Abstract
The feeling of body ownership is a fundamental aspect of self-consciousness. The underlying neural mechanisms can be studied by using the illusion where a person is made to feel that a rubber hand is his or her own hand by brushing the person's hidden real hand and synchronously brushing the artificial hand that is in full view. Here we show that threat to the rubber hand can induce a similar level of activity in the brain areas associated with anxiety and interoceptive awareness (insula and anterior cingulate cortex) as when the person's real hand is threatened. We further show that the stronger the feeling of ownership of the artificial hand, the stronger the threat-evoked neuronal responses in the areas reflecting anxiety. Furthermore, across subjects, activity in multisensory areas reflecting ownership predicted the activity in the interoceptive system when the hand was under threat. Finally, we show that there is activity in medial wall motor areas, reflecting an urge to withdraw the artificial hand when it is under threat. These findings suggest that artificial limbs can evoke the same feelings as real limbs and provide objective neurophysiological evidence that the rubber hand is fully incorporated into the body. These findings are of fundamental importance because they suggest that the feeling of body ownership is associated with changes in the interoceptive systems.
Keywords: body image, emotion, fMRI, multisensory, self
The experience of the body as part of the self is a fundamental aspect of self-consciousness. Neuroscientists have recently begun to investigate the neural mechanisms underlying this sense of body ownership (1–3). This research has a strong bearing on the recent developments in applied neuroscience to integrate the human body with artificial limb devices (4–6) and raises fundamental questions about how the brain represents the boundary between the self and the environment (7, 8).
It has been shown that people can be induced to experience an artificial arm as their own arm (9). By brushing a person's hand, while it is out of their sight, and synchronously brushing a visible rubber hand, the person experiences the feeling that the rubber hand senses the touch of the brush and that this artificial hand is a part of his or her own body. This illusory feeling of body ownership is associated with activity in multisensory areas such as premotor cortex, and it has been proposed that the key mechanism for this effect relates to integration of visual, tactile, and proprioceptive information (2, 3). However, the imaging experiment of the rubber-hand illusion has hitherto depended on subjective report, and it has not been established whether the feeling of ownership generalizes to domains beyond visual and tactile perception. Thus, it is unclear whether artificial limbs support the full range of feelings evoked by real limbs.
From an evolutionary perspective, it is critical to protect one's body from physical damage and to maintain homeostasis. Bodily threat typically evokes anxiety, change in autonomic arousal, and a withdrawal tendency (10–13). Thus, arguably, for an object to fully qualify as part of one's own body, it should be treated as such by the brain's homeostatic emotional systems. A key demonstration of this would be that a physical threat to the artificial limb evokes neuronal responses in areas related to the urge to withdraw and a feeling of anxiety. This hypothesis is supported by the finding that forceful bending of the finger of the rubber hand elicits enhanced sweating of the skin, as measured by skin conductance response, during the illusion of ownership (14). Here we used functional magnetic resonance imaging (fMRI) to provide objective neurophysiological evidence that the rubber hand is genuinely incorporated into a central representation of the body. We show that presentation of a stimulus threatening pain close to the rubber hand elicits cortical activity related to pain anticipation and anxiety, an effect related to a concurrent illusion of ownership. This finding indicates that artificial limbs, under appropriate circumstances, are fully incorporated into a bodily representation of the self.
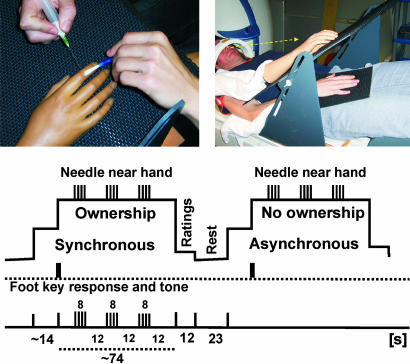
We used an experimental set-up whereby the participant's head was tilted so that he could see the rubber hand with direct vision (2) (see Fig. 1). We applied synchronous brushing to the rubber hand and the participant's own real hand (which was out of sight) to produce the illusion of ownership of the hand. As a control, we used asynchronous brush-strokes, which do not elicit the illusion. Coincident with these two patterns of stimulation, we occasionally made brisk stabbing movements with a sharp needle toward the rubber hand, without actually touching it. By comparing brain responses associated with the needle threats during the synchronous and asynchronous brushing (see Fig. 1 and Materials and Methods), we could test our hypothesis that the homeostatic defense responses, such as anxiety, would be greater when participants experienced the illusion of ownership. Specifically, we predicted activity in the anterior cingulate cortex (ACC) and insula, areas associated with anticipation of pain, empathic pain, and anxiety (15–18). Because the strength of the illusion varies between individuals (1–3), we also could test the prediction that a stronger illusion is correlated with a stronger threat-related neuronal response in these areas. Our design also enables us to compare the degree of activity evoked by this manipulation with that evoked when the participant's real hand was threatened. This allowed us to evaluate the extent to which the threat was comparable for real and illusory ownership conditions.
Fig. 1.
The setup used in the MRI experiments (Upper) and the experimental design of the rubber-hand runs (Lower) are shown. In separate runs, we examined the effects of threatening the real hand (see Materials and Methods for details).
Results
Subjective Ratings.
The participants reported that the illusion of ownership was significantly stronger during the synchronous brushing (“ownership” condition) than during the asynchronous stimulation (“no-ownership” condition) [P < 0.001, paired two-tailed t test; see supporting information (SI) Fig. 4a]. They also rated greater anxiety when the rubber hand was threatened during the ownership condition, as compared with no-ownership condition (P < 0.001, paired two-tailed t test; SI Fig. 4b). It is important to note that the level of anxiety when the rubber hand was threatened during the illusion was similar to the anxiety registered when the participants' real hand was threatened (P > 0.05, paired one-tailed t test; SI Fig. 4b). These results show that there is a strong link between the feeling of ownership of the seen hand and anxiety evoked when it is physically under threat (for further evidence, see Subjective Ratings: Additional Analyses in SI Text).
Brain Imaging.
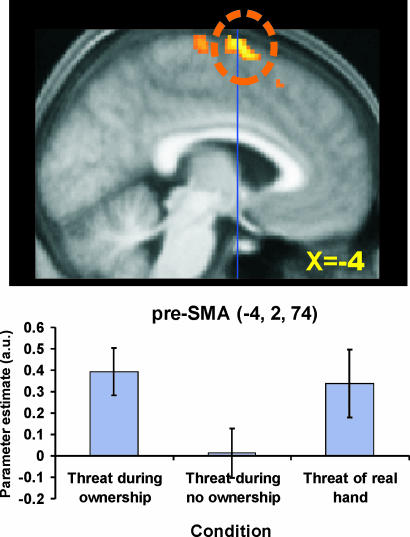
We first looked for areas that showed a stronger threat-related blood-oxygenation-level-dependent (BOLD) response when the participants felt the illusion of body ownership (“threat during ownership” compared with “threat during no ownership”). One significant peak of activation was found at the border zone between presupplementary motor area (pre-SMA) and supplementary motor area (SMA) proper (at x = −4, y = 2, z = 74; t value = 5.13; P < 0.05, corrected; see Fig. 2 and SI Table 1). In both the SMA and pre-SMA, the threat-related response was greater when the participants experienced the illusion than when they did not (Fig. 2). Fig. 2 also shows additional peaks that we found in the SMA proper {x = 0, y = −18, z = 74 [x, y, and z coordinates in Montréal Neurological Institute (MNI) space]} and pre-SMA (x = −8, y = 22, z = 56), but these did not survive correction for multiple comparisons (SMA: t = 3.95, P < 0.001 uncorrected; pre-SMA: t = 3.98, P < 0.001 uncorrected). We also observed trends for activation in the left insula (x = −54, y = 16, z = 0) and ACC (x = −8. y = 12, z = 44). However, these only reached the level of 0.01 uncorrected (insula: t = 2.05, P < 0.01, uncorrected; ACC: t = 2.19, P < 0.01, uncorrected; see also BOLD plots in SI Fig. 8). The reason why these effects were not more robust is that there was substantial intersubject variability in the strength of the rubber-hand illusion (1, 2) and in the anxiety elicited by seeing the needle.
Fig. 2.
Interaction between threat and ownership. (Upper) The activity in the pre-SMA (circled) was greater when the rubber hand was threatened during the illusion of ownership than when it just appeared to be a piece of rubber (threat during ownership − threat during no ownership). The significant activation is circled (P < 0.05 corrected). Additional activations of SMA-proper and a more anterior part of pre-SMA corresponds to P < 0.001 uncorrected. The coordinate in standard space for the displayed slice is indicated, as well as y = 0 (blue line). (Lower) The parameter estimates from the pre-SMA are plotted (error bars denote standard error). As can be seen, the level of activity was similar in the ownership and the real-hand conditions (P > 0.05 uncorrected).
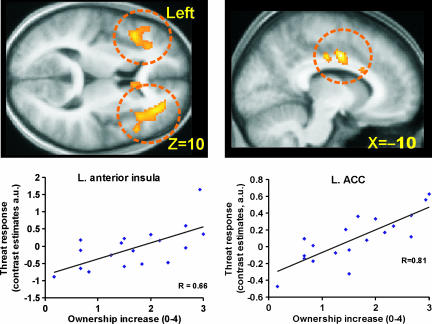
In the next set of analyses, we took advantage of this variability to run correlations across subjects. First, we directly examined the relationship between the feeling of body ownership and the threat-evoked cortical responses. We ran a regression analysis that related the strength of the ownership illusion (vividness ratings) to the activations in the insula and ACC that were evoked by threat (threat during ownership compared with threat during no ownership). As shown in Fig. 3, we found that the greater the vividness of the rubber-hand illusion, the greater the threat-related BOLD response in the left anterior insula cortex (x = −42, y = 20, z = 10, t = 3.63, R2 = 0.44, P < 0.05, corrected for multiple comparisons), right anterior insula (x = 40, y = 28, z = 6, t = 3.78, R2 = 0.46, P < 0.05, corrected for multiple comparisons), and bilateral ACC (x = −10, y = 4, z = 40, t = 5.65, R2 = 0.65; x = 8, y = 4, z = 36, t = 4.48, R2 = 0.54, both P < 0.05 corrected for multiple comparisons). Activity was also seen in SMA (P < 0.001) but did not reach significance when correcting for multiple comparisons. Thus, the more the participants felt the rubber hand to be their own hand, the greater the threat-evoked activation in these areas.
Fig. 3.
Linear relationship between ownership and the anxiety responses in the bilateral anterior insula and bilateral ACC (circled). A regression analysis identified a significant relationship between the vividness ratings of the rubber-hand illusion obtained during the scans and the parameter estimates for the contrast between threat during ownership and threat during no ownership in left insula (x = −42, y = 20, z = 10; R2 = 0.44; P < 0.05 corrected), right anterior insula (x = 40, y = 28, z = 6; R2 = 0.46, P < 0.05 corrected; data not shown), left ACC (x = −10, y = 4, z = 40; R2 = 0.66, P < 0.05 corrected), and right ACC (x = 8, y = 4, z = 36; R2 = 0.54; P < 0.05 corrected; data not shown). (For all plots: Pearson's correlation, 1-tailed, P < 0.001; Spearman's rho, 1-tailed, P < 0.01.) Plots for the right side are shown in SI Fig. 10.
We can conclude that the threat-evoked responses in ACC and insular cortices probably reflected anxiety because we found a correlation between the amplitude of the threat-evoked BOLD responses in these areas during the ownership condition (as compared with the no-ownership condition) and the increase in anxiety ratings in the ownership condition (as compared with the no-ownership condition; P < 0.05, corrected; SI Fig. 6 and SI Table 1).
We then asked whether a relationship existed between the activity in the areas associated with ownership and those associated with anxiety. We found a significant correlation between the activity in the bilateral ventral premotor cortex that reflects the rubber-hand illusion (2) and the threat-related BOLD response in the left anterior insular cortex (P < 0.05, correlation analysis; see SI Fig. 7). A similar correlation was observed between the insula and left intraparietal cortex (SI Fig. 7), which is another area related to the rubber-hand illusion (2, 3). Thus, the participants who had the strongest activity in the areas related to the illusion of ownership also showed the strongest anxiety response when the rubber hand was threatened.
Next we compared the degree of activity when the rubber hand was threatened during the illusion of ownership with that expressed when the participant's real hand was threatened. We found similar levels of activity (P > 0.05 uncorrected) in the bilateral anterior insula (SI Fig. 8; right insula not shown), ACC (SI Fig. 8), and pre-SMA cortex (Fig. 2) when we threatened the real hand and the rubber hand in the ownership condition. Thus, these areas were active in a conjunction analysis to highlight common activation in the two contrasts: threat during ownership (of the rubber hand) and threat to real hand (see SI Fig. 8).
Discussion
We have demonstrated that when people experience that an artificial limb is part of their own body, they display the same emotional responses and defense reactions as when their real limb is threatened. The fact that similar levels of activity are evoked in identical areas as when the person's real hand is threatened suggests that the feeling of ownership of the artificial limb is of such a degree that it fully “replaces” the real limb. Furthermore, our results reveal that the strength of the ownership-illusion (or the activity in multisensory areas reflecting this illusion) predicts the neuronal responses in areas associated with anxiety when the hand is under threat. Together these findings provide strong objective neurophysiological evidence that the rubber hand is incorporated into the body. Specifically, it shows that the physiological state of an owned artificial limb is subject to monitoring by the brain's emotional system, suggesting that artificial limbs can evoke the full range of feelings associated with real limbs.
We noted enhanced activity in medial motor areas when the rubber hand was threatened, but only when the rubber hand appeared to be part of the body. The peaks of activity lay in the SMA and pre-SMA. It is of interest that electrical stimulation of SMA in humans induces an urge to move limbs (19), and the BOLD signal in the pre-SMA increases when participants are asked to attend to the urge to produce a finger movement (20). Furthermore, activity in the SMA and pre-SMA increases before voluntary movement, reflecting the preparation to move (21–25). It is also commonly observed that medial wall motor areas are activated when humans experience or anticipate painful stimuli (16, 26–28). It has been suggested that this motor activity reflects the urge to remove the hand (27, 29) or preparation to generate an escape motor response (26). Indeed, in the present experiment, some participants spontaneously reported that they had an urge to withdraw the rubber hand in the ownership condition when the needle approached the hand. Thus, we suggest that the activity in SMA and pre-SMA in the present study reflects an urge to withdraw the hand, rather than anxiety, because this activity was not correlated with the anxiety ratings. An alternative interpretation is that this activity reflects the inhibition of the defensive motor response (30–33). Our data are consistent with both of these possibilities and indicate, whichever is correct, that the motor system is engaged when one's own body is perceived to be under physical threat. Finally, it is unlikely that medial wall activity reflects actual movement, because the participants were not observed to move their hand or fingers and there was no reliable contraction of their index finger muscle (see Monitoring of Hand Movement in SI Text) and no activation of the primary motor cortex (P > 0.001 uncorrected).
The most compelling finding in relation to our hypothesis was that the more strongly the participants felt the rubber hand to be their own hand, the greater the activity in the ACC and left insular cortex when the hand was under threat (Fig. 3). The peaks of activation in the ACC lay in the convexity cortex of the anterior cingulate and in the cingulate sulcus. This and the nearby surrounding cortex is known to be activated during anticipation and experience of pain (16–18, 16, 34) and when seeing other people receive pain (empathy for pain) (14). We also found activation in the anterior insular cortex, and this too is known to be activated during anticipation of pain, experience of pain, and empathy for pain (15–17). Coactivation of the ACC and anterior insula in imaging experiments is strongly linked to emotional processing and introceptive awareness (28, 35, 36). The activity in these areas probably reflected anxiety because there was a correlation between the increase in anxiety in the ownership condition and the increase in the threat-evoked BOLD responses in the ACC and insular cortex in this condition (SI Fig. 6).
Importantly, this anxiety-activity was specific to threats to one's own body and was not related to seeing the syringe or empathy for pain. Conclusive evidence for this was the direct correlation between ownership of the hand and the amplitude of the threat-evoked neuronal responses (Fig. 3). Furthermore, the participants who displayed the strongest activity in the bilateral ventral premotor cortex reflecting body ownership (2, 3) showed the strongest threat-related evoked responses in the insular cortex (SI Fig. 7). We did observe some activity in the ACC and insula that just reflected seeing the needle, as one would have expected (15) (see SI Results in SI Text and SI Fig. 9). However, over and above this basic activity we observed increases in anxiety-related activity during the ownership condition (SI Fig. 6), which was correlated with the ownership illusion (as described above; see Fig. 3).
The simple “subtraction” between the threat responses in the synchronous and asynchronous conditions only revealed statistical trends for activation in insula and ACC (see the BOLD plots in SI Fig. 8; P < 0.01, uncorrected). This probably just reflects the substantial variability between subjects in the strength of the illusion and the anxiety evoked by the needle. Indeed, it is this variability that is driving the significant correlations between the activity in insula and ACC and the subjective ratings of ownership and anxiety (described above).
Craig (35, 37) has argued that insula and ACC activity reflect engagement of an interoceptive system and that this system senses the physiological condition of the body. These areas mediate various feelings from the body such as pain, temperature, itch, and muscular sensations that are distinct from the exteroceptive systems. He further suggests that the distinction between self and nonself depends in part on the activation of this system (37) [a similar idea also was expressed by Damasio (38)]. Our results show that the feeling of ownership engendered by correlated visual, tactile, and proprioceptive information extends to engage the interoceptive system. This provides direct neurophysiological evidence for a link between the responsiveness of the ACC and insular regions and a crucial distinction between self and nonself, which supports the view that introception is intimately linked to self perception (37).
The engagement of interoceptive emotional systems demonstrates that the effects of the self-attribution during the rubber-hand illusion generalize beyond the multisensory areas. The natural feeling of body ownership might necessitate the engagement of the emotional systems, or the emotional responses may be a consequence of ownership mediated by multisensory mechanisms. We suggest that the recruitment of the emotional areas is an obligatory consequence of the self-attribution mediated by the multisensory mechanisms. This interoceptive activity enriches the experience of the limb as part of the body and gives it what one might call “a full sense of ownership,” that is, ownership that includes homeostatic feelings such as pain, pain anticipation, and temperature. Thus, it is the engagement of the interoceptive systems that gives the artificial hand the richness of feelings that makes the illusion so eerily vivid.
Our experimental design meant that we could compare brain activity when the rubber hand or the real hand was threatened. When the participants saw the needle approaching the rubber hand during the ownership condition, they reported similar levels of anxiety as when their real hand was threatened. This relationship was mirrored by similar levels of brain activity in the insula, ACC, and medial wall motor areas in the rubber-hand-ownership condition and the real-hand condition. This verifies the strength of the illusion of ownership and suggests the engagement of common neural substrates.
It has been suggested that tools may come to be included into the body representation during extensive tool use (7). Tool use may cause changes in visuo-tactile integration (39–41) and in the receptive fields of visuo-tactile cells in the parietal cortex (42), so that it appears as if peripersonal space extends from the hand to the tool. Further, tactile sensations can be projected to the tip of hand-held tools during manipulation (43–45). However, phenomenologically, we do not experience tools as part of our own body. Thus, the detection of visuo-tactile correlations is insufficient for a body part to be fully incorporated into the body image. Our results suggest that, perhaps unlike tool use, changes in body ownership require the changes in proprioceptive and interoceptive systems. To produce the illusion that an artificial limb belongs to oneself, it is necessary to correlate tactile stimulation of the real arm and visual stimulation of the artificial limb for an extended period. This leads to a recalibration of position sense for the person's own arm (3, 9, 46, 47). This recalibration means that as the needle approaches the rubber hand, it appears to be in peripersonal space and to represent a genuine threat.
Materials and Methods
Nineteen right-handed, healthy participants took part in the imaging experiments (11 males; all aged 19–33). Participation was limited to subjects who felt the rubber-hand illusion in preliminary testing; 9 other subjects were not scanned because they did not experience the illusion (see Prescanning Testing Phase in SI Text). All subjects had given their written consent, and the study was approved by the joint National Hospital for Neurology and Neurosurgery/Institute of Neurology Ethics Committee in the U.K.
Scanning Phase.
While the brain scans were being performed, the subjects rested comfortably in a supine position on the bed in the MRI scanner, with their right arm extended and placed on a support in a relaxed position (see Fig. 1). All subjects wore headphones to reduce noise and to enable them to receive auditory cues.
We used a setup where the participants could see the rubber hand with direct vision (2). Within the cylindrical head coil, the participant's head was tilted forward by ≈20° by placing foam wedges beneath the head (see Fig. 1). We used a life-size rubber prosthesis of a male or female right hand (gender matched). This rubber hand was placed on a tilted (30–45°) plastic table that was positioned over the participant's abdomen (see Fig. 1). The rubber hand was orientated in an anatomically plausible position on the table, pointing slightly left, toward the bodily midline (≈20–30°). The table was covered with a soft black material. The participant's own right hand was placed on a plastic support (30 × 30 cm), covered with the same soft material, which was below the table and hidden from the participant's view (30–40 cm away from the seen rubber hand). Finally, to reduce potential head movements, we fixed the position of the head by using foam pads.
One experimenter stood on the participant's right side so that he or she could apply brushstrokes to the participant's real right hand and the rubber hand by using small paintbrushes. This experimenter wore earphones to receive auditory cues about the onset and termination of the brushing periods. The brushstrokes were small (1 cm) and brisk (<400 ms) and applied to the upper parts of the index finger at a frequency of 1 Hz. To help the experimenter apply the same number of brushstrokes in the different conditions, he or she listened to an auditory metronome at 1 Hz over earphones and applied the stimuli with the same frequency.
The second experimenter was placed on the left side of the scanner bed. He or she held a syringe that was equipped with a sharp stainless steel needle (1.1 × 50 mm; Terum Europe N.V., Leuven, Belgium) (Fig. 1). The needle, which was slightly magnetic, was firmly attached to the syringe by using surgical tape, and the syringe was secured to the experimenter's arm. The experimenter could rest the hand that was holding the syringe on the table. In this position, the experimenter's hand was hidden behind a little screen on the table, 20 cm to the left of the rubber hand. The participant could not see the needle, the syringe, or the experimenter's hand when these were hidden in this way. The experimenter holding the needle wore earphones to receive auditory cues indicating when to threaten the rubber hand. When this occurred, the experimenter made brisk stabbing movements with the needle, moving it toward the index finger of the rubber hand in full view of the participant. Twelve such stabbing movements were produced in a period of 8 sec. The needle was moved close to the finger (≈10 mm) but not actually touching it. To minimize the risk of movement-related artefacts, both experimenters in the scanner room stood as still as possible during the scans and only made small movements of their hands to deliver the stimuli.
Four experimental runs were performed for each subject. In two of these runs, the participants were looking at the rubber hand as described above (“rubber-hand runs”; see Fig. 1 Lower). In two additional runs, the rubber hand was removed, and the participants looked at their own real right hand, which was placed on the table (“real-hand runs”). These two types of runs were conducted in a pseudorandomized order.
Rubber-Hand Runs.
In the two experimental runs with the rubber hand, there were two main conditions (or “contexts”) and short periods when we threatened the rubber hand during these contexts (see Fig. 1 Lower). We wanted to examine whether the needle threat evoked different neuronal responses in these different contexts (ownership or no ownership). During the ownership condition, the experimenter brushed the rubber hand and the subject's hidden hand as synchronously as possible with the two small paintbrushes. In the no-ownership condition, alternating brushstrokes were applied to the two hands. The periods of brushing the two hands synchronously or asynchronously lasted for 88 sec and were repeated 3 times in each run.
To indicate the onset of the ownership condition during the synchronous brushing, the subject was instructed to press a keypad with their left foot in a relaxed manner when they first started to feel that the rubber hand was their own (2, 3). The illusion of ownership of the rubber hand started after 14.3 ± 9.1 sec (mean ± SD across participants). When they pressed the key they heard a brief tone over the earphones (to match the tone presented in the no-ownership condition; see below). To control for the foot response, the participants were instructed to press the response key with their foot during the asynchronous brushing as well when they heard a tone. The timing of the presentation of these tones was yoked to the recorded times of the key response during the preceding ownership condition (Cogent 2000 software; Wellcome Department of Imaging Neuroscience, London, U.K.). After having made the responses, subjects were instructed to relax and to continue maintaining their gaze on the index finger of the rubber hand that was being brushed.
During both the ownership and no-ownership conditions, we occasionally threatened the hand with the needle. These threats were presented for periods of 8 sec (a miniblock), with periods lasting 12 sec of no threat between the threat miniblocks (Fig. 1). During each period of ownership or no-ownership condition, which lasted an average of 73.7 sec, two to four miniblocks of needle threat were presented, depending on how quickly the illusion started on that particular trial. The participants were instructed to keep looking at the index finger of the rubber hand and to avoid making any movements of their real hand throughout the experimental conditions.
After each period of synchronous or asynchronous brushing, the participants were asked to rate the following: (i) the vividness of the illusion of ownership, and (ii) the anxiety they had experienced when the hand was threatened by the needle. The participants were asked to press the foot-key 0–4 times, with 0 meaning no illusion or anxiety, respectively, and 4 meaning a very strong illusion or very strong anxiety. The participants were told to report the average feeling of ownership of the hand after the onset of the illusion (as indicated by the initial key press) and the average anxiety when the hand was being threatened. This rating procedure took 12 sec and was performed in a separate period after each period of brushing (Fig. 1). Before the next condition of synchronous or asynchronous brushing, there was a 23-sec rest period, serving as a baseline. This allowed for the illusion to disappear after the ownership condition and also gave the participants an opportunity to relax and close their eyes.
Real-Hand Runs.
The real hand was threatened in separate experimental runs. The rubber hand was removed, and the participant's right hand was positioned on the table in the same place where the rubber hand was normally placed. All of the other experimental procedures were kept identical to the rubber-hand runs with two variations. First, the participant pressed the foot-key when he or she heard a tone after 14.3 sec of stimulation (matched to the onset of the illusion in the rubber-hand runs). Second, after each period of brushing, the volunteers rated the anxiety associated with the needle threat but did not have to rate the vividness of the illusion.
Acquisition and Analysis of Functional Imaging Data.
The functional imaging was conducted by using a Sonata 1.5-T whole-body MRI Scanner (Siemens Medical, Erlangen, Germany) operated with a head-coil. Gradient echo T2*-weighted echo-planar images (EPIs) sensitive to the BOLD contrast were acquired as an index of local increases in synaptic activity (48) (see EPI Scanning Parameters in SI Text). A functional image volume comprised 44 continuous slices of 2-mm thickness (with a 1-mm interslice gap) covering the whole brain except the lower parts of the cerebellum [64 × 64 matrix, 3.0 ×3.0 mm in-plane resolution, Echo time (TE) = 49 msec, phase-encoding direction anterior − posterior].
The fMRI data were analyzed by using the Statistical Parametric Mapping Software 2 (SPM2; www.fil.ion.ucl.ac.uk/spm; Wellcome Department of Cognitive Neurology, London, U.K.). The functional images were realigned and unwarped to correct for head movements, coregistered with each subject's anatomical MRI, and transformed (linear and nonlinear transformation) to the space of the Montréal Neurological Institute (MNI) standard brain. The functional images were scaled to 100 and spatially smoothed with a 10-mm full-width at half-maximum (FWHM) isotropic Gaussian kernel and smoothed in time by a 4-sec FWHM Gaussian kernel.
For each individual participant, we fitted a linear regression model (general linear model) to the data (first level analysis). For each of the experimental conditions described above, we defined one regressor for the period before the key press and one regressor for the periods after the key press. The most relevant regressors were those that were defined for the miniblocks of needle threat (hereafter referred to as “threat during ownership,” “threat during no ownership,” and “threat during real hand”). Conditions of no interest corresponding to the 12-sec periods when the participants rated the illusion and anxiety also were defined. Each condition was modeled with a boxcar function convolved with the standard SPM2 hemodynamic response function. We defined linear contrasts in the general linear model (see below). The result from this analysis was a contrast estimate for each condition for each of the 19 participants (contrast images). To accommodate intersubject variability, the contrast images from all participants were entered into a random effect group analysis (second-level analysis). One-sample t tests were used (18 degrees of freedom). We first used the statistical threshold of P < 0.005 (uncorrected) and a cluster size of 10 voxels or more to generate activations maps. For statistical inference we then used the threshold of P < 0.05 after a correction for the number of multiple comparisons (Family-Wise-Error correction). Because we had an a priori hypothesis that threatening the hand would activate certain areas associated with pain anticipation and anxiety, we used a small volume correction in these regions (15, 17, 28, 36) (see Small Volume Correction in SI Text).
Contrasts.
Six main statistical analyses were performed (of which three are described in SI Text). First, we examined whether the neuronal response elicited by threatening the rubber hand was modulated by the illusion of ownership, which was done by examining the contrast (threat during ownership compared with threat during no ownership).
Second, we looked for areas that showed a correlation between the strength of the rubber-hand illusion and the threat-evoked BOLD responses. Using the second-level SPM2 simple regression (correlation), we related the increase in vividness ratings of the rubber-hand illusion (ownership compared with no ownership) to the contrast estimates for the contrast (threat during ownership compared with threat during no ownership).
Third, we investigated whether the same areas were activated when we threatened the participant's real hand as when we threatened the rubber hand during the illusion of ownership. For this, we used a conjunction analysis with the threshold of P < 0.005 (uncorrected) in each of the two contrasts used. This analysis, based on inclusive masking, constitutes a true logical “AND” operation (49) and identifies brain areas activated in both conditions.
Three additional analyses were performed which are described in SI Text (see Additional Analyses of the fMRI Data): (i) correlation between the activity in areas associated with ownership (premotor and parietal) and the threat-evoked activity in insula and ACC, (ii) correlations between the reported changes in anxiety and the threat-evoked BOLD responses in insula and ACC, and (iii) the main effect of threat.
Supplementary Material
Acknowledgments
We thank Peter Aston and Eric Featherstone for technical assistance with the experimental setup. H.H.E was supported by a long-term fellowship from the Human Frontier Science Program. The participation of K.W. was supported by the German Research Society Wi 1957/2-1. This work was supported by the Wellcome Trust and carried out as part of the PRESENCCIA project, a European Union-funded Integrated Project under the IST Program Project 27731.
Abbreviations
- ACC
anterior cingulate cortex
- BOLD
blood-oxygenation-level-dependent
- fMRI
functional MRI
- SMA
supplementary motor area
- pre-SMA
presupplementary motor area.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610011104/DC1.
References
- 1.Botvinick M. Science. 2004;305:782–783. doi: 10.1126/science.1101836. [DOI] [PubMed] [Google Scholar]
- 2.Ehrsson HH, Spence C, Passingham RE. Science. 2004;305:875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- 3.Ehrsson HH, Holmes NP, Passingham RE. J Neurosci. 2005;25:10564–10573. doi: 10.1523/JNEUROSCI.0800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friehs GM, Zerris VA, Ojakangas CL, Fellows MR, Donoghue JP. Stroke. 2004;35:2702–2705. doi: 10.1161/01.STR.0000143235.93497.03. [DOI] [PubMed] [Google Scholar]
- 5.Craelius W. Science. 2002;295:1018–1021. doi: 10.1126/science.295.5557.1018. [DOI] [PubMed] [Google Scholar]
- 6.Birchard K. Lancet. 1999;354:52. doi: 10.1016/S0140-6736(05)75321-6. [DOI] [PubMed] [Google Scholar]
- 7.Maravita A, Iriki A. Trends Cognit Sci. 2004;8:79–86. doi: 10.1016/j.tics.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Nicolelis MA. Nat Rev Neurosci. 2003;4:417–422. doi: 10.1038/nrn1105. [DOI] [PubMed] [Google Scholar]
- 9.Botvinick M, Cohen J. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- 10.Darwin C. The Expression of the Emotions in Man and Animals. Chicago: Univ of Chicago Press; 1872. reprinted (1965) (Univ of Chicago Press, Chicago) [Google Scholar]
- 11.Cooke DF, Graziano MS. J Neurophysiol. 2003;90:3317–3329. doi: 10.1152/jn.00513.2003. [DOI] [PubMed] [Google Scholar]
- 12.Kalisch R, Wiech K, Critchley HD, Seymour B, O'Doherty JP, Oakley DA, Allen P, Dolan RJ. J Cognit Neurosci. 2005;17:874–883. doi: 10.1162/0898929054021184. [DOI] [PubMed] [Google Scholar]
- 13.Kalisch R, Wiech K, Critchley HD, Dolan RJ. Neuroimage. 2006;30:1458–1466. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Armel KC, Ramachandran VS. Proc R Soc London Ser B. 2003;270:1499–1506. doi: 10.1098/rspb.2003.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 16.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 17.Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- 18.Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Brain. 2003;126:2139–2152. doi: 10.1093/brain/awg216. [DOI] [PubMed] [Google Scholar]
- 19.Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD. J Neurosci. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau HC, Rogers RD, Haggard P, Passingham RE. Science. 2004;303:1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- 21.Lee KM, Chang KH, Roh JK. Neuroimage. 1999;9:117–123. doi: 10.1006/nimg.1998.0393. [DOI] [PubMed] [Google Scholar]
- 22.Cunnington R, Windischberger C, Moser E. Hum Mov Sci. 2005;24:644–656. doi: 10.1016/j.humov.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Deecke L, Scheid P, Kornhuber HH. Exp Brain Res. 1969;7:158–168. doi: 10.1007/BF00235441. [DOI] [PubMed] [Google Scholar]
- 24.Shima K, Tanji J. J Neurophysiol. 1998;80:3247–3260. doi: 10.1152/jn.1998.80.6.3247. [DOI] [PubMed] [Google Scholar]
- 25.Tanji J, Kurata K. Neurosci Lett. 1979;12:201–206. doi: 10.1016/0304-3940(79)96062-2. [DOI] [PubMed] [Google Scholar]
- 26.Kwan CL, Crawley AP, Mikulis DJ, Davis KD. Pain. 2000;85:359–374. doi: 10.1016/S0304-3959(99)00287-0. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh JC, Stahle-Backdahl M, Hagermark O, Stone-Elander S, Rosenquist G, Ingvar M. Pain. 1996;64:303–314. doi: 10.1016/0304-3959(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 28.Farrell MJ, Laird AR, Egan GF. Hum Brain Mapp. 2005;25:129–139. doi: 10.1002/hbm.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh JC, Hagermark O, Stahle-Backdahl M, Ericson K, Eriksson L, Stone-Elander S, Ingvar M. J Neurophysiol. 1994;72:3004–3008. doi: 10.1152/jn.1994.72.6.3004. [DOI] [PubMed] [Google Scholar]
- 30.Farina S, Valeriani M, Rosso T, Aglioti S, Tamburin S, Fiaschi A, Tinazzi M. Neurosci Lett. 2001;314:97–101. doi: 10.1016/s0304-3940(01)02297-2. [DOI] [PubMed] [Google Scholar]
- 31.Farina S, Tinazzi M, Le Pera D, Valeriani M. Neurol Res. 2003;25:130–142. doi: 10.1179/016164103101201283. [DOI] [PubMed] [Google Scholar]
- 32.Svensson P, Miles TS, McKay D, Ridding MC. Eur J Pain. 2003;7:55–62. doi: 10.1016/s1090-3801(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 33.Avenanti A, Bueti D, Galati G, Aglioti SM. Nat Neurosci. 2005;8:955–960. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- 34.Craig AD, Reiman EM, Evans A, Bushnell MC. Nature. 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- 35.Craig AD. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 36.Peyron R, Laurent B, Garcia-Larrea L. Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 37.Craig AD. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 38.Damasio. Nature. 2003;423:227. doi: 10.1038/423227a. [DOI] [PubMed] [Google Scholar]
- 39.Maravita A, Spence C, Driver J. Curr Biol. 2003;13:R531–R539. doi: 10.1016/s0960-9822(03)00449-4. [DOI] [PubMed] [Google Scholar]
- 40.Holmes NP, Calvert GA, Spence C. Neurosci Lett. 2004;372:62–67. doi: 10.1016/j.neulet.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 41.Farne A, Iriki A, Ladavas E. Neuropsychologia. 2005;43:238–248. doi: 10.1016/j.neuropsychologia.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Iriki A, Tanaka M, Iwamura Y. NeuroReport. 1996;7:2325–2330. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto S, Moizumi S, Kitazawa S. J Neurophysiol. 2005;93:2856–2863. doi: 10.1152/jn.01015.2004. [DOI] [PubMed] [Google Scholar]
- 44.Gibson JJ. The Senses Considered as Perceptual Systems. Boston: Houghton Mifflin; 1966. [Google Scholar]
- 45.Paillard J. In: The Use of Tools by Human and Non-Human Primates. Berthelet A, Chavaillon J, editors. New York: Oxford Univ Press; 1993. pp. 36–46. [Google Scholar]
- 46.Tsakiris M, Haggard P. J Exp Psychol Hum Percept Perform. 2005;31:80–91. doi: 10.1037/0096-1523.31.1.80. [DOI] [PubMed] [Google Scholar]
- 47.Ehrsson HH, Holmes NP, Passingham RE. J Neurosci. 2005;25:10564–10573. doi: 10.1523/JNEUROSCI.0800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 49.Friston KJ, Penny WD, Glaser DE. Neuroimage. 2005;25:661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.