Abstract
Classic hallucinogens such as lysergic acid diethylamide are thought to elicit their psychotropic actions via serotonin receptors of the 5-hydroxytryptamine 2A subtype (5-HT2AR). One likely site for these effects is the prefrontal cortex (PFC). Previous studies have shown that activation of 5-HT2ARs in this region results in a robust increase in spontaneous glutamatergic synaptic activity, and these results have led to the widely held idea that hallucinogens elicit their effect by modulating synaptic transmission within the PFC. Here, we combine cellular and molecular biological approaches, including single-cell 5-HT2ARs inactivation and 5-HT2AR rescue over a 5-HT2AR knockout genetic background, to distinguish between competing hypotheses accounting for these effects. The results from these experiments do not support the idea that 5-HT2ARs elicit the release of an excitatory retrograde messenger nor that they activate thalamocortical afferents, the two dominant hypotheses. Rather, they suggest that 5-HT2ARs facilitate intrinsic networks within the PFC. Consistent with this idea, we locate a discrete subpopulation of pyramidal cells that is strongly excited by 5-HT2AR activation.
Keywords: gene gun, in vitro electrophysiology, organotypic slices, serotonin, hallucinogen
The idea that classic hallucinogens such as lysergic acid diethylamide and psylocibin act by interfering with serotonergic neurotransmission can be traced to the middle of the 20th century (1). It was, however, not until the 1980s that serotonin receptors of the 5-hydroxytryptamine 2A subtype (5-HT2AR) were identified as the molecular target for these agents (refs. 2, 3; reviewed in refs. 4, 5). Subsequent brain imaging studies in human subjects have extended these findings to identify the prefrontal cortex (PFC), which is highly enriched in these receptors, as a key brain region in mediating the effects of hallucinogens (6, 7). These findings have led to the now widely accepted view that activation of 5-HT2AR in the prefrontal is a key biological step leading to the psychological effects of hallucinogens (5, 8).
Our understanding of the mechanisms by which 5-HT2AR activation elicits the sensory and behavioral manifestation of hallucinogens would be enriched by a precise understanding of how these receptors modulate cellular and network excitability in the PFC. To that effect, a number of studies have addressed the electrophysiological effects signaled by 5-HT2ARs in this region. There is general concordance that the most robust cellular effect observed in pyramidal cell of the PFC on stimulation of 5-HT2ARs involves an increase in both the frequency and amplitude of glutamatergic spontaneous excitatory postsynaptic potentials/spontaneous excitatory postsynaptic currents (sEPSCs) (9–14). This observation thus points to 5-HT2ARs as powerful modulators of the excitability of PFC networks and reconciles evidence implicating both glutamatergic and serotonergic systems in the actions of hallucinogens (15).
Although multiple mechanistic interpretations have been proposed to account for the effect of 5-HT2AR activation on glutamatergic synaptic activity in the PFC, the now dominant view holds that 5-HT2ARs, which are overwhelmingly located postsynaptically on pyramidal neurons in this region, trigger the release of glutamate from thalamocortical fibers by mean of a yet-unidentified retrograde messenger (11, 14, 16) (but see ref. 17). From a conceptual standpoint, this idea, which has come to dominate the field (e.g., refs. 5, 14, 15, 18), forms a very attractive hypothesis that integrates results from a cellular level into the broader context of the thalamocortical gating hypothesis of psychotomimetic hallucinogens and schizophrenia.
Despite its conceptual attractiveness, this hypothesis has not been rigorously tested, partly because of the unknown identity of the postulated retrograde messenger. Here, we use various molecular and cellular strategies to directly test different aspects of this hypothesis. Our results are inconsistent with this view and instead indicate that 5-HT2ARs lead to an increase in glutamatergic recurrent network activity in the PFC.
Results
To study the cellular basis of the increase in synaptic activity induced by activation of 5-HT2ARs, we first sought to selectively activate 5-HT2ARs in our recording conditions because other 5-HT subtypes can also increase spontaneous synaptic activity in this region. Administration of the selective 5-HT2 agonist αm-5-HT (10 μM) elicited a robust increase in both the amplitude and the frequency of spontaneous synaptic activity recorded from PFC layer V pyramidal neurons (postnatal days 15–30; n = 18) (Fig. 1). This effect was blocked by the selective 5-HT2AR antagonist MDL 100907 (300 nM) (Fig. 1 B1 and B2) but not by the selective 5-HT2CR antagonist SB 242084 (100 nM; n = 4; P = 0.19, paired Student's t test; data not shown). Consistent with previous reports, this increase in synaptic activity was blocked by the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (30 μM; n = 3; data not shown) and by tetrodotoxin (1 μM; n > 10 cells; data not shown). In prefrontal cortical slices, administration of tetrodotoxin reduces the frequency of synaptic events indicating that a subset of neurons is spontaneously active (12). Together, these results recapitulate previous findings showing that activation of 5-HT2ARs in PFC induces an increase in glutamate-mediated sEPSCs recorded from layer V pyramidal neurons.
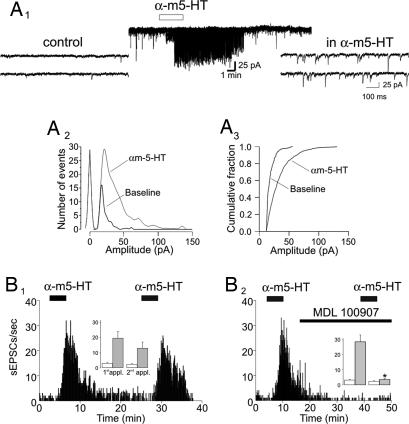
Fig. 1.
5-HT2ARs increase synaptic activity in the PFC. (A1) Administration of αm-5-HT (10 μM) results in a large increase in both the frequency and amplitude of sEPSCs recorded from a layer V pyramidal neuron. (A2) Histogram depicting the effect of αm-5-HT on the distribution of sEPSC amplitudes for the experiment illustrated in A1. The leftmost distribution centered at 0 pA depicts the noise distribution. (A3) Cumulative distribution of sEPSC amplitudes recorded before and after the administration of αm-5-HT for the same experiment. (B1) In a different cell, two consecutive administrations of αm-5-HT (10 μM) result in reliable increases in sEPSC activity. (Inset) Plot summarizing the effect of consecutive applications of αm-5-HT on sEPSCs (n = 6). White bars, baseline; gray bars, αm-5-HT. (B2) Administration of MDL 100907 (300 nM) blocks the ability of αm-5-HT to elicit an increase in sEPSC activity. (Inset) Plot summarizing the effect of MDL 100907 (n = 7). (∗, P < 0.01, paired Student's t test). White bars, baseline; gray bars, αm-5-HT.
Given that cortical pyramidal neurons are extensively interconnected, the simplest explanation for these observations would be that activation of 5-HT2ARs excites a subset of pyramidal neurons (presumably of layer V because they are highly enriched in 5-HT2ARs) whose activity is then detected in the recorded neuron as an increase in sEPSCs. This interpretation, however, has been generally rejected because 5-HT2ARs have not been found to excite (i.e., induce action potential firing) pyramidal neurons of PFC (4, 9, 11). This is contrary to the situation that prevails for muscarinic receptor activation, which, in addition to inducing a robust increase in sEPSCs in PFC, readily depolarizes and excites layer V pyramidal neurons (19). We thus next sought to address several alternative mechanisms that could account for this 5-HT2AR-mediated increase in sEPSCs.
Activation of 5-HT2AR Does Not Change Glutamate Release Probabilities or the Number or Function of Synaptic α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid Receptors.
First, a direct action of 5-HT2ARs on synaptic terminals could increase the probability of release of glutamate and thus contribute to the increase in frequency of sEPSCs. However, administration of αm-5-HT did not increase the frequency of mEPSCs (n = 13) [see supporting information (SI) Fig. 6], a measure of presynaptic release probabilities. This finding is consistent with the observation that 5-HT2ARs are located predominantly on postsynaptic dendritic structures and are generally undetectable at glutamate releasing terminals (18). Second, the amplitude of mEPSCs was unchanged by administration of αm-5-HT (SI Fig. 6). These findings, in conjunction with previous reports using similar, although not identical, conditions and approaches (9, 11, 12) indicate that neither an increase in the probability of release nor an increase in the number or function of synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors is likely to contribute significantly to the effect of αm-5-HT on sEPSCs.
Single-Cell Inactivation of 5-HT2AR-Mediated Signaling Does Not Block the 5-HT2AR-Mediated Increase in Spontaneous Synaptic Activity.
We next sought to determine whether activation of postsynaptic 5-HT2ARs elicited an increase in sEPSCs by inducing the release of a retrograde messenger that, in turn, would induce glutamate release from excitatory terminals, as was previously proposed (11, 14, 16). To this end, we envisioned a set of cellular and molecular strategies to be implemented by biolistic transfection of neurons (particle-mediated gene transfer) in organotypic slices of PFC. Organotypic slices from cortex maintain their general synaptic architecture for several days in culture (>2 weeks) and biolistically transfected neurons can routinely be recorded from such slices (20, 21). Recordings from layer V pyramidal neurons showed that administration of αm-5-HT to slices maintained in culture for up to 5–7 days induced an increase in the frequency and amplitude of sEPSCS (n > 40) (see SI Fig. 7) that was blocked by MDL 100907 (300 nM; n = 3; data not shown). Although the magnitude of this effect was generally smaller than that observed in acute slices, these observations indicate that this preparation can be used to address the mechanism underlying the 5-HT2AR-mediated increase in sEPSCs.
One prediction of the retrograde message hypothesis is that inactivation of postsynaptic 5-HT2ARs in a particular neuron should selectively suppress the increase in sEPSC activity onto that cell but not onto adjacent control neurons. To achieve single-cell suppression of 5-HT2AR signaling, we transfected pyramidal cells with a construct coding the C-terminal portion of phospholipase C β1 (PLCβ1) fused to GFP (PLCβ-ct). This construct binds to Gαq/11 and has been shown to act as a dominant negative capable of suppressing signaling by Gαq/11 (22). Paired recordings showed that αm-5-HT induced a small inward current in control nontransfected pyramidal neurons but not in neighboring neurons transfected with PLCβ-ct (Fig. 2C Upper). In addition, the ability of carbachol to induce an inward current (19) was also prevented in cells transfected with PLCβ-ct (Fig. 2D Upper). This is consistent with the view that carbachol acts through receptors coupled to Gαq-11 and PLCβ1 to induce an inward current (H.-D. Yan and R.A., unpublished work). Thus, these results indicate that Gαq/11 signaling in general, and 5-HT2AR signaling in particular, were effectively blocked in cells transfected with PLCβ-ct dominant negative.
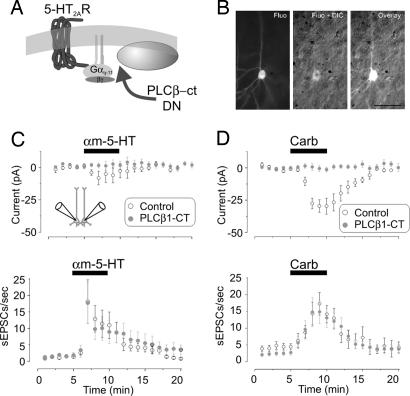
Fig. 2.
Single-cell inactivation of 5-HT2ARs by PLCβ-ct blocked the 5-HT2AR-induced inward current but not the increase in sEPSCs. (A) Diagram illustrating the site of action for the PLCβ-ct dominant negative. (B) Differential interference contrast (DIC)/fluorescence (Fluo) image of a neuron transfected with the PLCβ-ct dominant negative in an organotypic cortical slice (postnatal day 12; 3 days in vitro). (Scale bar: 50 μm.) (C) Paired recordings from neighboring neurons (Inset; n = 14) showing that the inward current elicited by αm-5-HT in control neurons was blocked in neurons expressing the PLCβ-ct construct (Upper; P < 0.05, paired Student's t test) but not the increase in sEPSCs (Lower; P = 0.59, paired Student's t test; n = 14 pairs). (D) Administration of carbachol (Carb) induced a slow inward current in control, nontransfected neurons (−29.9 ± 6.1 pA; n = 11) but not in neurons transfected with the PLCβ-ct construct (−0.4 ± 2.4 pA; n = 20; P < 0.01, unpaired Student's t test) (Upper). The ability of carbachol to induce an increase in sEPSCs was indistinguishable between control and transfected neurons (Lower; P = 0.41, unpaired Student's t test).
If postsynaptic 5-HT2ARs induce the release of a retrograde messenger that then induces glutamate release from presynaptic terminals, inhibition of postsynaptic 5-HT2AR signaling can be expected to inhibit the ability of these receptors to increase sEPSC activity. However, we found that the ability of αm-5-HT to increase the frequency of sEPSCs was indistinguishable between controls and neurons transfected with PLCβ-ct (Fig. 2C Lower). Likewise, the ability of carbachol to increase frequency of sEPSCs was unaltered by PLCβ-ct (Fig. 2D Lower). These results are inconsistent with the idea that postsynaptic 5-HT2ARs signal the release of a retrograde messenger capable of inducing glutamate release.
Single-Cell Rescue of 5-HT2AR Signaling Rescues the Ability of αm-5-HT to Signal an Inward Current but Not to Increase Synaptic Activity.
In the previous experiments, the pyramidal neurons whose 5-HT2ARs were inactivated by transfection with PLCβ-ct were surrounded by neurons presumably expressing control levels of functional 5-HT2ARs. Activation of 5-HT2ARs in these untransfected neurons could, in principle, have released a retrograde messenger capable of diffusing away from the site of release to act on excitatory terminals releasing glutamate onto the transfected neuron. To control for this possibility, we sought to carry out essentially the reverse experiments, i.e., to record from a neuron that expresses 5-HT2ARs but that is surrounded by neurons devoid of 5-HT2ARs. To this end, we used biolistic transfection procedures to rescue expression of 5-HT2ARs in prefrontal cortical neurons derived from 5-HT2AR knockout (5-HT2AR−/−) mice (23). We reasoned that if 5-HT2ARs signaled the increase in sEPSCs by the release of a retrograde messenger, then we should be able to selectively rescue the ability of αm-5-HT to increase sEPSCs only in cells whose expression of 5-HT2ARs has been rescued.
We first characterized the effect of αm-5-HT on acute slices derived from wild-type and 5-HT2AR−/− mice. In wild-type mice, αm-5-HT increases sEPSCs frequency and this effect was blocked by the selective 5-HT2A antagonist MDL 100907 (300 nM; n = 4) (Fig. 3A1 and A2). The ability of αm-5-HT to increase sEPSCs was greatly, but surprisingly not completely, suppressed in 5-HT2AR−/− mice (Fig. 3 A1 and A2). Indeed, in 9 of 39 cells tested from 5-HT2AR−/− mice, a generally small, but clearly distinguishable, increase in the frequency of sEPSCs could be detected after administration of αm-5-HT. This residual response to αm-5-HT was blocked by the selective 5-HT2C receptor antagonist SB 242084 (100 nM; n = 4; P < 0.05, paired Student's t test; data not shown). Because the effect of αm-5-HT appeared to be entirely mediated by 5-HT2ARs in wild-type mice, we interpreted this finding as reflecting a possible small adaptive up-regulation of 5-HT2C receptors in the 5-HT2AR−/− mice. However, because this residual increase in sEPSC activity was very small, these findings validate the use of this knockout mouse as a suitable physiological null background.
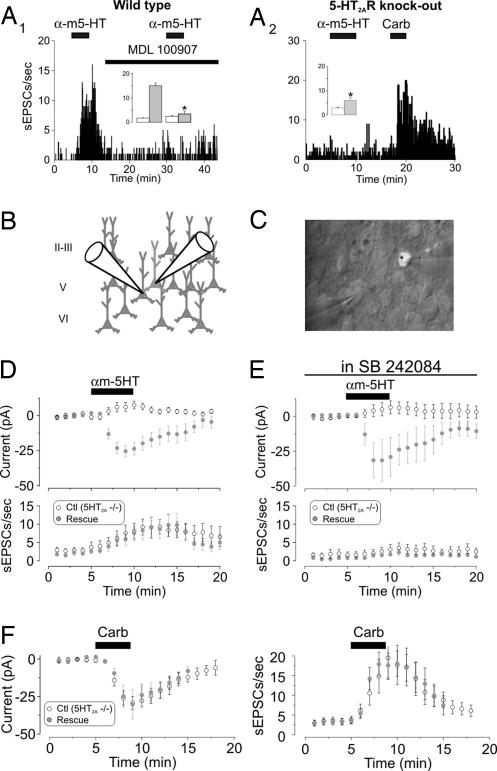
Fig. 3.
5-HT2AR expression in cells derived from 5-HT2AR knockout (KO) mice rescues the ability of αm-5-HT to induce an inward current but not to increase sEPSC activity. (A1) Administration of αm-5-HT (10 μM) increases sEPSC activity in wild-type mice, and this effect was blocked by MDL 100907 (300 nM). (Inset) Plot summarizing the effect of this experiment in four neurons (∗, P < 0.01, paired Student's t test). White bars, baseline; gray bars, αm-5-HT. (A2) The ability of αm-5-HT to increase sEPSC activity is blocked in this recording derived from a 5-HT2AR KO mouse. In this same cell, carbachol (Carb) (30 μM) elicited a robust increase in sEPSC activity. On average, the ability of αm-5-HT to increase sEPSCs activity was largely, although not completely, blocked in the KO (Inset) (n = 39; residual increase in sEPCS by αm-5-HT; ∗, P < 0.01 compared with baseline, paired Student's t test). White bars, baseline; gray bars, αm-5-HT. (B) Diagram illustrating the approach used for this experiment. 5-HT2ARs were expressed in cultured cortical brain slices derived from 5-HT2AR KO mice to rescue 5-HT2AR expression in a small subset of neurons. (C) Differential interference contrast/fluorescence image illustrating a paired recording from a control neuron and one transfected with 5-HT2ARs and GFP. (D) Results from paired recordings showing that expression of 5-HT2ARs rescued the ability of αm-5-HT to induce an inward current (Upper; P < 0.01, paired Student's t test; n = 16 pairs) but had no effect on the ability of this agonist to increase sEPSC activity (Lower). (E) In slices bathed in the 5-HT2C receptor antagonist SB 242084 (100 nM; >20 min), paired recordings from control and transfected neurons showed that expression of 5-HT2ARs rescued the ability of αm-5-HT to induce an inward current (Upper; P < 0.01, paired Student's t test; n = 10 pairs) but had no effect on the ability of this agonist to increase sEPSC activity (Lower). (F) The ability of carbachol to induce an inward current (Left) and an increase in sEPSCs (Right) was indistinguishable between controls (n = 16) and neurons transfected with 5-HT2ARs (n = 15) in slices derived from 5-HT2AR KO mice.
A key requirement for this experiment is that 5-HT2AR function in pyramidal neurons be effectively “rescued” by the transfection of 5-HT2ARs. We thus monitored the ability of 5-HT2AR activation to elicit an inward current in pyramidal neurons. Paired recordings in slices derived from 5-HT2AR−/− mice showed that αm-5-HT did not induce an inward current in control (nontransfected) cells but elicited a clear inward current in neighboring neurons transfected with 5-HT2ARs (Fig. 3D Upper). Thus, 5-HT2AR-mediated signaling was effectively rescued in 5-HT2AR−/− cells transfected with 5-HT2ARs.
If activation of 5-HT2AR were to result in the generation of a retrograde messenger capable of inducing the release of glutamate, the ability of αm-5-HT to increase the frequency of sEPSCs should be rescued in 5-HT2AR−/− cells transfected with 5-HT2ARs. We found that αm-5-HT elicited a small increase in sEPSC frequency in control untransfected cells from 5-HT2AR−/− mice and this small increase was of similar magnitude in neighboring neurons transfected with 5-HT2ARs (Fig. 3D Lower; n = 16 pairs).
Admittedly, the interpretation of this experiment is complicated by the small increase in sEPSCs induced by αm-5-HT in slices derived from 5-HT2AR−/− mice. We had earlier attributed this residual response to a possible up-regulation of 5-HT2C receptor function in 5-HT2AR−/− mice. To resolve this remaining ambiguity, we repeated the rescue experiment in the presence of the 5-HT2C receptor antagonist SB 242084 (100 nM) (Fig. 3E). Consistent with our observation in acute slices, in the presence SB 242084, αm-5-HT did not increase the frequency of sEPSCs in control (untransfected) neurons in cultured slices from 5-HT2AR−/− mice (Fig. 3E Lower; n = 10 pairs). Importantly, αm-5-HT also did not increase the frequency of sEPSCs in neighboring cells rescued with 5-HT2ARs in these conditions (Fig. 3E Lower; n = 10 pairs). Again, 5-HT2ARs were functional in the transfected neurons in these experiments because αm-5-HT induced a robust inward current only in transfected neurons (Fig. 3E Upper). Finally, this general failure to observe changes in the frequency of sEPSCs was likely not the result of nonspecific changes brought by the transfection procedure or from 5-HT2AR gene deletion per se, because the ability of carbachol to induce an inward current and to increase the frequency of sEPSCs was indistinguishable between control cells and cells transfected with 5-HT2ARs (Fig. 3F). Together, these results indicate that expression of 5-HT2ARs in pyramidal cells from 5-HT2AR−/− mice effectively rescued the ability of 5-HT2ARs to signal an inward current, but not the ability to increase the frequency of sEPSCs. As such, they failed to support the retrograde message hypothesis.
GTPγS Selectively Renders Irreversible the 5-HT2AR-Induced Inward Current but Not the Increase in sEPSCs.
To control for a potential confound resulting from the culturing procedure, we sought an alternative strategy to test for the presence of a retrograde messenger induced by activation of 5-HT2ARs in acute slices. We reasoned that a manipulation that would alter G protein-mediated signaling in the recorded cell should equally disrupt the ability of 5-HT2ARs to induce an inward current and the release of a retrograde messenger. To test this prediction, we used the poorly hydrolysable GTP analog GTPγS, a compound that limits the rate of GTP hydrolysis by Gα subunits and thus renders G protein-mediated responses irreversible. A previous study has successfully used this strategy to disclose a G protein-mediated release of a cannabinoid retrograde messenger (24).
Consistent with a previous report (25), paired recordings from layer V pyramidal neurons of acute slices showed that αm-5-HT induced a small inward current that fully recovered on agonist removal in control cells, while inducing an irreversible inward current in neighboring cells loaded with GTPγS (Fig. 4A Lower; n = 13 pairs). Administration of αm-5-HT induced comparable increases in sEPSCs in control and GTPγS-loaded neurons. However, contrary to what was observed for the inward current, the increases in sEPSC for both conditions recovered on agonist removal with identical time courses (Fig. 4A Upper). Similar results were obtained in slices derived from adult animals by using 5-HT as the agonist, with the exception that the inward current induced by 5-HT was larger than that induced by αm-5-HT (Fig. 4B). These findings in acute slices are again inconsistent with the idea that activation of 5-HT2ARs in the PFC lead to an increase in sEPSCs by inducing the release of a retrograde messenger.
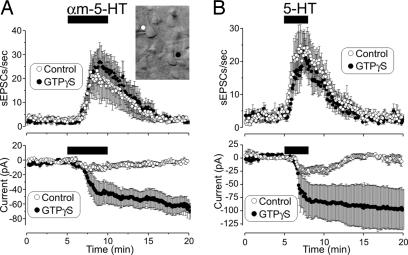
Fig. 4.
Effects of intracellular GTPγS on the 5-HT2AR-induced inward current and increase in sEPSCs. (A) Cell pairs were patched with electrodes filled with intracellular solution containing either GTP or GTPγS. Intracellular perfusion with GTPγS (>10 min) rendered the inward current induced by αm-5-HT (10 μM) effectively irreversible (Upper) but had no detectable effect on the increase in sEPSCs (n = 13 pairs) (Lower). (Inset) Image illustrating the recording configuration used for this experiment. (B) Essentially identical results were observed in cortical slices derived from adult (postnatal day > 33) rats by using 5-HT as an agonist (10 μM, n = 7 control and n = 6 GTPγS-loaded neurons).
A Subpopulation of Neurons Within the PFC Is Depolarized and Excited by 5-HT2AR Activation and Represents the Likely Source of the sEPSCs Induced by Activation of These Receptors.
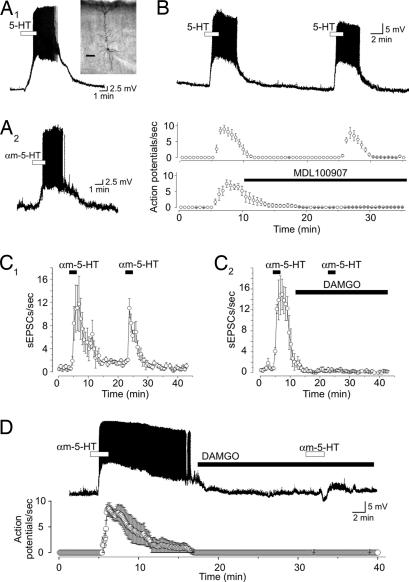
One of the key finding leading to the retrograde messenger hypothesis has been the failure to locate, within PFC, neurons capable of being robustly depolarized to fire action potential by the activation of 5-HT2ARs (4, 9, 11). The very small inward current induced by αm-5-HT in our recordings, both in acute and cultured slices, is consistent with these observations. However, we noticed in current-clamp recordings in acute rat PFC slices that a subpopulation of large neurons in the deep layers was highly sensitive to the administration of 5-HT and responded with strong membrane depolarizations capable of initiating spiking activity (Fig. 5). Focusing on the largest cells in the deep layer of the PFC, we found that approximately one-third of our recorded cells were excited by 5-HT (10–30 μM; 84 of 260 cells sampled). Biocytin labeling in some of these recordings identified post hoc these cells as pyramidal (i.e., glutamate releasing; n > 20) (Fig. 5A1 Inset) neurons.
Fig. 5.
A subpopulation of pyramidal neurons in acute rat PFC slices is excited by activation of 5-HT2ARs. (A1) Administration of 5-HT (30 μM) depolarized and excited this pyramidal neuron in PFC. (Inset) Image of this cell after filling with biocytin. (Scale bar: 150 μm.) (A2) Administration of αm-5-HT (10 μM) similarly depolarized and excited another pyramidal neuron. (B) Consecutive applications of 5-HT (30 μM) elicited comparable (i.e., nondesensitizing) excitation of this subpopulation of neurons. The excitation induced by 5-HT was blocked by MDL 100907. Upper, n = 6; Lower, n = 7. (C) DAMGO suppresses the 5-HT2AR-induced increase in spontaneous activity. (C1) Consecutive administration of αm-5-HT (30 μM) elicited comparable (i.e., nondesensitizing) increase in frequency of sEPSCs (n = 6). (C2) The increase in sEPSCs induced by αm-5-HT is blocked by administration of DAMGO (10 μM; n = 6; P < 0.01, paired Student's t test). (D) The ability of αm-5-HT (30 μM) to excite pyramidal neurons is blocked by administration of DAMGO (10 μM; n = 5; P < 0.01, paired Student's t test).
This 5-HT-induced depolarization/excitation was blocked by MDL 100907 (100 nM to 1 μM; n = 5 cells) (Fig. 5B) but not by GR113808 (1 μM) and SB 269970 (1 μM), blockers of 5-HT4 and 5-HT7 receptors, respectively, two receptor subtypes previously shown capable of depolarizing pyramidal cells (n = 4 cells; data not shown). Administration of αm-5-HT (n = 10) was also found to be capable of inducing a strong membrane depolarization leading to action potential spiking (Fig. 5A2), whereas administration of the hallucinogen 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane (10 μM), a selective 5-HT2 partial agonist, also resulted in a strong membrane depolarization (mean depolarization, 6.4 ± 0.9 mV; data not shown). Thus, activation of 5-HT2ARs in cortex both depolarizes and excites a subpopulation of deep neurons and increases the frequency of sEPSCs in layer V pyramidal neurons.
It has been shown previously that activation of μ-opioid receptors can abolish the ability of 5-HT to increase sEPSC activity (26). If the 5-HT2AR-induced action potential firing of these deep, large pyramidal neurons is the substrate of the 5-HT2AR-induced increase in sEPSCs in canonical layer V pyramidal neurons, both these responses should be abolished by μ-opioid receptor activation. We first replicated the initial finding and found that the selective μ-opioid receptor agonist [d-Ala2,N-MePhe4,Gly5-ol]enkephalin (DAMGO) (10 μM) completely suppressed the ability of αm-5-HT to increase sEPSCs activity from layer V pyramidal neurons (Fig. 5 C1 and C2). We then carried out current-clamp recordings from the deep, large cells identified above and found that administration of DAMGO completely suppressed the ability of αm-5-HT to depolarize and excite these neurons (Fig. 5D). Consistent with these findings, αm-5-HT induced a robust inward current in these cells, which was blocked by DAMGO, and this effect of DAMGO was blocked by the opioid antagonist naloxone (1 μM; n = 6; data not shown). Together, these results support the idea that the increase in sEPSC activity observed in PFC in response to 5-HT2AR activation results from the excitation of a subpopulation of pyramidal cells in the deep layers of PFC and not through activation of thalamocortical axons by a retrograde messenger.
Discussion
Studies on pyramidal neurons of the PFC have consistently shown that one of the most robust effects signaled by 5-HT2ARs is a marked increase in spontaneous glutamate-mediated synaptic activity. Although the mechanism underlying this increase is generally believed to involve the release of a retrograde messenger capable of exciting presynaptic thalamocortical terminals impinging on 5-HT2AR expressing pyramidal neurons, this idea has not been rigorously tested. Here, we report on a number of experiments whose results are inconsistent with the involvement of a retrograde messenger. Rather, our results suggest that 5-HT2AR activation leads to an increase in glutamatergic recurrent network activity by directly exciting a subpopulation of pyramidal cells located in the deep layers of the PFC.
Our strategy for testing the retrograde message hypothesis was to design experiments aimed at detecting the involvement of a retrograde messenger independent of any assumptions about its identity. In the first of these, we used a Gαq-11 dominant negative to block 5-HT2AR signaling in a limited number of cells within the cortical network. We reasoned that, if 5-HT2AR signaled the release of a retrograde message capable of activating glutamate releasing terminals impinging on the cell, then the dominant negative should reduce or block the response. Although 5-HT2AR signaling was effectively blocked in the cells transfected with the dominant negative, the 5-HT2AR-induced increase in sEPSCs was comparable to that seen in control cells. Because in this experiment control pyramidal neurons surrounding the transfected cell could have released a retrograde message (27), we also carried out essentially the reverse experiments and rescued 5-HT2ARs in a small number of neurons in slices derived from 5-HT2AR knockout mice. In this experiment, transfection with 5-HT2AR effectively rescued the ability of αm-5-HT to signal an inward current, but failed to rescue its ability to increase EPSC activity. Both of these results are inconsistent with the retrograde message hypothesis.
By necessity, the molecular intervention strategies used for the above experiments were implemented on organotypic slices held in cultures for several days. In this preparation, only synaptic networks from within the cortical slice are likely to be preserved, whereas axons originating from neurons outside the plane of cut can be expected to have degenerated. Thus, it remains possible that the culturing procedure resulted in functional changes impacting the ability of 5-HT2AR activation to trigger the release of a retrograde messenger. To test this possibility, we took advantage of the ability of GTPγS to allow for the irreversible activation of G protein signaling cascades. Intracellular injection of GTPγS, however, had no effect on the ability of αm-5-HT to elicit an increase in sEPSCs while rendering the inward current elicited by 5-HT2AR activation effectively irreversible. As such, these results again failed to support the idea that postsynaptic 5-HT2ARs in pyramidal cell of the rat PFC regulate sEPSCs by releasing a retrograde message.
Historically, acceptance of the retrograde messenger hypothesis emerged from the failure of simpler mechanisms to explain the increase the ability of 5-HT2ARs to increase sEPSC activity. Specifically, 5-HT2AR activation did not appear to excite pyramidal neurons (4, 9, 11, 25) but involved an increase in the release of glutamate by afferent presynaptic terminals (9, 11, 12, 14). As such, the retrograde messenger hypothesis did seem to offer the only possible explanation for the phenomenon. We have now identified a discrete subpopulation of neurons in the PFC that is excited by 5-HT2AR activations and that can, in principle, represent the cellular elements responsible for the increase in sEPSCs. Although it is difficult to test experimentally this conjecture, previous studies have reported that μ-opioid agonist suppress the ability of 5-HT2ARs to increase sEPSC activity. Because these receptors are expressed at very low levels in cortex, it has also been suggested this effect could help identify the presynaptic cellular elements mediating the 5-HT2AR induce increase in glutamate release. We found that activation of μ-opioid receptors completely blocked the ability of 5-HT2ARs to excite this subpopulation of pyramidal cells. These physiological results are consistent with the presence of scattered μ-opioid receptor expressing cells in the frontal cortex, especially in the deep layers (28, 29). As such, these results support the view that 5-HT2ARs in PFC enhance the overall excitability of PFC network by regulating the properties of a key subpopulation of pyramidal neurons.
Although 5-HT2ARs are expressed robustly in the PFC, they are also expressed in other regions of the brain (30) where they mediate membrane depolarization and neuronal excitation (31). However, selective rescue of 5-HT2AR expression in cortex is sufficient to rescue 5-HT2AR-induced head shaking, a behavioral proxy for hallucinogenic activity (32) and as such provide experimental support to the idea that cortical 5-HT2ARs mediate the psychotropic effects of hallucinogens. Interestingly, activation of μ-opioid receptors suppresses the 5-HT2AR-induced excitation of the subpopulation of neurons identified in the current study, the increase in sEPSCs recorded from canonical layer V neurons, and the 1-[2,5-dimethoxy-4-iodophenyl]-2-aminopropane-induced head shaking behavior (33). This congruence across different levels of biological organization supports the idea that the 5-HT2AR-mediated increase in glutamate synaptic activity analyzed in the current work bears a causal relationship to the mechanism of action of hallucinogens.
The results outlined in the current work lead to a reconceptualization of the mechanism of action of hallucinogens away from the idea that they facilitate thalamocortical excitatory synaptic transmission and toward the idea that they directly modulate recurrent intrinsic networks, perhaps regulating the gain of recurrent circuits in the PFC. A corollary implication of this view hold that excessive stimulation of 5-HT2ARs, such as during hallucinogen use, might destabilize PFC recurrent circuits and thus give rise to the sensory manifestation of hallucinogens. The present results thus suggest that the “breakdown” of cortical function brought by hallucinogens does not result from an excessive stimulation of thalamocortical innervation but rather from altered function of PFC intrinsic circuitry. Future studies will be required to further dissect the behavioral consequences predicted by this model.
Methods
Acute slices containing PFC were prepared following standard procedures, and cultured slices were prepared as previously described. Whole-cell voltage- and current-clamp recordings were obtained from layer V pyramidal neurons following standard procedures. These procedures are described in greater detail in SI Methods.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health Grant MH43985 (to R.A.) and by a grant from the Mental Illness Research Association (to J.-C.B.). J.-C.B. was in receipt of a postdoctoral fellowship from the Canadian Institutes for Health Research.
Abbreviations
- 5-HT2AR
5-hydroxytryptamine 2A receptor
- PFC
prefrontal cortex
- sEPSC
spontaneous excitatory postsynaptic current
- PLC
phospholipase C
- DAMGO
[d-Ala2,N-MePhe4,Gly5-ol]enkephalin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700436104/DC1.
References
- 1.Wooley DW, Shaw E. Proc Natl Acad Sci USA. 1954;40:228–231. doi: 10.1073/pnas.40.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glennon RA, Titeler M, McKenney JD. Life Sci. 1984;35:2505–2511. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- 3.Titeler M, Lyon RA, Glennon RA. Psychopharmacology (Berl) 1988;94:213–216. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- 4.Aghajanian GK, Marek GJ. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- 5.Nichols DE. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. NeuroReport. 1998;9:3897–3902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 7.Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Neuropsychopharmacology. 1997;16:357–372. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- 8.Vollenweider FX, Geyer MA. Brain Res Bull. 2001;56:495–507. doi: 10.1016/s0361-9230(01)00646-3. [DOI] [PubMed] [Google Scholar]
- 9.Aghajanian GK, Marek GJ. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 10.Marek GJ, Aghajanian GK. Eur J Pharmacol. 1999;367:197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- 11.Zhou FM, Hablitz JJ. J Neurophysiol. 1999;82:2989–2999. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]
- 12.Béïque JC, Chapin-Penick EM, Mladenovic L, Andrade R. J Physiol. 2004;556:739–754. doi: 10.1113/jphysiol.2003.051284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambe EK, Goldman-Rakic PS, Aghajanian GK. Cereb Cortex. 2000;10:974–980. doi: 10.1093/cercor/10.10.974. [DOI] [PubMed] [Google Scholar]
- 14.Lambe EK, Aghajanian GK. J Neurosci. 2001;21:9955–9963. doi: 10.1523/JNEUROSCI.21-24-09955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aghajanian GK, Marek GJ. Brain Res Brain Res Rev. 2000;31:302–312. doi: 10.1016/s0165-0173(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 16.Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. Neuroscience. 2001;105:379–392. doi: 10.1016/s0306-4522(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. J Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miner LA, Backstrom JR, Sanders-Bush E, Sesack SR. Neuroscience. 2003;116:107–117. doi: 10.1016/s0306-4522(02)00580-8. [DOI] [PubMed] [Google Scholar]
- 19.Haj-Dahmane S, Andrade R. J Neurosci. 1996;16:3848–3861. doi: 10.1523/JNEUROSCI.16-12-03848.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Béïque JC, Andrade R. J Physiol. 2003;546:859–867. doi: 10.1113/jphysiol.2002.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villalobos C, Shakkottai VG, Chandy KG, Michelhaugh SK, Andrade R. J Neurosci. 2004;24:3537–3542. doi: 10.1523/JNEUROSCI.0380-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kammermeier PJ, Ikeda SR. Neuron. 1999;22:819–829. doi: 10.1016/s0896-6273(00)80740-0. [DOI] [PubMed] [Google Scholar]
- 23.Weisstaub NV, Zhou M, Lira A, Lambe E, Gonzalez-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, et al. Science. 2006;313:536–540. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- 24.Haj-Dahmane S, Shen RY. J Neurosci. 2005;25:896–905. doi: 10.1523/JNEUROSCI.3258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Béïque JC, Campbell B, Perring P, Hamblin MW, Walker P, Mladenovic L, Andrade R. J Neurosci. 2004;24:4807–4817. doi: 10.1523/JNEUROSCI.5113-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marek GJ, Aghajanian GK. Neuroscience. 1998;86:485–497. doi: 10.1016/s0306-4522(98)00043-8. [DOI] [PubMed] [Google Scholar]
- 27.Wilson RI, Nicoll RA. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 28.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 29.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Gimenez JF, Mengod G, Palacios JM, Vilaro MT. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:446–454. doi: 10.1007/pl00005075. [DOI] [PubMed] [Google Scholar]
- 31.Barnes NM, Sharp T. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, et al. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Marek GJ. Eur J Pharmacol. 2003;474:77–83. doi: 10.1016/s0014-2999(03)01971-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.