Abstract
In dopaminoceptive neurons, dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) plays a central role in integrating the effects of dopamine and other neurotransmitters. Phosphorylation of DARPP-32 at Thr-34 by protein kinase A results in inhibition of protein phosphatase 1 (PP1), and phosphorylation at Thr-75 by Cdk5 (cyclin-dependent kinase 5) results in inhibition of protein kinase A. Dephosphorylation at Thr-34 involves primarily the Ca2+-dependent protein phosphatase, PP2B (calcineurin), whereas dephosphorylation of Thr-75 involves primarily PP2A, the latter being subject to control by both cAMP- and Ca2+-dependent regulatory mechanisms. In the present study, we have investigated the mechanism of Ca2+-dependent regulation of Thr-75 by PP2A. We show that the PR72 (or B″ or PPP2R3A) regulatory subunit of PP2A is highly expressed in striatum. Through the use of overexpression and down-regulation by using RNAi, we show that PP2A, in a heterotrimeric complex with the PR72 subunit, mediates Ca2+-dependent dephosphorylation at Thr-75 of DARPP-32. The PR72 subunit contains two Ca2+ binding sites formed by E and F helices (EF-hands 1 and 2), and we show that the former is necessary for the ability of PP2A activity to be regulated by Ca2+, both in vitro and in vivo. Our studies also indicate that the PR72-containing form of PP2A is necessary for the ability of glutamate acting at α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid and NMDA receptors to regulate Thr-75 dephosphorylation. These studies further our understanding of the complex signal transduction pathways that regulate DARPP-32. In addition, our studies reveal an alternative intracellular mechanism whereby Ca2+ can activate serine/threonine phosphatase activity.
Keywords: calcium, protein phosphorylation, dopamine, glutamate
Dopamine and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) is a phosphoprotein that is selectively enriched in medium spiny neurons in the neostriatum (1, 2). When phosphorylated at Thr-34 by protein kinase A (PKA), DARPP-32 is converted into a potent, high-affinity inhibitor of the broad specificity serine/threonine protein phosphatase, protein phosphatase 1 (PP1), leading to increased phosphorylation of many physiologically important substrates in medium spiny neurons, including neurotransmitter receptors, voltage-gated ion channels, ion pumps, protein kinases, and transcription factors (1, 2). These biochemical studies, as well as targeted deletion and mutation of DARPP-32 in mice, have shown that the protein plays a critical role in the actions of dopamine, as well as in the actions of antipsychotic drugs, drugs of abuse, and other agents that modulate dopamine levels in the brain (2–5). DARPP-32 is also phosphorylated at other sites by the protein kinases CK1, CK2, and Cdk5 (cyclin-dependent kinase 5), which serve to modulate the phosphorylation and dephosphorylation of Thr-34 (6–9). For example, phosphorylation at Thr-75 by Cdk5 inhibits PKA and blocks phosphorylation at Thr-34, thereby attenuating the dopamine/D1/cAMP/PKA/DARPP-32/PP1 signaling cascade (8).
The mechanisms involved in the dephosphorylation of the various sites of DARPP-32 are also complex, involving the actions of various serine/threonine protein phosphatases. Phospho-Thr-34 is largely under the control of PP2B (or calcineurin), a Ca2+/calmodulin-dependent enzyme that is regulated by glutamate acting at both NMDA and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors. Activation of AMPA or NMDA receptors also results in Ca2+-dependent dephosphorylation of Thr-75, but our previous studies indicated that this appeared to involve a distinct PP2A-dependent pathway (10, 11). Phospho-Thr-75 is also dephosphorylated by a cAMP/PKA-dependent pathway that regulates PP2A activity (12, 13). The ability of cAMP to increase phosphorylation of Thr-34 and to decrease phosphorylation of Thr-75 contributes to the reciprocal relationship between the phosphorylation status of these two critical sites in DARPP-32, and plays an important role in coordinating the efficacy of dopaminergic neurotransmission in striatal neurons (12). The balance between dopamine/cAMP-dependent pathways, and glutamate/Ca2+-dependent pathways also plays a critical role in regulating the function of DARPP-32 in striatal neurons (2).
PP2A is ubiquitously expressed in eukaryotic cells where it exists as a heterotrimeric enzyme composed of a 36-kDa catalytic C subunit, a 64-kDa scaffolding A subunit, and multiple regulatory B subunits. The B subunits are thought to influence enzyme activity, substrate specificity, and subcellular localization (14–20). We have recently shown that dephosphorylation at Thr-75 of DARPP-32 involves a distinct heterotrimeric form of PP2A that includes the B56δ subunit. These studies have found that PKA phosphorylates B56δ, thereby activating the enzyme, and that this mechanism is responsible for dopamine/cAMP-dependent dephosphorylation of Thr-75 of DARPP-32. In the current study, we have investigated the molecular basis for the Ca2+ and PP2A-dependent regulation of Thr-75 of DARPP-32. Previous studies have found that the B″ subunits (also known as the PPP2R3 family) contain two Ca2+ binding sites formed by E and F helices (EF-hands) (21), small helix–loop–helix motifs that are found in calmodulin, troponin C, S100, etc., that bind Ca2+ with high affinity. Five different B″ isoforms have been described, including PR72 and PR130, which are spliced products from the same gene (22, 23). Our preliminary studies indicated that the PR72 subunit was expressed at relatively high levels in striatal tissue, raising the possibility that it might play a role in the regulation of DARPP-32 dephosphorylation by PP2A. The results obtained in the present study indicate that Ca2+ directly regulates PR72-containing PP2A, and that the PR72-containing form of PP2A is necessary for the ability of glutamate acting at AMPA and NMDA receptors to regulate Thr-75 dephosphorylation.
Results
Selective Ca2+-Dependent Dephosphorylation of Phospho-Thr-75 of DARPP-32 in Cells Expressing the PR72 Regulatory Subunit of PP2A.
In initial studies, we analyzed the expression of the PR72 subunit in brain and also in a neuroblastoma cell line. The protein was found by immunoblotting to be expressed in highest levels in striatum and hippocampus, but was not detected in frontal cortex or Neuro-2a (N2a) cells (Fig. 1a). PR130, a splice variant of PR72, was not detected in any rat brain region examined, or in N2a cells (data not shown).
Fig. 1.
PP2A containing the PR72 subunit selectively regulates dephosphorylation of Thr-75 of DARPP-32. (a) Protein (50 μg per lane) from frontal cortex, striatum, and hippocampus of 7- to 8-week-old Sprague–Dawley rats, or from N2a cells, was separated by SDS/PAGE (4–20% acrylamide), and PR72 expression was measured by immunoblotting using a polyclonal anti-PR72 antibody. (b) N2a cells were transfected with Myc-tagged DARPP-32 without (vector) or with PR72 or B56δ subunit. Cells were treated without (−) or with (+) ionomycin (5 μM) for 10 min. (Upper) Phosphorylation at Thr-34 or Thr-75 of DARPP-32 was analyzed by immunoblotting using phosphospecific antibodies corresponding to each site. Total DARPP-32 expression was analyzed by using anti-Myc antibody. (Lower) Phosphorylation levels of Thr-34 (Left) and Thr-75 (Right) were normalized to values obtained for untreated cells expressing only DARPP-32. Data represent means ± SEM (n = 3). ∗, P < 0.001 compared with vehicle-treated vector control by two-way ANOVA with Bonferroni's posttest.
We took advantage of the low level of endogenous PR72 in N2a cells and cortical neurons to investigate the consequences of PR72 overexpression on DARPP-32 dephosphorylation. Myc-tagged DARPP-32 was first expressed in N2a cells in the absence or presence of PR72 (or B56δ as a control for an alternative type of B subunit). Previous studies have confirmed that exogenously expressed B subunits, including PR72 and B56δ, form heterotrimeric PP2A complexes (13, 21) (see also below). Cells were then incubated in the absence or presence of the Ca2+ ionophore, ionomycin, and the phosphorylation levels of Thr-34 and Thr-75 of DARPP-32 were analyzed by immunoblotting (Fig. 1b). Ionomycin treatment stimulated dephosphorylation of phospho-Thr-75 in cells expressing PR72 but not the vector control. However, there was no effect of PR72 expression on the level of phospho-Thr-34 in the absence or presence of ionomycin. In addition, ionomycin treatment had no effect on phosphorylation of Thr-75 of DARPP-32 in N2a cells expressing the B56δ subunit.
We then carried out similar studies in cortical neurons (Fig. 2). Treatment of cortical neurons with ionomycin resulted in dephosphorylation of Thr-75 of DARPP-32 in cells expressing PR72 but not the vector control, or the B56δ or Bα subunits. This effect of ionomycin was attenuated by pretreatment with the Ca2+ chelator, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester (BAPTA-AM). We have previously found in striatal neurons that activation of the AMPA or NMDA subclasses of ionotropic glutamate receptor decreases the level of phospho-Thr-75 DARPP-32 via a Ca2+-dependent mechanism that involves PP2A (10, 11). Treatment of PR72-expressing cortical neurons with AMPA plus NMDA resulted in dephosphorylation of Thr-75, an effect that was blocked by pretreatment with BAPTA-AM (Fig. 2). As expected, treatment of cortical neurons with ionomycin or AMPA/NMDA resulted in dephosphorylation of Thr-34, but this was unaffected by expression of PR72 or the other B subunits (data not shown).
Fig. 2.
Ca2+-dependent regulation of PR72-containing PP2A in cortical neurons. Rat cortical neurons were cotransfected with Myc-DARPP-32 and either vector, PR72, B56δ, or Bα subunit by electroporation. Cells were pretreated without or with BAPTA-AM (50 μM) for 30 min as indicated and then treated with DMSO vehicle, AMPA (50 μM) plus NMDA (100 μM), or ionomycin (10 μM) for an additional 5 min. (Upper) The phosphorylation at Thr-75 of DARPP-32 was analyzed by immunoblotting using a phosphospecific antibody. Total DARPP-32 expression was analyzed by using anti-Myc antibody. (Lower) Phosphorylation levels of Thr-75 were normalized to values obtained for untreated cells in each transfection set. Data represent means ± SEM (n = 3). ∗, P < 0.001 compared with vehicle-treated control by two-way ANOVA with Bonferroni's posttest.
cAMP also can regulate Ca2+ signaling in neurons and other cell types via a variety of mechanisms, including the regulation of Ca2+ channels by PKA-dependent phosphorylation, as well as via non-PKA-dependent pathways such as EPAC (24, 25). We found that, after expression of PR72 in N2a cells, forskolin treatment resulted in dephosphorylation of Thr-75 [see supporting information (SI) Fig. 6].
PR72 Is Required for AMPA- and NMDA-Mediated Dephosphorylation at Thr-75 of DARPP-32.
To directly address the role of endogenous PR72 subunit in DARPP-32 dephosphorylation in intact neurons, we generated an adeno-associated virus (AAV)/RNAi construct to suppress PR72 expression. In control studies, PR72 RNAi expression in N2a cells strongly inhibited the expression of PR72 but had no effect on expression of the B56δ subunit (Fig. 3a). We next examined the effect of PR72 RNAi in rat hippocampal neurons. DARPP-32 level is normally low in hippocampal neurons in culture, and therefore, DARPP-32 was also overexpressed by using a separate AAV construct. The expression of PR72 was reduced by PR72 RNAi but not by a control AAV (Fig. 3b). DARPP-32 expression was not affected by the coinfected RNAi. After PR72 knockdown, both the AMPA- and NMDA-mediated decreases in phospho-Thr-75 DARPP-32 were prevented. As a control, we also examined the effect of cAMP stimulation on the regulation of DARPP-32 dephosphorylation. Forskolin treatment resulted in a reduction of phospho-Thr-75 levels in the absence or presence of expression of PR72 RNAi, as a result of cAMP-dependent regulation of endogenous B56δ (data not shown; see ref. 13). Down-regulation of PR72 had no effect on the ability of AMPA, NMDA, or forskolin to regulate phosphorylation of Thr-34.
Fig. 3.
AMPA- and NMDA-mediated dephosphorylation at Thr-75 of DARPP-32 requires the PR72 subunit. (a) N2a cells were transfected with vector, FLAG-PR72 or FLAG-B56δ, and a pAAV-H1/PR72 RNAi construct as indicated. Expression levels of PR72 or B56δ were measured by immunoblotting using anti-Flag antibody. Note that PR72 is slightly smaller than B56δ (529 vs. 602 aa excluding the Flag tag), but the difference was hardly visible using SDS/PAGE (4–20% acrylamide). (b) Rat hippocampal neurons were infected with AAV containing myc-tagged DARPP-32 in the absence (no RNAi) or after coinfection with control AAV (control) or AAV containing PR72 RNAi (PR72 RNAi) (for coinfection, viruses were used at a 1:1 pfu ratio). After 5 days of infection, cells were treated without or with AMPA (50 μM), NMDA (100 μM), or forskolin (10 μM) for 5 min. The phosphorylation at Thr-34 or Thr-75 of DARPP-32 was analyzed by immunoblotting using phosphospecific antibodies corresponding to each site. PR72 and DARPP-32 immunoblots were performed with anti-PR72 antibody and anti-myc antibody, respectively. The phosphorylation levels of Thr-34 (Upper) and Thr-75 (Lower) were normalized to values obtained for untreated cells. Data represent means ± SEM (n = 3). ∗, P < 0.001; ∗∗, P < 0.01 compared with vehicle-treated control by two-way ANOVA with Bonferroni's posttest.
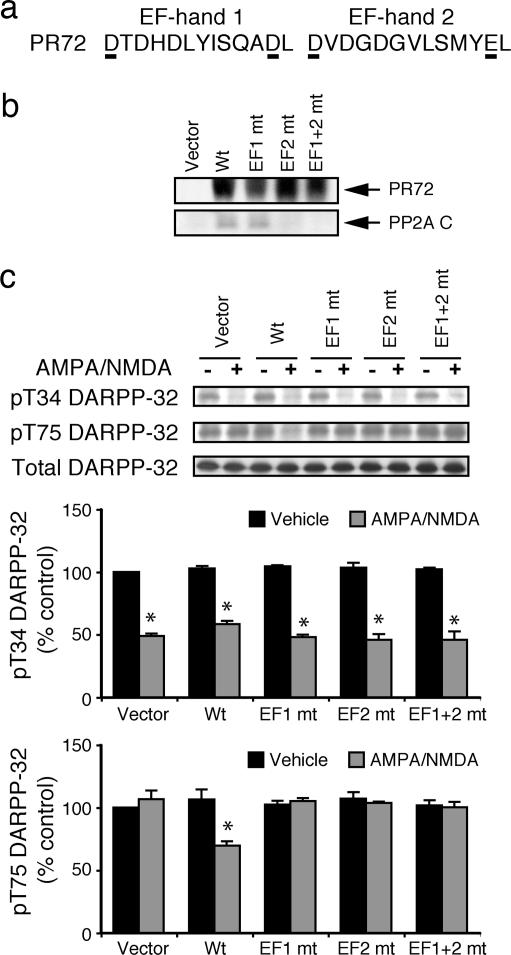
The Ca2+-Binding EF-Hands in PR72 Are Required for PP2A-Dependent Dephosphorylation of Phospho-Thr-75 DARPP-32.
The EF-hand motif is often found in Ca2+ sensor proteins such as calmodulin and troponin C (26). PR72 contains two EF hands, EF1 and EF2, and previous studies have implicated a role for these Ca2+-binding domains in heterotrimeric assembly of PR72/PP2A, as well as in regulation of PP2A activity (21). To further investigate the role of the EF-hands, we mutated two acid amino acid residues that are implicated in Ca2+ binding in each EF-hand motif (Fig. 4a). Flag-tagged wild-type or mutant PR72 proteins were then expressed in N2a cells, and PR72 was immunoprecipitated. Consistent with a previous result (21), wild-type PR72 and the EF-hand 1 mutant formed a heterotrimeric PP2A complex as demonstrated by the presence of the C subunit in the immunprecipitates (Fig. 4b). However, the PR72 mutants in which EF-hand 2 or both EF-hands were mutated, failed to interact with the C subunit.
Fig. 4.
The EF-hands of PR72 are required for Ca2+-dependent regulation of PP2A. (a) Amino acid sequences of EF-hands 1 and 2 in human PR72. The EF-hands were mutated by changing the underlined aspartate and glutamate residues to alanine. (b) N2a cells were transfected with vector, or wild-type or mutant FLAG-tagged PR72 subunit (as indicated). Cells were lysed and immunoprecipitated with agarose-conjugated anti-Flag antibody. Immune complexes were analyzed by SDS/PAGE (4–20% acrylamide) and immunoblotting. PP2A C subunit was detected by using anti-PP2A C antibody, and PR72 subunits were detected with anti-PR72 antibody. (c) Cortical neurons were cotransfected with Myc-DARPP-32 and either control vector, or wild-type (Wt) or mutant PR72, as indicated. After 5 days, cells were treated without or with AMPA (50 μM) plus NMDA (100 μM) for 5 min. The phosphorylation at Thr-34 or Thr-75 of DARPP-32 was analyzed by immunoblotting using phosphospecific antibodies corresponding to each site. Total DARPP-32 expression was analyzed by using anti-Myc antibody. Phosphorylation levels of Thr-34 (Upper) and Thr-75 (Lower) were normalized to values obtained for vector-transfected, untreated cells. Data represent means ± SEM (n = 3). ∗, P < 0.001 compared with vehicle-treated vector control by two-way ANOVA with Bonferroni's posttest.
Next, we expressed wild-type or mutant PR72 in rat cortical neurons and examined the ability of AMPA plus NMDA to stimulate dephosphorylation of DARPP-32 (also expressed in cortical neurons). AMPA/NMDA-dependent dephosphorylation at Thr-75 of DARPP-32 was observed following transfection with wild-type PR72, but not with any of the PR72 mutants (Fig. 4c). Expression of wild-type and mutant PR72 had no effect on AMPA/NMDA-dependent dephosphorylation at Thr-34 of DARPP-32, which is known to be mediated by PP2B (11, 27).
We also examined the role of the EF hands in vitro. Flag-tagged wild-type PR72 or the EF-hand 1 mutant protein were expressed in HEK293 cells, and PR72 was immunoprecipitated to obtain the heterotrimeric PP2A/PR72 enzyme complexes. The presence of both the A and C subunits in the heterotrimeric complex was confirmed by both immunoblotting and Coomassie blue staining (Fig. 5Inset and data not shown). The immune complexes were then used to dephosphorylate in vitro phospho-DARPP-32, phospho-Histone H1, or a phosphopeptide as substrates in the absence or presence of Ca2+. By using phospho-Thr-75 DARPP-32 as substrate, the PP2A preparation containing wild-type PR72 was activated ≈2-fold by addition of 50 μM or higher concentrations of Ca2+ (Fig. 5). Ca2+ also increased dephosphorylation of phospho-Histone H1 or the phosphopeptide substrate, although to a lesser extent than for phospho-DARPP-32. In contrast, Ca2+ had no effect on the activity of a PP2A heterotrimeric complex in which the EF1-hand was mutated. The basal phosphatase activity (in the absence of added Ca2+) of PP2A containing either the wild-type or EF1-hand mutant were very similar by using DARPP-32, Histone H1, or phosphopeptide as substrate (data not shown).
Fig. 5.
In vitro analysis of Ca2+-dependent regulation of PP2A containing the PR72 subunit. HEK293 cells were transfected with either wild-type PR72 or PR72 in which the EF1-hand was mutated. Cells were lysed and wild-type (Wt) or mutant PR72 (EF1 mt) was immunoprecipitated with agarose-conjugated anti-Flag antibody (Inset shows immunoblot showing approximately equal amounts of C subunit in the immunoprecipitated samples). PP2A immune complexes were incubated with each substrate [32P-Thr-75 DARPP-32 (Left), 32P-Histone H1 (Center), nonradioactive phosphopeptide (P-peptide) (Right)] in the absence or presence of various concentrations of Ca2+ as indicated. Phosphatase activity was normalized to that measured in the absence of Ca2+. Data represent means ± SEM (n = 3). ∗, P < 0.001; ∗∗, P < 0.01 compared with zero-calcium by two-way ANOVA with Bonferroni's posttest.
Discussion
The results obtained in this study suggest a mechanism whereby Ca2+ can activate PP2A via an interaction with the PR72 subunit. These studies expand on a previous report that indicated that the PR72 subunit of PP2A contains two functional EF-hands that were required for normal heterotrimeric assembly (21). Our results confirm an essential role for EF-hand 2 in heterotrimeric assembly, and now clearly indicate that Ca2+ binding to EF-hand 1 is able to activate the PR72/PP2A complex by using phospho-Thr-75 of DARPP-32, and other model substrates. Our previous studies have indicated that glutamate regulates the phosphorylation and dephosphorylation of multiple sites on DARPP-32 through a variety of signaling pathways (11). In particular, stimulation of AMPA and NMDA receptors leads to dephosphorylation of both Thr-34 and Thr-75 via PP2B and PP2A pathways, respectively (10, 11, 27). Our present results indicate that the regulation of PP2A via Ca2+-dependent regulation of PR72 is required for the ability of AMPA and NMDA receptors to stimulate dephosphorylation of Thr-75 of DARPP-32. The results also indicate that PR72 plays no role in the Ca2+-dependent regulation of Thr-34 dephosphorylation. These studies add to the complexity of the signaling pathways that control DARPP-32 function, and illustrate an alternative to PP2B, whereby Ca2+ can activate protein dephosphorylation through the activation of a specific heterotrimeric form of PP2A.
The Ca2+-dependent regulation of PP2A activity is likely to be widespread in various cell and tissue types. mRNA for PR72 and the alternatively spliced PR130 are widely distributed, with PR72 expression being high in skeletal muscle and heart, whereas PR130 is more ubiquitous (22). Our studies indicate that PR72 is relatively highly expressed in striatum but not in cortex, consistent with its role in the dephosphorylation of DARPP-32, which is also highly expressed in striatum. Notably, PP2B is also highly expressed in this brain region, although this phosphatase is also found in high levels in cortex and other brain regions (28). Previous studies have shown that EGFP-PR72 is localized to the nucleus and that an intact EF-hand 2 is required for nuclear localization. However, treatment of cells with a Ca2+ ionophore or a Ca2+ chelator did not influence nuclear localization (21), suggesting that the nuclear localization is a reflection of the role of EF-hand 2 in assembly of the PR72/PP2A heterotrimer. The subcellular localization of PR72 in striatum has not been examined in detail. However, our preliminary studies suggest it is present in both cytoplasm and nucleus (data not shown). Other studies suggest that substrates for PR72/PR130/PP2A are likely to be present in both the cytoplasm and nucleus (21, 29). DARPP-32 has been well characterized as a cytosolic protein, and it is likely that the effects of glutamate on Thr-75 dephosphorylation take place primarily in the cytoplasm. Our recent studies have revealed that DARPP-32 can readily translocate to the nucleus (A. Stipanovich, E. Valjent, M. Sanchez y Matamales, A.N., J.-H.A., K. Brami-Cherrier, H. Enslen, A.C.N., P.C., D. Hervé, and J.-A. Girault, unpublished results). PR72/PP2A may be involved in regulation of dephosphorylation of Thr-75 in nuclear DARPP-32.
Before the present studies of DARPP-32 dephosphorylation by PR72/PP2A, most of the protein targets for the PR72 family of proteins have been implicated in the control of the cell cycle (21, 30, 31). PR72, PR48, and PR59 all influence G1/S progression, with PR59 likely to target PP2A to the retinoblastoma-like protein, p107, and PR48 to target PP2A to Cdc6, an ATPase involved in initiation of DNA replication. At the present time, there is no known regulation of p107 and Cdc6 by Ca2+, although Ca2+ has been implicated in G1/S progression (32). One other target for PR72 and PR130 is the protein termed Naked cuticle (Nkd) (29, 33). Nkd is able to both positively and negatively influence Wnt signaling, through the actions of PR130 and PR72, respectively. However, it is notable that Nkd also contains an EF-hand, suggesting additional levels of regulation by Ca2+ in this signaling system. Another potential target for Ca2+-dependent PP2A activity might be the Cav1.2 L-type Ca2+ channel that is known to bind PP2A heterotrimers that include the PR59 subunit (34, 35). Conceivably, Ca2+ could play a negative-feedback role in dephosphorylation of Cav1.2.
An important question raised by this study is how Ca2+ binding to the PR72 subunit results in the regulation of PP2A. The recent elucidation of crystal structures of the C/A/B56γ PP2A heterotrimer has provided important insight into holoenzyme assembly, and the role of the B56 subunit in substrate recognition (36–38). The central core of the B56 subunit contains a number of HEAT-like repeats that make multiple contacts with both the C and A subunits. The interaction of the B and C subunits appears likely to create a unique substrate recognition surface that serves to recruit specific substrates. There are no direct contacts between the B56γ subunit and the active site. However, it is possible that interactions of the B56γ subunit with the β12/13 loop of the C subunit, which overhangs the active site, could indirectly influence enzyme activity, as has been suggested by studies of the analogous region of PP1 (39). Although there is no extensive homology between the PR72 and B56 proteins, Li and Virshup (23) have suggested based on amino acid sequence comparison that two ASBDs (A subunit binding domains) present in B56 proteins may also be contained in PR72 family subunits. There is no evidence that PR72 or other types of B subunits contain HEAT repeats. However, it seems possible that they may interact with the C and A subunits and function in a similar way to B56γ. The two EF-hands in the PR72 isoforms are located near the C-terminal end of each of the two ASBDs, placing them in positions that would potentially influence PP2A substrate interactions.
The EF-hands of PR72 family members appear to function in at least two different ways. As shown by Janssens et al. (21) and confirmed in this study, a functional EF-hand 2 is necessary for heterotrimeric assembly. However, increased Ca2+ levels or chelation of Ca2+ in intact cells, or the absence or presence of Ca2+ in vitro, had little influence on the interaction of PR72 and the A subunit (21). These results raise the possibility that EF-hand 2 plays a structural role, but that Ca2+ binding is not required for heterotrimeric assembly. However, Janssens et al. did find in vitro that the intrinsic fluorescence of PR72 is unaffected by mutation of the two EF-hands, supporting the idea that the mutations do not have a large effect on PR72 structure. Irrespective of the precise role of Ca2+ binding to EF-hand 2, our results clearly show that Ca2+ binding to EF-hand 1 is able to activate PP2A activity. Mutation of EF-hand 1 prevents the ability of AMPA plus NMDA to stimulate DARPP-32 Thr-75 dephosphorylation in intact cells and blocks the ability of Ca2+ to activate the PR72/PP2A heterotrimer in vitro. DARPP-32 and the other substrates used in our in vitro assays are all efficiently dephosphorylated by PR72/PP2A in the absence of Ca2+, with addition of Ca2+ being able to increase PP2A activity in vitro ≈2-fold. Previous studies of a native preparation of PR72/PP2A did not find any effect of Ca2+ on phosphatase activity by using phosphorylase a as substrate (21), raising the possibility that the effects of Ca2+ are substrate specific. Ca2+ binding causes a conformational change in PR72 in vitro (21). Ca2+ binding to EF-hand 1 may be able to further increase PP2A affinity for certain substrates like Thr-75-DARPP-32, or to alter the orientation of phospho-serine or -threonine in the active site of the C subunit.
The B subunits of PP2A are generally considered to be involved in recruitment of specific substrates, and there is growing evidence to support this view. However, the results from this and another recent study (13) have indicated that Thr-75 of DARPP-32 is subject to dephosphorylation by two distinct heterotrimeric forms of PP2A, namely those containing PR72 and B56δ. It appears that phospho-Thr-75 of DARPP-32 is intrinsically a good substrate for PP2A, with the B subunits in the case of DARPP-32 being less critical for substrate recruitment, but more important for conferring regulation by distinct signaling pathways. Phospho-Thr-75 plays an important role in controlling the ability of DARPP-32 to be phosphorylated at Thr-34 and to regulate PP1 (2, 8). Our previous studies have also indicated that DARPP-32 is involved in integrating convergent dopamine and glutamate inputs in striatal neurons (40). Activation of cAMP/PKA/B56δ by dopamine and Ca2+-dependent activation of PR72 by glutamate may result in a synergistic dephosphorylation of Thr-75 of DARPP-32, which could contribute to the convergent actions of these two neurotransmitters in striatal neurons.
Materials and Methods
Cell Culture and Transfection.
For cortical and hippocampal neurons, newly prepared neurons prepared by using standard methods were transfected by using a Nucleofector kit (Amaxa, Gaithersburg, MD). Neurons (5 × 106 cells) were mixed with 3 μg of DNA in Nucleofector solution, and then transferred into a cuvette and electroporated with the program O-03 by using the Nucleofector V2.4 apparatus. Neurons were transferred to DMEM with 10% FBS and incubated in a humidified CO2 incubator. After 24 h, medium was replaced with Neurobasal medium containing B-27 (1×), N-2 (1×), glutamine (1.5 mM), and one-third of the medium was replaced with fresh medium on every third day. Experiments were done after 5 days of transfection. Other cell culture, transfection, and immunoblotting were carried out as described (13).
Immunoprecipitation of PP2A Complex and PP2A Activity Assay.
PP2A complexes were prepared and assayed essentially as described (13).
Viral Production and Purification of AAV-RNAi.
Oligonucleotides for PR72 RNAi that recognized both human and rat sequences, GATCCCCGCAGAATGGCTCACATCTTCTTCCTG T C A AAGATGTGAGCCATTCTGCTTTTTTGGAAT and TAG A T TCCAAAAAAGCAGAATGGCTCACATCTTTG A C A G GAAGAAGATGTGAGCCATTCTGCGGG, were hybridized in buffer [100 mM Tris·HCl (pH 8.0), 10 mM MgCl2, 150 mM NaCl], and then ligated into pAAV-H1 (a gift from Michael Kaplitt, Weill Medical College of Cornell University, New York, NY). HEK293 cells were cultured in 10 150 × 25-mm dishes and triple-transfected with pAAV-H1/PR72 RNAi or empty pAAV-H1 (control AAV) and pHelper and pAAV-RC plasmids (Stratagene, La Jolla, CA) by using a standard calcium phosphate method. Cells were collected, pelleted and resuspended in buffer [0.15 M NaCl and 50 mM Tris (pH 8.0)] 66–70 h after transfection.
Additional detailed methods are described in SI Text.
Supplementary Material
Acknowledgments
We thank Dr. Michael Kaplitt for the pAAV-H1 vector used for RNAi expression. This work was supported by National Institutes of Health Grants DA 10044 and MH 74866 (to P.G. and A.C.N.).
Abbreviations
- DARPP-32
dopamine- and cAMP-regulated phosphoprotein of 32 kDa
- PKA
protein kinase A
- PP1
protein phosphatase 1
- AMPA
α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- N2a
Neuro-2a cells
- BAPTA-AM
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester
- AAV
adeno-associated virus
- EF-hand
Ca2+ binding site formed by E and F helices.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703589104/DC1.
References
- 1.Greengard P, Allen PB, Nairn AC. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 2.Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 3.Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, et al. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- 4.Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, McKinzie DL, Fienberg AA, Nomikos GG, Greengard P. Science. 2003;302:1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- 5.Svenningsson P, Nairn AC, Greengard P. AAPS J. 2005;7:E353–E360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desdouits F, Cohen D, Nairn AC, Greengard P, Girault JA. J Biol Chem. 1995;270:8772–8778. doi: 10.1074/jbc.270.15.8772. [DOI] [PubMed] [Google Scholar]
- 7.Girault J-A, Hemmings HC, Jr, Williams KR, Nairn AC, Greengard P. J Biol Chem. 1989;264:21748–21759. [PubMed] [Google Scholar]
- 8.Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, et al. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- 9.Jin M, Bateup H, Padovan JC, Greengard P, Nairn AC, Chait BT. Anal Chem. 2005;77:7845–7851. doi: 10.1021/ac051519m. [DOI] [PubMed] [Google Scholar]
- 10.Nishi A, Bibb JA, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P. J Neurochem. 2002;81:832–841. doi: 10.1046/j.1471-4159.2002.00876.x. [DOI] [PubMed] [Google Scholar]
- 11.Nishi A, Watanabe Y, Higashi H, Tanaka M, Nairn AC, Greengard P. Proc Natl Acad Sci USA. 2005;102:1199–1204. doi: 10.1073/pnas.0409138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P. Proc Natl Acad Sci USA. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn JH, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC. Proc Natl Acad Sci USA. 2007;104:2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Virshup DM. Curr Opin Cell Biol. 2000;12:180–185. doi: 10.1016/s0955-0674(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 15.Janssens V, Goris J. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price NE, Mumby MC. Curr Opin Neurobiol. 1999;9:336–342. doi: 10.1016/s0959-4388(99)80049-x. [DOI] [PubMed] [Google Scholar]
- 17.Price NE, Mumby MC. Biochemistry. 2000;39:11312–11318. doi: 10.1021/bi0008478. [DOI] [PubMed] [Google Scholar]
- 18.Van Kanegan MJ, Adams DG, Wadzinski BE, Strack S. J Biol Chem. 2005;280:36029–36036. doi: 10.1074/jbc.M506986200. [DOI] [PubMed] [Google Scholar]
- 19.Letourneux C, Rocher G, Porteu F. EMBO J. 2006;25:727–738. doi: 10.1038/sj.emboj.7600980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saraf A, Virshup DM, Strack S. J Biol Chem. 2007;282:573–580. doi: 10.1074/jbc.M607407200. [DOI] [PubMed] [Google Scholar]
- 21.Janssens V, Jordens J, Stevens I, Van HC, Martens E, De SH, Engelborghs Y, Waelkens E, Goris J. J Biol Chem. 2003;278:10697–10706. doi: 10.1074/jbc.M211717200. [DOI] [PubMed] [Google Scholar]
- 22.Hendrix P, Mayer-Jackel RE, Cron P, Goris J, Hofsteenge J, Merlevede W, Hemmings BA. J Biol Chem. 1993;268:15267–15276. [PubMed] [Google Scholar]
- 23.Li X, Virshup DM. Eur J Biochem. 2002;269:546–552. doi: 10.1046/j.0014-2956.2001.02680.x. [DOI] [PubMed] [Google Scholar]
- 24.Ster J, De BF, Guerineau NC, Janossy A, Barrere-Lemaire S, Bos JL, Bockaert J, Fagni L. Proc Natl Acad Sci USA. 2007;104:2519–2524. doi: 10.1073/pnas.0611031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bos JL. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 26.Ikura M. Trends Biochem Sci. 1996;21:14–17. [PubMed] [Google Scholar]
- 27.Nishi A, Snyder GL, Greengard P. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goto S, Matsukado Y, Mihara Y, Inoue N, Miyamoto E. Brain Res. 1986;397:161–172. doi: 10.1016/0006-8993(86)91381-8. [DOI] [PubMed] [Google Scholar]
- 29.Creyghton MP, Roel G, Eichhorn PJ, Hijmans EM, Maurer I, Destree O, Bernards R. Genes Dev. 2005;19:376–386. doi: 10.1101/gad.328905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan Z, Fedorov SA, Mumby MC, Williams RS. Mol Cell Biol. 2000;20:1021–1029. doi: 10.1128/mcb.20.3.1021-1029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Voorhoeve PM, Hijmans EM, Bernards R. Oncogene. 1999;18:515–524. doi: 10.1038/sj.onc.1202316. [DOI] [PubMed] [Google Scholar]
- 32.Berridge MJ. BioEssays. 1995;17:491–500. doi: 10.1002/bies.950170605. [DOI] [PubMed] [Google Scholar]
- 33.Creyghton MP, Roel G, Eichhorn PJ, Vredeveld LC, Destree O, Bernards R. Proc Natl Acad Sci USA. 2006;103:5397–5402. doi: 10.1073/pnas.0507237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davare MA, Horne MC, Hell JW. J Biol Chem. 2000;275:39710–39717. doi: 10.1074/jbc.M005462200. [DOI] [PubMed] [Google Scholar]
- 35.Hall DD, Feekes JA, Arachchige Don AS, Shi M, Hamid J, Chen L, Strack S, Zamponi GW, Horne MC, Hell JW. Biochemistry. 2006;45:3448–3459. doi: 10.1021/bi051593z. [DOI] [PubMed] [Google Scholar]
- 36.Cho US, Xu W. Nature. 2007;445:53–57. doi: 10.1038/nature05351. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Xing Y, Chen Y, Chao Y, Lin Z, Fan E, Yu JW, Strack S, Jeffrey PD, Shi Y. Cell. 2006;127:1239–1251. doi: 10.1016/j.cell.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 38.Mumby M. ACS Chem Biol. 2007;2:99–103. doi: 10.1021/cb700021z. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe T, Huang HB, Horiuchi A, da Cruze Silva EF, Hsieh-Wilson L, Allen PB, Shenolikar S, Greengard P, Nairn AC. Proc Natl Acad Sci USA. 2001;98:3080–3085. doi: 10.1073/pnas.051003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, et al. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.