Abstract
We investigated the therapeutic effects of two different versions of Aβ1–15 (16) liposome-based vaccines. Inoculation of APP-V717IxPS-1 (APPxPS-1) double-transgenic mice with tetra-palmitoylated amyloid 1–15 peptide (palmAβ1–15), or with amyloid 1–16 peptide (PEG-Aβ1–16) linked to a polyethyleneglycol spacer at each end, and embedded within a liposome membrane, elicited fast immune responses with identical binding epitopes. PalmAβ1–15 liposomal vaccine elicited an immune response that restored the memory defect of the mice, whereas that of PEG-Aβ1–16 had no such effect. Immunoglobulins that were generated were predominantly of the IgG class with palmAβ1–15, whereas those elicited by PEG-Aβ1–16 were primarily of the IgM class. The IgG subclasses of the antibodies generated by both vaccines were mostly IgG2b indicating noninflammatory Th2 isotype. CD and NMR revealed predominantly β-sheet conformation of palmAβ1–15 and random coil of PEG-Aβ1–16. We conclude that the association with liposomes induced a variation of the immunogenic structures and thereby different immunogenicities. This finding supports the hypothesis that Alzheimer's disease is a “conformational” disease, implying that antibodies against amyloid sequences in the β-sheet conformation are preferred as potential therapeutic agents.
Keywords: Alzheimer's disease, β-amyloid, vaccine
Clinical manifestations of Alzheimer's disease (AD) include progressive memory loss, cognitive impairment, confusion, and personality changes. The major neuropathological changes in the brains of AD patients are senile plaques and neurofibrillar tangles causing progressive neuronal dysfunction. These pathological alterations are likely causally involved in eventual neuronal death, particularly in brain regions related to memory and cognition (1–4). Senile plaques are formed predominantly by the β-amyloid peptide Aβ1–42 that is coiled and α-helical in its soluble form but, upon conformational transition, aggregates into β-sheeted multimers. The monomeric Aβ peptide is a physiological metabolite of the large amyloid precursor protein (APP, 695–770 aa) that undergoes processing by several sequential proteolytic steps (5). The Aβ1–42 aggregates are proposed to play the key role in the pathogenesis of AD (6). In transgenic animals that overexpress mutant human APP, anti-Aβ-specific antibodies decreased the Aβ burden and improved memory after either passive (7–11) or active (12–18) immunization.
We previously demonstrated that i.p. inoculation of tetrapalmitoylated Aβ1–16 reconstituted in liposomes to transgenic NORBA mice elicited significant titers of anti-Aβ antibodies, that solubilized amyloid fibers in vitro and pancreatic Aβ plaques in vivo (19). To circumvent T cell-mediated immune responses known to be causatively involved in the adverse events of meningoencephalitis of AD patients immunized with Aβ1–42 (20–22), the Aβ1–16 and 1–15 sequences were used for immunization of APPxPS1 double-transgenic mice (23) because strong T cell epitopes are located more toward the C-terminal region for mice (24) and humans (25). To gain a sufficient yield and purity of the chemical synthesis of the PEGylated Aβ, we added a lysine residue at the C terminus of the Aβ1–15.
The purpose of this study was to investigate (i) the safety and therapeutic effect of antibodies elicited by conformation-stabilized Aβ1–15 (16) reconstituted in liposomes as evaluated by memory recovery in F1 FVBxC57Bl6 APP-V717IxPS-1 (APPxPS-1) transgenic mice, and (ii) to define the relationship between therapeutic efficacy and conformation of the antigenic peptide in the liposomal antigen.
Results
Design of Liposomal Vaccines and Analysis of the Conformation of the Reconstituted Antigens.
To anchor the antigen Aβ 1–15 on the liposomal surface (ACI-24, Fig. 1A), we used a palmitoylated lysine tandem at each end of the peptide as described (19). Sixteen-carbon palmitic acid has the appropriate length for stable insertion into the liposomal bilayer (26). In this construct, the peptide is closely apposed to the surface of the liposome. In an attempt to prolong the immune response, we synthesized the peptide Aβ1–16 in which polyethylene glycol (PEG) spacers with 77 repetitive units were introduced between the peptide termini and liposomal anchors (ACI-01, Fig. 1A). It was envisaged that the PEG spacers might enhance liposome stability in vivo (27). The influence of the spacer between the liposomal anchor and the Aβ peptide on the secondary conformation of the amyloid sequence reconstituted in liposomes was equally measured by CD. PEGylated Aβ1–16 appears to be in a random coil or unstructured protein conformation (negative signal up to 210 nm, crossing the x axis and slowly reapproaching the zero axis up to 260 nm (Fig. 1C). On the other hand, palmitoylated peptide Aβ1–15 contains a significant proportion of β-sheet conformation (positive signal up to 210 nm, downward crossing zero axis and approaching it again in the region of 260 nm (Fig. 1C). The closer proximity of the palmitoylated peptide to the liposomal surface appears to impose a defined secondary conformation. This configuration is potentially due to interactions of the peptide with the surface of the liposome that apparently are not possible with the PEGylated peptide.
Fig. 1.
Design and biophysical characterization of the two liposomal vaccines containing peptide immunogens with the first 16 (ACI-01, Aβ1–16) and 15 (ACI-24, Aβ1–15) amino acids of the full length Aβ1–42 peptide. (A) ACI-01 contains Aβ1–16 flanked with one PEGylated lysine residue on each side that utilizes DSPE as liposomal anchor of the PEG chain. For ACI-24, two terminal palmitoylated lysine residues were covalently linked at each end of Aβ1–15 to reconstitute and anchor the antigen into the liposome. (B) Sequence of the peptides integrated into the liposomal vaccines ACI-01 and ACI-24. (C) CD spectra of ACI-01 at 10 and 20 μM peptide concentration (Upper) and ACI-24 at 20 μM (Lower).
NMR Spectroscopy.
To gain additional insight into the molecular structure of the polypeptide chains, MAS NMR experiments were performed (28–30). The spectral region of the peptide backbone and aromatic side chains were monitored for ACI-01 and ACI-24 when reconstituted into liposomes [supporting information (SI) Fig. 5]. For comparison, the MAS NMR spectra of the PEGylated and palmitoylated as well as the peptide chain alone in PBS buffer were measured. Whereas the NMR line-width observed for the peptide alone is indicative of the formation of small aggregates in aqueous solution (31), larger complexes are formed in the presence of the linker sequences (SI Fig. 5). Still-larger complexes are formed after reconstitution of the peptides into liposomes. Although the spectral lines are broadened after reconstitution and do not allow the assignment of individual resonances, the spectral differences in the 6- to 9-ppm region are indicative of conformational alterations when the linker that anchors the antigen in the bilayer is modified. The line-broadening observed for the ACI-01 and ACI-24 peptides carrying their respective linker is between that observed for the free peptide and when attached to the liposomes. These findings also indicate the formation of macromolecular aggregates, probably micelles, for the peptides of ACI-01 and ACI-24 in the absence of lipids. The spectral features of these “peptide micelles” exhibit many similarities to the ones obtained from the respective peptides in the presence of liposomes. This observation suggests that similar conformational determinants already exist in the peptidic micelles.
Anti-Aβ Antibody Response in APPxPS-1 Mice.
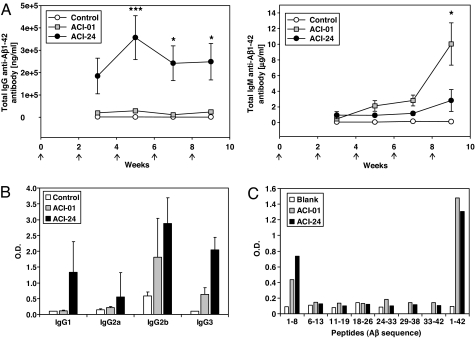
To evaluate the immune response to the liposomal antigens, double-transgenic APPxPS-1 mice received a total of five i.p. inoculations of 200 μl of either liposomal vaccine ACI-01 or ACI-24 administered at 2-wk intervals. Blood was collected after the second inoculation and then every second week to determine antisera titers. ACI-01 elicited primarily antibodies of the IgM class and at higher levels than those elicited by ACI-24. In contrast, the anti-Aβ1–42-specific IgG immune response to ACI-01 immunization was much lower compared with ACI-24 that increased rapidly and reached a peak at 5 wk (Fig. 2A).
Fig. 2.
ELISA analysis of anti-β-amyloid antibody response after immunization with PEGylated (ACI-01) or palmitoylated (ACI-24) liposomal antigens. (A) Analysis of amyloid-specific titers [IgG, (Left) and IgM (Right)] in the sera of APPxPS1 mice immunized (arrows) with PEGylated (ACI-01) or palmitoylated (ACI-24) liposomal antigens compared with mice immunized with empty liposomes (control). (B) IgG isotype analysis after 9-week treatment of ACI-01 or ACI-24. (C) Epitope-mapping of serum antibodies generated by ACI-01 or ACI-24 taken 1 week after the final boost. Results are shown as means ± SEM obtained in groups of 7–8 immunized mice.
The final blood samples from all of the animals were also analyzed for their IgG isotype. Immunization with ACI-24 resulted mainly in isotypes IgG1 and IgG2b, which are associated with noninflammatory Th2 response, and also in IgG3, which is a T cell-independent IgG subclass (32) (Fig. 2B). With the exception of one animal vaccinated with ACI-24, both vaccines induced only very low levels of IgG2a (Th1).
Epitope-mapping of the resulting antibodies was performed by ELISA using a peptide library comprising a total of 33 biotinylated peptides covering the complete amino acid sequence of Aβ1–42. A biotinylated complete Aβ1–42 peptide served as positive control. Immunization with both vaccines resulted in anti-Aβ antibodies with the same epitopes defined by amino acids 1–8 of Aβ (Fig. 2C). Introduction of the additional lysine residue at position 16 for synthetic reasons in the amyloid peptide in ACI-01 exerted no influence on the binding function of the elicited antibodies. Both vaccines showed significantly higher binding to the entire Aβ1–42 protein than to the peptide Aβ1–8. We analyzed the dependency on the conformation of the peptide by measuring specific binding of the resulting anti-Aβ antisera to polymeric Aβ by adapting the ELISA with Aβ1–42 fibers. Immunization with ACI-24 raised significantly higher titers of anti-Aβ antibodies recognizing Aβ1–42 fibers than antisera raised by immunization with ACI-01 (Fig. 3). Thus, immunization with ACI-01 and ACI-24 produce immune responses that differ not only in their titer, subclasses, and Ig-isotypes but also in their conformational specificity.
Fig. 3.
ELISA analysis of Aβ1–42 fiber-specific antibody titers in the sera of APPxPS1 mice immunized with PEGylated (ACI-01) or palmitoylated (ACI-24) antigens in liposomes compared with mice immunized with empty liposomes (control). Data are expressed in mean ± SEM and ∗, P < 0.05 and ∗∗ with P < 0.01 by ANOVA (Turkey–Kramer multiple comparison test obtained in groups of 7–8 immunized mice).
Immunization with ACI-24 but Not ACI-01 Restores Cognitive Memory and Reduces Brain Amyloid Load.
To analyze the effect of immunization on nonspatial, hippocampus-dependent cognitive memory in the APPxPS1 mouse model, a 3-month immunization was carried out consisting of six inoculations with ACI-01 or ACI-24, at 2-wk intervals. One group of mice received empty liposomes as control. The cognitive memory capacity of APPxPS-1 mice in the novel object recognition test was significantly increased by immunization with ACI-24 compared with APPxPS-1 mice treated with empty liposomes (Fig. 4A). Mice immunized with ACI-24 recognized and remembered the original object for at least 3 h, similar to healthy mice matched for age, gender, and genetic background. In contrast, immunization with ACI-01 did not result in any restoration of memory.
Fig. 4.
Effect of immunization of APPxPS-1 mice with ACI-01 and ACI-24 on memory capacity and brain amyloid load. (A) Analysis of cognition assessed by ORT of 6-month-old APPxPS-1 mice immunized with PEGylated (ACI-01) and palmitoylated (ACI-24) liposomal vaccines. Data are expressed in mean ± SEM in groups of 5–8 mice. (B) Analysis of individual correlation (nonlinear regression, order of three) of anti Aβ1–42-specific IgG antibody titer (Left) and of anti Aβ1–42-specific IgM antibody titer (Right) with ORT Index (ORT individual − ORT mean of control). (C) Analysis of soluble and insoluble Aβ1–40 and Aβ1–42 of brain homogenates of ACI-24 immunized APPxPS-1 mice compared with empty liposome-immunized control group by Aβ1–40- and Aβ1–42-specific ELISA. Data are percent means ± SD of the values of 7–8 mice. ∗, P < 0.05; ∗∗, P < 0.01 by ANOVA (Turkey–Kramer multiple-comparison test).
To decipher the potential contribution of the IgM and IgG antibodies to cognitive function, a correlation analysis was performed. IgM antibodies did not correlate with the memory capacity (r2 = 0.2333), but antibodies of IgG class correlated roughly (r2 = 0.857) and directly to the memory capacity (ORT index). Between an ORT Index of 0 to 20, an essentially linear relationship between antibody titer and memory improvement was observed (Fig. 4B). At an ORT index >20, the Ig antibody titer enters a saturation phase, and the ORT index is not further improved. This result could indicate that IgM antibodies, which cannot pass the blood–brain barrier, do not contribute to the restoration of memory. In contrast, depending on their subclass, IgG antibodies cross the blood–brain barrier and result in the improvement of memory (7).
The effect of immunization with ACI-24 on the quantity of soluble and insoluble amyloid peptides in the brain of the APPxPS-1 mice, human Aβ1–40 and Aβ1–42 was evaluated by ELISA. Immunization with ACI-24 led to a significant decrease of insoluble, plaque-related Aβ1–40 and Aβ1–42 (Fig. 4C). Soluble Aβ1–42 was significantly reduced, whereas soluble Aβ1–40 showed only a trend to decrease.
Immunization with ACI-01 and ACI-24 Does Not Cause Inflammation.
The safety of liposomal vaccines ACI-01 and ACI-24 was assessed by measuring the local production of the inflammatory cytokines IL-1β, IL-6, IFN-γ, and TNFα by ELISA. The amount of activated microglial cells (MHCII) and astrogliosis (GFAP) in the brain in the plaque-predominant subiculum region was assessed by quantitative immunohistochemistry (see SI Methods for additional details) (23). Immunization with either ACI-01 or ACI-24 did not significantly increase the levels of IL-1β, IL-6, IFN-γ, and TNFα in the brain (SI Table 1). Similarly, no differences in astrogliosis were observed upon immunization with ACI-24, whereas the extent of activated microglia showed a tendency to decrease after three month period of immunization (SI Table 1).
Discussion
Two liposome-based vaccines directed toward Aβ1–15 (16) amyloid peptides were synthesized, and immune response, reduction of amyloid, memory improvement, and inflammatory response were examined in APPxPS1 double-transgenic mice. The vaccines were identical in terms of liposomes, amount of antigen, the ratio of lipid to antigen, and the Aβ-binding epitope of the antibodies they elicited in mice. They differed, however, in the linker technology used and, consequently, the distance between the antigen and the lipid anchor in the liposome.
We investigated the effect of antigen conformation on the safety and efficacy of anti-β-amyloid liposome-based vaccines. The aim of the study was to enhance the effectiveness of vaccine therapy for AD. We developed a vaccine that preferentially generates antibodies against amyloid sequences in a β-sheet conformation. This construct (ACI-24) exhibited increased affinity for aggregated β-amyloid compared with ACI-01, an Ig isotype that enabled passage across the blood–brain barrier and a negligible inflammatory response.
These liposomal vaccines were highly immunogenic in APPxPS1 double-transgenic mice in terms of kinetics and antibody titers. After two intraperitoneal injections, and by 3 wk after the start of the immunization, significant levels of systemic anti-Aβ1–42 antibodies were observed. The epitope of the resulting immunoglobulins was identical for ACI-01 and ACI-24, indicating its independence of the 16th amino acid used to create ACI-01. By way of comparison, a significant titer of Aβ-specific antibodies by intranasal administration of Aβ1–15 tandem-peptides with and without covalently linked T helper epitope was elicited only after 6 wk and six intranasal applications (12). Peripheral administration of a Aβ1–42 peptide immunogen likewise took 11 months to reach a “therapeutic titer” (14), comparable with that achieved by ACI-24 after only 3 months.
Introduction of a spacer between the antigen peptide and the surface of the liposome appeared to have a major impact on the immune response. First, PEGylated ACI-01 elicited lower IgG antibody titers than palmitoylated ACI-24 and, second, the Aβ antigen is in a random coil conformation in ACI-01, whereas the antigen in ACI-24 is predominantly in β-sheet conformation. The resulting dominating IgG subclasses are the noninflammatory Th2 isotypes (IgG1 and IgG2b). This finding is in accordance with recently published results (33) indicating that vaccines that do not contain the strong T cell epitopes located in the C terminus (24, 25) can induce predominantly Th2-associated antibodies (IgG1, Ig2b). Immunization of the APPxPS-1 double-transgenic mice with ACI-24 led to complete restoration of cognitive, nonspatial memory as measured by ORT. ACI-24-immunized mice had a significantly improved memory over those vaccinated with ACI-01.
Because the only significant structural difference between the two vaccines that could account for their different function in vivo is the linker chemistry, the resulting difference in conformation of the antigenic peptide seems most likely to be the key for the specificity and efficacy of the resulting antibodies. Both CD and MAS solid-state NMR spectroscopy indicated that the secondary structures of the ACI-01 and ACI-24 antigens exhibit significant differences when reconstituted into liposomes. Whereas, in aqueous solution, the Aβ1–16 peptide, alone or in the context of the ACI-01 vaccine, exhibited predominantly random-coil conformation, a predominant β-sheet conformation was observed in the ACI-24 vaccine, probably because of closer proximity to the liposomal surface (34, 35). At present, we can only speculate on the reason for the β-sheet-like structure of Aβ1–15 on the liposome surface. Can electrostatic interactions or hydrogen bonding between the hydrophilic termini of the lipids and the peptide stabilize the structure? Alternatively, does the peptide form an intramolecular hairpin of two antiparallel β-strands with a β-turn stabilized at both ends by the fatty acid groups inserted into the membrane (36)? An alternative model might be that several Aβ-peptides form a two-dimensional array of parallel or antiparallel β-strands that would be stabilized by hydrogen bonds between the strands, contact with the liposome surface, and fixation of the termini by fatty acids inserted into the liposome. The antibodies formed against such structures would be expected to more readily interact with the β-sheet structures of the amyloid oligomers or fibrils found in the brain than with nonaggregated Aβ1–40/42 (37).
The memory restoration and higher specificity of antibodies for aggregated Aβ by ACI-24 points to an improved therapeutic effect of antibodies against amyloid sequences in β-sheet conformation that target Aβ in either oligomers or deposited plaques (38). This assumption is supported by our findings that antibodies resulting from the ACI-24 vaccination were effective in decreasing insoluble amyloid deposits as well as soluble Aβ1–42 in the brains of vaccinated mice.
An additional factor for the high biological activity of ACI-24 compared with ACI-01 appears to be related to the Ig classes of the anti-Aβ antibodies. Whereas ACI-24 elicited predominantly an IgG-based immune response, antibodies produced by immunization with ACI-01 were mainly of the IgM class. Although anti-Aβ IgM antibodies can contribute to the clearance of Aβ plaques in the brain, probably by the so-called “sink-effect” (39), we did not observe a significant improvement of memory impairment with the IgM anti-β-amyloid antibodies.
We did not detect any significant signs of inflammation, measured by the proinflammatory TNFα, IL-1β, IL-6, IFN-γ cytokines or by an increase of the MHCII marker for activated microglial cells or the GFAP marker for astrogliosis (40) (see SI Methods for details). The absence of induction of TNFα secretion in the brain of immunized mice is an additional important safety criterion. The lack of inflammation as indicated by the specific markers examined and specifically by the trend of a reduction in the number of activated microglial cells identifies the liposomal vaccine ACI-24 as a potential candidate for additional preclinical and possibly clinical investigations.
The findings reported here demonstrate that the liposomal antigen vaccines examined in this investigation elicited therapeutic antibodies against β-amyloid only when the antigenic peptide was in a predominantly β-sheet conformation. This deduction is substantiated by the restoration of the memory defect in the APPxPS1 mice to that of wild-type mice with this specific type of vaccination. The dependency of the therapeutic activity of the antibodies on antigen conformation provides support for the hypothesis that Alzheimer's disease is a “conformational” disease and that antibodies against amyloid sequences in β-sheet conformation will be preferred as therapeutic agents. The study also raises the question whether these observations apply specifically to the amyloid peptide vaccine or whether they can be applied to the development of vaccines against other proteins whose pathogenicity is linked to a particular conformation.
Methods
Preparation of Antigens.
Liposomes were prepared as described (19). For the synthesis of the palmitoylated amyloid 1–15 peptide in ACI-24, the orthogonally protected amino acid Fmoc-Lys(Mtt)-OH (Merck, Darmstadt, Germany) was coupled to an H2N-Lys(Mtt)-Wang resin. Selective deprotection of the Mtt groups was carried out by addition of 1% TFA in dichloromethane. Palmitic acid was then coupled to the two lysine residue side chains, followed by 15 rounds of standard solid-phase peptide synthesis. Fmoc-Lys(Mtt)-OH was incorporated as the last two amino acids and again the Mtt groups deprotected and palmitic acid was coupled by using PyBOP. Simultaneous side-chain deprotections and resin cleavage was carried out by using TFA/TIPS to give the required lipopeptide. The N- and C- terminal lipid-PEG β-amyloid peptide antigen of ACI-01 was synthesized by using a similar methodology to that described above except using a H-Gly-2-chlorotrityl resin and an orthogonally protected lysine residue Fmoc-Lys(ivDde)-OH flanking the peptide at both the N- and C-termini. After peptide synthesis was complete, the N terminus was blocked with an acetyl group, and then the two ivDde protecting groups were simultaneously deprotected by using 3% hydrazine in DMF. The peptide was then cleaved from the resin by using 30% trifluoroethanol in dichloromethane so as not to deprotect the other amino acid side chains and then coupled in solution with DSPE-PEG-SPA (PEG DP = 77;Nektar Therapeutics, Huntsville, AL) at 40°C for 18 h in DMSO. The reaction was quenched, lyophilized, side-chain deprotected by using TFA, and purified by rpHPLC. PEGylated Aβ1–16 (for ACI-01) and palmitoylated Aβ1–15 (for ACI-24) were reconstituted in liposomes consisting of dimyristoyl phosphatidyl choline (DMPC), dimyristoyl phosphatidyl glycerol (DMPG), and cholesterol (Avanti Polar Lipids, Alabaster, AL) at molar ratios 9:1:7. Monophosphoryl lipid A (Sigma-Aldrich, St Louis, MO) was added at 40 mg/mmol phospholipids.
CD Spectra.
ACI-01 and ACI-2 samples were diluted either 2- or 4-fold in PBS and then sonicated for 3 min at 170–260 W by using 0.5-s pulses immediately before analysis. Spectra were recorded on a Dichrograph (J-715; Jasco, Easton, MD) with a quartz cell cuvette of 0.1-cm optical path length. The spectral window was scanned between 195 and 260 nm at a speed of 50 nm/min at 25°C. Spectral data of control empty liposomes was analyzed at corresponding dilutions and subtracted from sample data. CDPro package was used to estimate the secondary structure of the samples. A protein database of 56 entries, of which 13 were membrane proteins, was used for deconvolution by CONTINLL software (41).
Blood Collection.
For the analyses of the antiamyloid titers, blood was collected at six different time points, 5 days after each inoculation. The blood (≈30 μl) was obtained from the tail vein, and ≈5 μl of heparin was immediately added. A final blood collection was performed by a heart puncture during anesthesia at the moment of killing. Blood was centrifuged at 4000 × g for 5 min at 4°C. The sera were separated from the blood cells and used immediately in an ELISA or frozen at −20°C.
Quantification of Antigen-Specific Antibodies.
Aβ 1–42-specific IgG and IgM antibodies were measured by ELISA. IgG subclasses were analyzed in serum samples 1 wk after the last boost by Aβ1–42-specific ELISA at eight different dilutions. Plates were coated with 10 μg/ml Aβ1–42 overnight at 4°C. After washing each well with PBS-0.05%/Tween 20 and blocking with 1% BSA, serial dilutions of sera were added to the plates and incubated at 37°C for 2 h. After washing, the plates were incubated with a phosphatase-conjugated anti-mouse Ig (IgG, whole antibody, Jackson ImmunoResearch, West Grove, PA) or isotype-specific antibodies (IgM, IgG1, IgG2a, and IgG3 (Pharmingen BD, San Diego, CA) and Ig2b (Zymed, San Francisco, CA) for 2 h at 37°C. After washing, plates were incubated with pNPP and read at 405 nm by using an ELISA plate reader. Results are expressed with reference to serial dilutions of a titrated pool of serum from immunized adult mice or from serial dilutions of a commercially available antibody (6E10; Chemicon International, Temecula, CA). Alternatively, results are expressed as O.D. at a dilution where no sera were at saturation level (1:100 for IgG1, 2a, and IgG3 and 1:400 for IgG2b).
Epitope Mapping.
Epitope mapping was performed by ELISA by using a peptide library consisting of 33 biotinylated peptides covering the complete amino acid sequence of Aβ 1–42 (produced by Mimotopes, Clayton, Victoria, Australia and purchased from ANAWA Trading, Wangen, Switzerland). A biotinylated complete Aβ1–42 peptide was used as positive control (Bachem, Bubendorf, Switzerland). Epitope mapping was done according to the manufacturer's (Mimotopes) instructions.
Animal Care and Treatment.
All treatments were approved by the Local Committee for Animal Use and were carried out in accordance to state and federal regulations. Double-transgenic 3- to 4-month-old female mice of F1 (FVB × C57BL) genetic background that expressed both mutant human amyloid precursor protein (APP-V717I) and mutant human presenilin-1 (PS1-A246E) both under the control of the mouse thy1 gene promoter were used (42, 43). All mice were genotyped by PCR at the age of 3 wk, and each mouse was uniquely labeled. All mice were then genotyped by a second PCR performed at the onset of the study, before blind randomization into different experimental groups. Mice had free access to water and standard mouse chow (Muracon-G; Trouw Nutrition, Gent, Belgium) and were housed under a reversed day–night rhythm in standard metal cages in accordance with local legislation on animal welfare. Five days before the initiation of the behavior test, mice were caged in macrolon Type 2 cages and transported to the behavior laboratory to familiarize them with the testing environment.
For immunization of the APPxPS1 double-transgenic mice, the PEGylated (ACI-01) and palmitoylated (ACI-24) liposomal antigens were injected at 2-wk intervals. In each experimental group, 10 animals were injected i.p. with vaccine (200 μl per injection, containing 8 nmol of the peptide), whereas “empty” liposomes served as control. Sera were taken at regular intervals and also 5 days after final boosting for analysis of antibody titers by ELISA.
Object Recognition Test.
To analyze the memory capacity of the APPxPS1 double-transgenic mice, the novel object recognition test (ORT) was performed as described (44, 45). Three-month-old mice received five i.p. inoculations of ACI-01 or ACI-24 with 2-wk intervals between injections. At an age of 6 months, the object recognition test was performed. Recognition memory is expressed as exploratory preference in the retention test (time of new object exploration in percentage of total exploration time). Retention was measured 3 h after training (additional details are provided in SI Methods). Statistical analysis was done by using ANOVA (46). For the analysis of Ig titer correlation and cognitive memory, we used a nonlinear regression calculation with SigmaPlot for Windows version 10 (Systat, San Jose, CA).
Quantification of Soluble and Insoluble Aβ-40 and Aβ-42 in Brain Homogenates.
To quantify the amount of human Aβ-40 and human Aβ-42 in the soluble fraction of mouse brain homogenates, commercially available ELISA kits were used (Amyloid β40 and β42 ELISA, The Genetics Company, Zurich, Switzerland). ELISA was performed according to the manufacturer's protocol. Quantification of the Aβ content of the samples was obtained by comparing absorbance to the standard curve made with synthetic Aβ1–40 or Aβ1–42 (additional information is provided in SI Methods).
Quantification of Inflammatory Cytokines.
The levels of TNF-α, IFN-γ, IL-6, and IL1-β were measured in total-brain homogenates by using sandwich ELISA according to manufacture's directions (R & D Systems, Minneapolis, MN). Results are expressed in pg/ml by reference to serial dilutions of the recombinant cytokines.
Statistical Evaluations.
For statistical analysis, the ANOVA Turkey–Kramer multiple-comparison test was used. This test was performed by using InStat version 3.06 for Windows, (GraphPad, San Diego CA).
Supplementary Material
Acknowledgments
We thank Dr. Jésus Raya (Université Louis Pasteur) for help with the NMR spectroscopy.
Abbreviations
- Aβ
β-amyloid protein
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- ivDde
1-(4,4-dimethyl-2,6-dioxo-cyclohexylidene)-3-methyl-butyl
- DMF
dimethylformamide
- DP
degree of polymerization
- DSPE
1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine
- MAS
magic angle spinning
- Mtt
4-methyltrityl
- NORBA
transgenic mice expressing 99 residues of the carboxyl-terminal fragment (CTF) of amyloid precursor protein under control of the cytomegalovirus enhancer/chicken beta-actin promoter
- ORT
novel object recognition task
- pNPP
para-nitro-phenyl-phosphate
- PyBOP
benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate
- SPA
succinimidyl ester of propionic acid
- TFA
trifluoroacetic acid
- TIPS
triisopropylsilane.
Footnotes
Conflict of interest statement: R.O.B. is a member of the Scientific Advisory Board, AC Immune SA.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703137104/DC1.
References
- 1.Braak H, Braak E, Bohl J. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 2.Jellinger KA, Bancher C. Neurology. 1996;46:1186–1187. doi: 10.1212/wnl.46.4.1186-b. [DOI] [PubMed] [Google Scholar]
- 3.Soto C. Mol Med Today. 1999;5:343–350. doi: 10.1016/s1357-4310(99)01508-7. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Trends Neurosci. 1993;16:403–409. doi: 10.1016/0166-2236(93)90008-a. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J, Selkoe DJ. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Walsh DM, Selkoe DJ. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 8.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Proc Natl Acad Sci USA. 2001;98:8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodart JC, Bales KR, Gannon KS, Greene SJ, DeMattos RB, Mathis C, DeLong CA, Wu S, Wu X, Holtzman DM, et al. Nat Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- 10.Lee EB, Leng LZ, Zhang B, Kwong L, Trojanowski JQ, Abel T, Lee VM. J Biol Chem. 2006;281:4292–4299. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- 11.Levites Y, Das P, Price RW, Rochette MJ, Kostura LA, McGowan EM, Murphy MP, Golde TE. J Clin Invest. 2006;116:193–201. doi: 10.1172/JCI25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, Lemere CA. J Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seabrook TJ, Thomas K, Jiang L, Bloom J, Spooner E, Maier M, Bitan G, Lemere CA. Neurobiol Aging. 2007;28:813–823. doi: 10.1016/j.neurobiolaging.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 15.Weiner HL, Lemere CA, Maron R, Spooner ET, Grenfell TJ, Mori C, Issazadeh S, Hancock WW, Selkoe DJ. Ann Neurol. 2000;48:567–579. [PubMed] [Google Scholar]
- 16.Qu B, Boyer PJ, Johnston SA, Hynan LS, Rosenberg RN. J Neurol Sci. 2006;244:151–158. doi: 10.1016/j.jns.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu B, Rosenberg RN, Li L, Boyer PJ, Johnston SA. Arch Neurol. 2004;61:1859–1864. doi: 10.1001/archneur.61.12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, Hardy J, Duff K, Jantzen P, DiCarlo G, Wilcock D, et al. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 19.Nicolau C, Greferath R, Balaban TS, Lazarte JE, Hopkins RJ. Proc Natl Acad Sci USA. 2002;99:2332–2337. doi: 10.1073/pnas.022627199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orgogozo JM, Gilman S, Dartigues JF, Laurent B, Puel M, Kirby LC, Jouanny P, Dubois B, Eisner L, Flitman, et al. Neurology. 2003;61:46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 21.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 22.Ferrer I, Boada RM, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewachter I, Van DJ, Smeijers L, Gilis M, Kuiperi C, Laenen I, Caluwaerts N, Moechars D, Checler F, Vanderstichele H, et al. J Neurosci. 2000;20:6452–6458. doi: 10.1523/JNEUROSCI.20-17-06452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monsonego A, Maron R, Zota V, Selkoe DJ, Weiner HL. Proc Natl Acad Sci USA. 2001;98:10273–10278. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monsonego A, Zota V, Karni A, Krieger JI, Bar-Or A, Bitan G, Budson AE, Sperling R, Selkoe DJ, Weiner HL. J Clin Invest. 2003;112:415–422. doi: 10.1172/JCI18104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tosi PF, Radu D, Nicolau C. Biochem Biophys Res Commun. 1995;212:494–500. doi: 10.1006/bbrc.1995.1997. [DOI] [PubMed] [Google Scholar]
- 27.Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Biochim Biophys Acta. 1991;1066:29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 28.Furrer J, Piotto M, Bourdonneau M, Limal D, Guichard G, Elbayed K, Raya J, Briand JP, Bianco A. J Am Chem Soc. 2001;123:4130–4138. doi: 10.1021/ja003566w. [DOI] [PubMed] [Google Scholar]
- 29.Bechinger B, Aisenbrey C, Bertani P. Biochim Biophys Acta. 2004;1666:190–204. doi: 10.1016/j.bbamem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Cruciani O, Mannina L, Sobolev AP, Segre A, Luisi P. Langmuir. 2004;20:1144–1151. doi: 10.1021/la035804h. [DOI] [PubMed] [Google Scholar]
- 31.Zirah S, Kozin SA, Mazur A, Blond A, Cheminant M, Segalas-Milazzo I, Debey P, Rebuffat S. J Biol Chem. 2006;27:2151–2161. doi: 10.1074/jbc.M504454200. [DOI] [PubMed] [Google Scholar]
- 32.Gavin AL, Barnes N, Dijstelbloem HM, Hogarth PM. J Immunol. 1998;160:20–23. [PubMed] [Google Scholar]
- 33.Maier M, Seabrook TJ, Lemere CA. Vaccine. 2005;23:5149–5159. doi: 10.1016/j.vaccine.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 34.De Gioia L, Selvaggini C, Ghibaudi E, Diomede L, Bugiani O, Forloni G, Tagliavini F, Salmona M. J Biol Chem. 1994;269:7859–7862. [PubMed] [Google Scholar]
- 35.Ono S, Lee S, Mihara H, Aoyagi H, Kato T, Yamasaki N. Biochim Biophys Acta. 1990;1022:237–244. doi: 10.1016/0005-2736(90)90119-9. [DOI] [PubMed] [Google Scholar]
- 36.Lowik DW, Linhardt JG, Adams PJ, van Hest JC. Org Biomol Chem. 2003;1:1827–1829. doi: 10.1039/b303749e. [DOI] [PubMed] [Google Scholar]
- 37.Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, Tycko R. Proc Natl Acad Sci USA. 2002;99:16742–16747. doi: 10.1073/pnas.262663499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 39.Sigurdsson EM, Knudsen E, Asuni A, Fitzer-Attas C, Sage D, Quartermain D, Goni F, Frangione B, Wisniewski T. J Neurosci. 2004;24:6277–6282. doi: 10.1523/JNEUROSCI.1344-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiner HL, Frenkel D. Nat Rev Immunol. 2006;6:404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- 41.Sreerama N, Woody RW. Anal Biochem. 2000;287:252–260. doi: 10.1006/abio.2000.4880. [DOI] [PubMed] [Google Scholar]
- 42.Moechars D, Lorent K, De Strooper B, Dewachter I, van Leuven F. EMBO J. 1996;15:1265–1274. [PMC free article] [PubMed] [Google Scholar]
- 43.Dewachter I, Van DJ, Smeijers L, Gilis M, Kuiperi C, Laenen I, Caluwaerts N, Moechars D, Checler F, Vanderstichele H, et al. J Neurosci. 2000;20:6452–6458. doi: 10.1523/JNEUROSCI.20-17-06452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 45.Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 46.Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler, et al. J Biol Chem. 1999;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.