈12 months of age. This ad hoc explanation is highly unlikely given differences in size and presumed foraging success between YOY and adult females (4). We favor the final explanation: that YOY from breeding colonies in the eastern Aleutians and Pacific Northwest weaned at a much older age than their modern Bering Sea counterparts.
To determine whether the divergence in NFS maternal strategy reflects interspecific differences, we extracted ancient mitochondrial DNA sequences and compared them with published sequences from northeast Pacific otariids, including modern NFS (24). Although genetically diverse [H = 0.8 (where “H” indicates haplotypic diversity)], prehistoric NFS from three temperate-latitude archaeological sites group among modern NFS to the exclusion of other sympatric otariids (SI Fig. 6); archaeological NFS are thus not a different species than modern NFS.
Our results suggest that in the absence of strong selection for early weaning imposed by the onset of severe winter conditions in the Bering Sea, weaning at an older age may have been adaptive for NFS populations breeding at temperate latitudes. Most otariids (12 of 14 species; refs. 19 and 25) utilize a long-term lactation strategy in which pups are gradually weaned sometime between 10 and 14 months of age depending on species and breeding locality. The long-term strategy is thought to buffer populations against interannual resource fluctuations resulting from short-term climatic events, such as El Niño–Southern Oscillation (ENSO) events, that negatively affect seasonal productivity in many temperate- and tropical-latitude marine ecosystems (25, 26). Today on SMI (Fig. 1), NFS pup production drastically declines during ENSO events (27), and it takes several years (≥8) for production to recover to pre-ENSO levels, implying that recruitment and/or adult reproduction are also negatively impacted by these short-term resource failures. If maternal strategy has a genetic foundation, as suggested by observation of otariid interspecific hybridization (28), the recently established NFS populations on SMI and the Farallon Islands provide a unique opportunity to test hypotheses relating to the evolution of otariid maternal strategies (29). Finally, the antiquity of the NFS breeding population on the Pribilof Islands is unknown, but we assume it would have followed the same rapid weaning schedule as modern NFS.
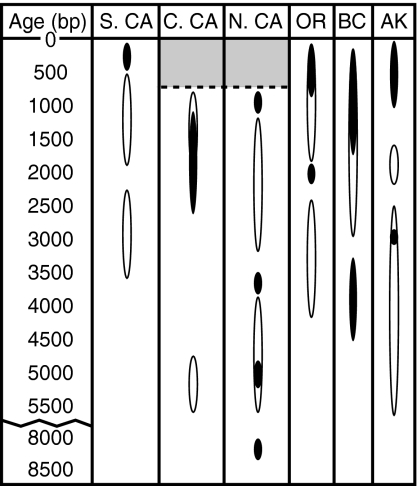
The loss of NFS breeding populations at temperate latitudes is part of a broader pattern of declines in marine mammal abundances along the eastern Pacific margin. Researchers studying ecosystem and human cultural change on the Pacific margin have offered several explanations for drops in marine mammal abundance in archaeological sites. These include predation by Europeans in historic times (30), intensifying human predation spanning several millennia before European contact (5), climate change on land that drove humans to unsustainably harvest marine prey (31), or climatically driven reductions in marine productivity, with humans playing no major role (32). Our accelerator mass spectrometry (AMS) 14C dates on NFS and published radiocarbon data on archaeological material firmly associated with NFS provide constraints on these hypotheses (Fig. 4 and SI Table 2) (for review, see refs. 5, 6, and 8). First, NFS are consistent, and relatively abundant, components of coastal archaeological faunal assemblages that date from the middle Holocene (≈5,000 years B.P.) to the historical period (≈200 years B.P.) in nearly all regions along the northeast Pacific margin (8). These radiocarbon data suggest that the rapid and substantial enlargement of NFS breeding distribution that has occurred over the past several decades is unprecedented in comparison with the prehistoric scenario; it may be a response to the intense, well documented, historic population bottleneck that the species suffered before protection in the early 20th century (30, 33). Second, the apparent declines in NFS were asynchronous across its prehistoric breeding range. In southern California, the Pacific Northwest, and the eastern Aleutian Islands, NFS remained abundant in the archaeological record up to the historical period; thus, their regional collapse is plausibly attributed to historical hunting or some other anthropogenic ecosystem disturbance. In contrast, NFS dates from central and northern California range from ≈8,400 to 800 years B.P., with population collapse before European contact. The Medieval Climatic Anomaly, which approximately coincides with the last dates for NFS in central and northern California (≈800–1,000 years B.P.), was a period of drought in western North America (31, 34). Paleoceanographic records from central and northern California (35) do not reveal any substantial, permanent shifts in marine conditions at the time, but more data, particularly on El Niño–Southern Oscillation frequency and intensity, are needed. The alternative, that drought on land might have precipitated unsustainable use of marine resources by human populations, is intriguing, but likewise must be tested with additional terrestrial paleoclimate and archaeological data from the coastal zone.
Fig. 4.
Calibrated AMS 14C results on NFS (filled areas within ovals; SI Table 2) and associated material (open areas within ovals) from northeast Pacific archaeological sites in which NFS are the first or second most abundant pinniped species (for review, see refs. 5, 6, 8, and 46). The gray-shaded region represents dated sites from central and northern California in which NFS are rare (5, 6, 8).
NFS provide one of the clearest examples of major ecological change in the recent past leading to present-day remnant distributions. Our study demonstrates that NFS had more temperate-latitude breeding colonies in the past, with breeding populations in California, the Pacific Northwest (Washington and/or British Columbia), and the eastern Aleutian Islands, and that these populations used a different reproductive strategy than modern populations. Over the past several decades, NFS migrants sourced from high-latitude breeding colonies (Pribilof and Commander Islands, Bering Sea) have reestablished breeding colonies in some of these areas (3, 12, 13), despite ongoing and unexplained population declines in the source population in the eastern Bering Sea. Further establishment of temperate-latitude breeding colonies could help buffer the global population of this species, through diversification of population and genetic structure, and by diffusing potential threats to population viability (anthropogenic or climatic) over a larger geographical area. Our results also have implications for questions regarding the behavioral plasticity of marine mammals in response to environmental gradients. As NFS reestablish temperate-latitude breeding colonies, our data suggest that their reproductive behavior (attendance behavior and weaning age) may change as they adapt to a different set of environmental selection pressures. Our multidisciplinary study highlights the importance of understanding preexploitation biogeography and behavior of species whose current ecology may be shaped by recent exploitation and/or environmental change, which is only possible through examination of ancient material.
Materials and Methods
Stable Isotope and AMS Radiocarbon Methods.
For δ13C and δ15N analysis, an ≈100-mg sample of compact bone was removed from each specimen with a low-speed cutting tool. Bone fragments were cleaned of sediment and demineralized in 0.5 M HCl for ≈12–15 h at 5°C. The resulting material was treated repeatedly with a chloroform/methanol (2:1) mixture to remove lipids and then lyophilized. Dried samples (≈1.0 mg) were sealed in tin boats and analyzed by using a Carlo-Erba elemental analyzer interfaced with an Optima gas source mass spectrometer (Department of Earth and Planetary Sciences, University of California Santa Cruz).
Results are expressed as δ values, δ13C or δ15N = 1,000[(Rsample/Rstandard) − 1], where Rsample and Rstandard are the 13C/12C or 15N/14N ratios of the sample and standard, respectively. The standards are Vienna-Pee Dee Belemnite limestone (V-PDB) for carbon and atmospheric N2 for nitrogen. The units are expressed as parts per thousand (‰). Repeated measurements of a gelatin standard (n = 100) yielded an SD of <0.2‰ for both δ13C and δ15N values. Duplicate isotopic measurements were performed on ≈20% of all unknown samples and yielded a mean absolute difference of 0.2‰ for δ13C and δ15N values.
For AMS 14C analysis of bone collagen (36), raw bone samples were crushed and demineralized in 0.5 M HCl at 5°C for ≈12–15 h. The resulting organic matter was gelatinized in 0.1 M HCl at ≈60°C for 12–15 h. Gelatinized samples were filtered by using glass microfiber filters to remove large particulate matter. The resulting mixture was then ultra-filtered by using centrifugal filters to remove low-molecular-weight (<30 kDa) fragments and vacuum-concentrated. Collagen was combusted to CO2 via combustion and then graphitized for AMS 14C analysis at the Center for AMS at the Lawrence Livermore National Laboratory (LLNL). We applied a marine reservoir correction (ΔR) of 250 ± 35 years to the δ13C-corrected 14C ages to report the calibrated 2σ age range (Fig. 4 and SI Table 2) (37, 38).
Statistical Analyses.
Statistical tests were calculated by using the software program JMP (version 5.0; SAS Institute, Cary, NC). NFS subgroups were determined via a multivariate ANOVA (MANOVA) model of site-specific δ13C and δ15N values and an identity response function. Post hoc Wilks' lambda and Pillai's trace multivariate tests yielded significant differences (P < 0.001). Differences in bone collagen δ13C and δ15N values among the subgroups discussed in the text (Fig. 1 and SI Table 1) were assessed by using a post hoc F test comparison of subgroups identified by the MANOVA model (F = 1.055; P < 0.001). Northern and southern HS groups did not differ significantly from one another (P > 0.10).
Statistically significant differences among NFS bone collagen δ15N values for the ontogenetic series were assessed by using a one-way ANOVA followed by a post hoc Tukey honestly significant difference (HSD) pairwise comparison test. For the modern Pribilof Island ontogenetic series, significant differences in mean δ15N values were found between the 6- to 9-month and 9- to 12-month age classes (P < 0.001), but the 2- to 6-month and 6- to 9-month age classes are not significantly different from one another (P > 0.10). The most likely reason why the 2- to 6-month and 6- to 9-month age classes do not differ in mean δ15N values is because isotopic turnover in bone collagen is relatively slow, taking ≈8–10 months in NFS YOY (22). Furthermore, the 9- to 12-month, 12- to 15-month, and 15- to 30-month age classes are not significantly different from one another (P > 0.10). For the archaeological series from Umnak Island, statistical tests yielded two groups. All classes younger than 12 months of age were not significantly different from one another (P > 0.10); however, these groups were significantly different from the 15- to 30-month age class (P < 0.001). Unfortunately, the 12- to 15-month age class was not present in our sample from Umnak Island, AK. For Ozette, all classes younger than 15 months of age are not significantly different from one another (P > 0.10); however, these groups were significantly different from the 15- to 30-month and adult female age classes (P < 0.001). Given the small SDs on the age estimates for 6- to 12-month-old animals from Umnak and Ozette (1–2 months) (Fig. 3), it is highly unlikely that the high δ15N values seen in these individuals are attributable to misclassification of animals that are <4 months of age.
Reference NFS Bone Regressions and Error in Age Estimation.
The age-at-death of modern (Pribilof Islands, AK) reference specimens was estimated from tags and/or brands applied at birth. Age was calculated in fractions of years, with an age of zero corresponding to the date of birth. Virtually all births occur within a few weeks of the mean birthing date, which ranges from July 3rd to July 11th on breeding colonies in the Pribilof Islands (39, 40). We assume that collection date is synonymous with the date of death because the large majority (>90%) of the specimens were not stranded individuals but directly harvested for commercial, subsistence, or scientific purposes.
Age-at-death estimates for archaeological specimens are based on regressions of bone length versus age developed from known-age reference specimens (7). Bone regressions were developed for 16 different element metrics (e.g., total length, proximal width, distal width, etc.) on 10 NFS skeletal elements, such as mandibles, humeri, and femora. The metric with the highest R2 value was used when possible. However, if the unknown specimens were fragmented or incomplete, the next best available measurement was used. Bone length versus age regressions followed Schnute's (41) formulation of von Bertalanffy growth curves (42, 43):
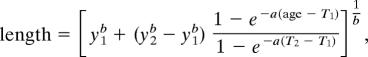 |
where T1 and T2 are specified a priori and represent the youngest and oldest ages over which growth rates are calculated. Rearranging this equation to express age as a function of length yields:
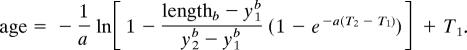 |
To determine the precision of age estimation, an approximation for the variance of age for a given length measurement was calculated by using a first-order Taylor series expansion:
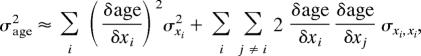 |
where the xs represent the regression parameters a, b, y1, and y2 (44). For each bone-length measurement, the age SD (SD = square root of σage2) was calculated, and the median SD was determined for all of the age estimates in each age class (Figs. 2 and 3).
Ancient DNA Methods.
To determine whether the high behavioral variability in maternal strategy that we observed signifies taxonomic differences at the species level, we extracted DNA from teeth and/or bone for 19 archaeological specimens. We then compared these data with sequences of modern NFS from the Pribilof Islands as well as the sympatric Guadalupe fur seal (Arctocephalus townsendi), California sea lion (Zalophus californianus), and Steller sea lion (Eumetopias jubatus) available in the public GenBank database (SI Fig. 6). We analyzed archaeological specimens from Chaluka (Umnak Island, AK; n = 7), Ozette (Olympic Peninsula, WA; n = 4), and SMI (Channel Islands, CA; n = 8). E.A.H.'s laboratory at Stanford University, in which ancient DNA (aDNA) was extracted and amplified, had no prior or concurrent history of working with modern pinniped DNA.
The following primers were used to obtain a 156-years-B.P. region of the mitochondrial control region from DNA extracted from teeth: 5′-3′ CalloCR1, CTCCCCCTATGTACTTCGTGCA; 3′-5′ CalloCR2, CAGCAACCCTTGTGAAAAGTGTAC. This region was chosen because it was targeted in published studies and shown to be informative within and above the species level for otariids (24). Final PCR concentrations were AmpliTaq Gold polymerase (0.035 units/ml), Taq Gold buffer (1×), MgCl2 (5 mM), dNTPs (1 mM each), primers (20 μM), sterile water, spermidine (1 mM), and 5 μl of DNA template in a total volume of 50 μl. We used the following PCR conditions: 95°C for 10 min followed by 45 cycles of 95°C for 30 s, 45°C for 30 s, and 72°C for 1 min. Successfully amplified fragments were cleaned with the Qiagen (Valencia, CA) Qiaquick PCR purification kit protocol and served as templates in the sequencing thermocycling. To corroborate results and resolve ambiguous sites on single strands, fragments were sequenced in both directions.
Sequences were aligned in Sequencher 3.1.1 (Gene Codes, Ann Arbor, MI) and checked by eye for potential polymorphic sites. For tree-building, we used MEGA 3.0 (MEGA, Tempe, AZ) to perform neighbor-joining analyses and PAUP 4.0 (Sinaur Associates, Sunderland, MA) for maximum likelihood analyses and parameter estimation. We used the Akaike Information Criterion in Modeltest 3.7 (David Posada, Vigo, Spain) to select the most likely model of DNA substitution (TVM+G, using substitution code abcdbe and ∝ = 0.3655). Martes and Procyon were used as outgroup taxa.
Bootstrapping of the data by using both likelihood and neighbor-joining methods yielded significant support for the monophyly of ancient and modern NFS (98%) and for each of the sympatric species. This result was not affected by the inclusion of available DNA sequences from additional nonsympatric Arctocephalus taxa (data not shown). The clustering of most ancient sequences among modern NFS (SI Fig. 6) to the exclusion of other species further supports the notion that prehistoric temperate-latitude NFS did not form a distinct genetic unit separate from modern NFS. We also acknowledge that the genetic distinction between NFS and A. townsendi is based on a small sample size (n = 2) for Arctocephalus, which experienced a severe loss of genetic diversity because of a population bottleneck after historic exploitation (45). In addition to the significant support for reciprocal monophyly mentioned above, the specific distinction is strengthened by the fact that NFS and A. townsendi are not in the same genus and thus are not expected to share similar haplotypes that closely related species may share with rapid molecular markers like the control region. It would indeed be possible that primitive haplotypes of one species with high intrinsic genetic variability could be misidentified if a closely related species within the same genus went through a genetic bottleneck, but it would be very unlikely to make a genetic misdiagnosis at the generic level with fast-evolving molecular markers. The phylogenetic tree shows similar intergenus distances for Arctocephalus–Callorhinus and Arctocephalus–Zalophus/Eumetopias, which are significantly larger than the largest intra-Callorhinus distance.
Supplementary Material
Acknowledgments
We thank the following individuals and institutions for access to samples: D. Corbett (United States Geological Survey, Anchorage, AK); R. Knecht (Museum of the Aleutians); E. Pillaert (University of Wisconsin Zoological Museum, Madison, WI); A. MacMillan, I. McKechnie, and R. Carlson (Simon Fraser University, Burnaby, BC, Canada); G. Keddie (Royal British Columbia Museum, Victoria, BC, Canada); S. Crockford and G. Frederick (Pacific Identifications, Victoria, BC, Canada); D. Fedje and I. Sumpter (Parks Canada); J. Bowchop (Makah Cultural and Research Center, Neah Bay, WA); D. Brauner (Oregon State University, Corvallis, OR); and T. Wake (University of California, Los Angeles, CA). We also thank J. A. Estes, R. L. DeLong, A. C. Jakle, and M. L. Fogel for comments on earlier drafts of this manuscript. This research was funded by National Science Foundation Grants EAR-0000895 and OCE-0345943 and by student research grants from the Myers Oceanographic and Marine Biology Trust, the Long Marine Laboratory, and the Professional Association of Diving Instructors Foundation. Radiocarbon analyses were performed under the auspices of the United States Department of Energy by the University of California, Lawrence Livermore National Laboratory under Contract W-7405-Eng-48.
Abbreviations
- AMS
accelerator mass spectrometry
- HS
harbor seals
- NFS
northern fur seal(s)
- SMI
San Miguel Island
- YOY
young-of-the-year.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610986104/DC1.
References
- 1.Jackson JB, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, et al. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 2.Willis KJ, Birks HJB. Science. 2006;314:1261–1265. doi: 10.1126/science.1122667. [DOI] [PubMed] [Google Scholar]
- 3.Pyle P, Long D, Schonewald J, Jones R, Roletto J. Mar Mammal Sci. 2001;17:397–402. [Google Scholar]
- 4.Gentry R. Behavior and Ecology of the Northern Fur Seal. Princeton: Princeton Univ Press; 1998. [Google Scholar]
- 5.Hildebrandt W, Jones T. J Anthropol Archaeol. 1992;11:360–401. [Google Scholar]
- 6.Lyman R. J Anthropol Archaeol. 1995;14:45–77. [Google Scholar]
- 7.Etnier MA. Seattle: Univ of Washington; 2002. PhD thesis. [Google Scholar]
- 8.Gifford-Gonzalez D, Newsome SD, Koch PL, Guilderson TP, Snodgrass JJ, Burton RK. In: The Exploitation and Cultural Importance of Sea Mammals. Monks G, editor. New York: Oxbow Books; 2005. pp. 19–38. [Google Scholar]
- 9.Towell R, Ream R, York A. Mar Mammal Sci. 2006;22:486–491. [Google Scholar]
- 10.Kajimura H. Opportunistic Feeding of the Northern Fur Seal, Callorhinus ursinus, in the Eastern North Pacific and Eastern Bering Sea. Seattle: Natl Oceanic Atmospheric Admin; 1994. Tech Rep NMFS-SSRF-779. [Google Scholar]
- 11.Loughlin TR, Ingraham WJ, Jr, Baba N, Robson BW, Loughlin TR, Ohtani K. Loughlin TR, Ohtani K. Dynamics of the Bering Sea: A Summary of Physical, Chemical, and Biological Characteristics, and a Synopsis of Research on the Bering Sea. Vol AK-SG-99-03. Fairbanks, AK: Univ of Alaska Sea Grant; 1999. pp. 615–630. [Google Scholar]
- 12.Peterson R, LeBoeuf B, DeLong R. Nature. 1968;219:899–901. doi: 10.1038/219899a0. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd DS, McRoy CP, Day RH. Arctic. 1981;24:318–320. [Google Scholar]
- 14.Hobson K, Sease J, Merrick R, Piatt J. Mar Mammal Sci. 1997;13:114–132. [Google Scholar]
- 15.Burton R, Koch P. Oecologia. 1999;119:578–585. doi: 10.1007/s004420050822. [DOI] [PubMed] [Google Scholar]
- 16.Goericke R, Fry B. Global Biochem Cycles. 1994;8:85–90. [Google Scholar]
- 17.Rau G, Chavez F, Friederich G. Deep-Sea Res. 2001;48:79–94. [Google Scholar]
- 18.Burton RK, Snodgrass JJ, Gifford-Gonzalez D, Guilderson T, Brown T, Koch PL. Oecologia. 2001;128:107–115. doi: 10.1007/s004420100631. [DOI] [PubMed] [Google Scholar]
- 19.Perrin W, Wursig B, Thewissen J, editors. Encyclopedia of Marine Mammals. San Diego: Academic; 2002. p. 1414. [Google Scholar]
- 20.Schell D, Barnett B, Vinette K. Mar Ecol Prog Ser. 1998;162:11–23. [Google Scholar]
- 21.Altabet MA, Pilskaln C, Thunell R, Pride C, Sigman D, Chavez F, Francois R. Deep-Sea Res. 1999;46:655–679. [Google Scholar]
- 22.Newsome S, Etnier M, Aurioles-Gamboa D, Koch P. Mar Mammal Sci. 2006;22:556–572. [Google Scholar]
- 23.Hobson K, Alisauskas R, Clark R. Condor. 1993;95:388–394. [Google Scholar]
- 24.Wynen L, Goldsworthy SD, Insley SJ, Adams M, Bickham JW, Francis J, Gallo JP, Hoelzel AR, Majluf P, White RW, et al. Mol Phylogenet Evol. 2001;21:270–284. doi: 10.1006/mpev.2001.1012. [DOI] [PubMed] [Google Scholar]
- 25.Gentry R, Kooyman G, editors. Fur Seals: Maternal Strategies on Land and at Sea. Princeton: Princeton Univ Press; 1986. [Google Scholar]
- 26.Trillmich F, Dellinger T. In: Pinnipeds and El Niño. Trillmich F, et al., editors. Berlin: Springer-Verlag; 1991. pp. 247–270. [Google Scholar]
- 27.Melin S, DeLong R, Orr A. In: Fur Seal Investigations, 2000–2001. Robson B, editor. Seattle: Natl Oceanic Atmospheric Admin; 2002. pp. 51–58. Tech Memorandum NMFS-AFSC-134. [Google Scholar]
- 28.Kerley G. S Afr J Zool. 1983;18:388–392. [Google Scholar]
- 29.Costa D. Symp Zool Soc London. 1993;66:293–314. [Google Scholar]
- 30.Busch B. The War Against the Seals: A History of the North American Seal Fishery. Kingston, Ontario, Canada: McGill–Queens Univ Press; 1985. p. 374. [Google Scholar]
- 31.Kennett D, Kennett J. Am Antiq. 2000;65:379–395. [Google Scholar]
- 32.Colten R, Arnold J. Am Antiq. 1998;63:679–701. [Google Scholar]
- 33.York AE. In: Status, Biology, and Ecology of Fur Seals: Proceedings of an International Symposium and Workshop. Croxall JP, Gentry RL, editors. Seattle, WA: Natl Oceanic Atmospheric Admin; 1987. pp. 9–21. Tech Rep NMFS 51. [Google Scholar]
- 34.Stine S. Nature. 1994;369:546–549. [Google Scholar]
- 35.Barron J, Heusser L, Herbert T, Lyle M. Paleoceanography. 2003;18:1020. [Google Scholar]
- 36.Brown T, Nelson D, Vogel J, Southon J. Radiocarbon. 1988;30:171–177. [Google Scholar]
- 37.Stuiver M, Reimer P. Radiocarbon. 1993;35:215–230. [Google Scholar]
- 38.Stuiver M, Reimer P, Reimer R. CALIB 14C Calibration Program, Version 5.0.1. 2005 Available at http://calib.qub.ac.uk/calib/.
- 39.Bartholomew G, Hoel P. J Mammal. 1953;34:417–436. [Google Scholar]
- 40.Trites A. Mar Mammal Sci. 1992;8:44–56. [Google Scholar]
- 41.Schnute J. Can J Fish Aquat Sci. 1981;38:1128–1140. [Google Scholar]
- 42.von Bertalanffy L. Hum Biol. 1938;10:181–213. [Google Scholar]
- 43.von Bertalanffy L. In: Fundamental Aspects of Malignant and Normal Growth. Nowinski W, editor. Amsterdam: Elsevier; 1960. pp. 137–259. [Google Scholar]
- 44.Taylor J. An Introduction to Error Analysis: The Study of Uncertainties in Physical Measurements. Oxford, UK: Oxford Univ Press; 1997. [Google Scholar]
- 45.Weber D, Stewart B, Lehman N. J Hered. 2004;95:144–153. doi: 10.1093/jhered/esh018. [DOI] [PubMed] [Google Scholar]
- 46.Knecht R, Davis R. In: Archaeology in the Aleut Zone of Alaska. Dumond D, editor. Eugene, OR: Univ of Oregon Press; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.