Abstract
We previously performed mutant screens in the medaka for defects in gonadal development and identified a mutant of interest in this regard, which was designated as hotei (hot). This mutant manifests a number of remarkable phenotypic abnormalities including: (i) excessive proliferation of germ cells that initiates at around the hatching stage regardless of the genetic sex of the fish; (ii) initiation of premature meiosis in phenotypically male hot homozygotes; (iii) one-half of the hot-homozygous XY fish undergo sex reversal, which accompanies the expression of the female-characteristic aromatase gene in the somatic cells of the gonad; and (iv) in phenotypically female homozygotes, follicular development is arrested at an early stage. We have also performed genetic mapping, chromosome walking, and candidate gene sequencing analysis of hot and demonstrate that the underlying mutation occurs in the recently identified medaka anti-Müllerian hormone (Amh) receptor type II (amhrII) gene. Moreover, this gene was found to be responsible for each of the hot phenotypes, as an amhrII transgene rescues these abnormalities. In addition, the amhrII gene is expressed in the somatic cells of the gonads of both sexes. The phenotypes of the hot homozygotes indicate that there are multiple regulatory functions of the AMH/AMHRII signaling system in the development of the gonad, including the sex-dependent regulation of germ cell proliferation and follicular development. These presumably represent the basic roles of Amh, which precede Müllerian duct evolution during phylogeny.
Keywords: gonad, sex differentiation, hotei mutant
Medaka, Oryzias latipes, is a small freshwater fish native to Japan and eastern Asia and has been cultivated in recent years as a genetically refined laboratory animal and model organism (1). An advantage of using the medaka in sexual differentiation studies is that the sex of this organism is primarily determined genetically, by the XX/XY chromosome system (2), which is analogous to mammals. In addition, the DMY/dmrt1bY gene on the Y chromosome of this fish species has been identified as the male-determining gene (3, 4), which further facilitates the investigation of gonadal and germ cell development in conjunction with sex differentiation. In both medaka and mammals, germ cells develop through various stages of mihotic proliferation and by entry into meiosis, reductive division, and also entry into a quiescent state awaiting specific fertilization signals that have different timings, depending on the sex. In females, germ cells begin to proliferate at around the hatching stage, and soon enter into meiosis and develop as oocytes. In males, germ cells remain in mihotic arrest for 2 weeks and initiate meiosis only 4 weeks after hatching. The sex of a normal medaka of a few weeks old can thus be judged by the abundance of germ cells in its gonads (5, 6).
In the developing gonad, multiple interactions occur between germ cells and somatic cells. Recent studies have also identified several genes that are essential for mammalian early gonadal development (7, 8). Anti-Müllerian hormone (Amh), also known as Müllerian inhibiting substance/factor (Mis/Mif), is one such gene and is a secreted intercellular signaling protein belonging to the TGF-β superfamily. Amh expression is restricted to the Sertoli cells in the testis and granulosa cells in the ovary (9–13). In amniotes, a hallmark function of Amh is to induce regression of the Müllerian ducts. Amh functions primarily through the anti-Müllerian hormone receptor type II (AmhrII) (14), a member of the type II receptors for the TGF-β -related protein family that possesses a single transmembrane domain and a serine/threonine kinase domain and functions in conjunction with a type I receptor of TGF-β superfamily (15–17). Bmpr1a (Alk3) has been shown to function as an AMH type I receptor (16). Interestingly, however, Amh is also expressed in lower vertebrates lacking a Müllerian duct (18–20), suggesting that there are evolutionally conserved functions of the Amh/Amhr signaling network, such as ovarian folliculogenesis and gonadal steroidogenesis.
Aromatase is an enzyme that is expressed in the follicular layer surrounding the oocytes in the ovary and catalyzes the conversion of androgen to estrogen (21). Although estrogen has been suggested to contribute to sexual differentiation of the gonads in lower vertebrates, the actual roles of aromatase and Amh in this process have not been fully clarified. The inhibition of the estrogen action results in the development of testicular structures that express amh (22), whereas Amh inhibits the aromatase-dependent estrogenic activity that is present in cultured fetal gonads (23, 24), suggesting that a reciprocal interaction occurs between these two pathways.
Recently, the medaka amhrII gene was identified, which represents the first characterization of this receptor in nonmammalian vertebrates (25). In fish, Wolffian duct is associated with the pronephros regardless of the sex, and sperm duct and oviduct are derived from the coelomic epithelium (26) analogous to amniote Müllerian duct. In medaka, amh and amhrII are expressed in somatic cells of the developing gonads identically between the sexes during larval and juvenile stages. These results suggest that the medaka amh and amhrII genes are involved in gonadal development including the regulation of germ cells in both sexes.
To further elucidate the genes involved in gonadal development, we performed a screen of medaka mutants displaying abnormal gonad development (27, 28). As a result of this analysis, we successfully identified a number of mutants showing gonadal defects (27), among which a mutant of particular interest was hotei (hot), causing excessive germ cell proliferation and male-to-female sex reversal. Here, we show that the hot mutation resides in the amhrII gene and thus reveal important functions of Amh signaling that have not been characterized in amniotes.
Results
hotei Homozygous Mutants Develop Hypertrophic Gonads.
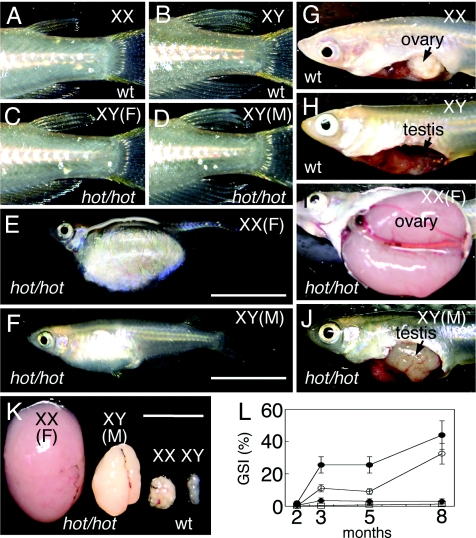
The hot mutation, which causes gonadal hypertrophy, behaves in an autosomal recessive manner [supporting information (SI) Fig. 7 and SI Table 2]. In wild-type fish, XX females and XY males are distinguishable by the shapes of their dorsal and anal fins (Fig. 1 A and B). Adult XX hot-homozygotes (hot/hot) show an enlarged abdomen (Fig. 1E). Adult XY hot-homozygous fish can be classified into two groups (Table 1), one-half of which have female characteristics (Fig. 1C) with an enlarged abdomen (as shown in Fig. 1E), and the remaining half of which show a male fin phenotype (Fig. 1D) with an abdominal enlargement (Fig. 1F), although this is less pronounced when compared with mutants of phenotypic females. The abdomens of the female hot homozygotes of either the XX or XY genotypes were occupied with hypertrophic ovaries (Fig. 1I) that had pushed the other internal organs against the abdominal wall. The hot homozygotes of XY males also display hypertrophic testis (Fig. 1J).
Fig. 1.
Adult hot-homozygous mutant phenotypes. Primary sexual phenotypes of hot homozygotes are indicated by F (female) and M (male) in parentheses with the sex chromosome composition. (A–D) Sex-dependent fin morphologies. (A) Wild-type XX female. (B) Wild-type XY male. Male fins have rougher edges and are larger than female fins. (C) Phenotypically female XY hot homozygote. (D) Phenotypically male XY hot homozygote. (E and F) External appearance of the hot-homozygous fish at 6 months. (Scale bar, 10 mm.) (E) XX hot homozygote, showing an expanded abdomen. (F) Phenotypically male XY hot mutant, with a less pronounced abdominal expansion. (G–J) Exposed gonads in wild-type and hot-homozygous fish. (G) Wild-type ovary. (H) Testis. (I) The abdominal cavity of a female hot homozygote occupied by a hypertrophic ovary. (J) Enlarged testis in a phenotypically male homozygote. (K) Comparisons of the gonads isolated from wild-type and hot homozygotes fish at 6 months; from left to right, XX hot ovary, XY hot testis, wild-type ovary and wild-type testis. (Scale bar, 10 mm.) (L) Changes in the gonad-somatic index (GSI = gonad weight/body weight in percentage) were plotted for wild-type and hot-homozygous mutant fish at 2, 3, 5, and 8 months after hatching. The averages measurements for at least four fish are indicated. Filled diamond, wild-type XX; open square, wild-type XY; filled circle, ovary-containing hot homozygotes of either XX or XY; open circle, XY hot homozygotes with testes.
Table 1.
Phenotypes of the hot homozygous fish at 6 months after hatching
| Genotype | XX | XY | |
|---|---|---|---|
| Fin phenotypes | Female | Female | Male |
| Gonad development | Ovary | Ovary | Testis |
| The number of fish | 35 | 14 | 13 |
At 3 months after hatching, wild-type medaka are sexually mature, and their gonads reach a maximum size. In contrast, the gonads of hot mutants were found to continue growing at 5 months after hatching (Fig. 1 K and L). Although most of the hot-homozygous mutants are sterile, a few female mutant fish were found to be fertile until 5 months of age, which contrasts with wild-type fish that show fertility for 1 year. Phenotypically, male hot-homozygous fish produced sperm, although the fertility of these fish is low. A fraction of the heterozygous males were also found to develop enlarged testis past 6 months after hatching. Very few of the hot homozygotes survive past one year, compared with the normal life span of around two years for wild-type medaka.
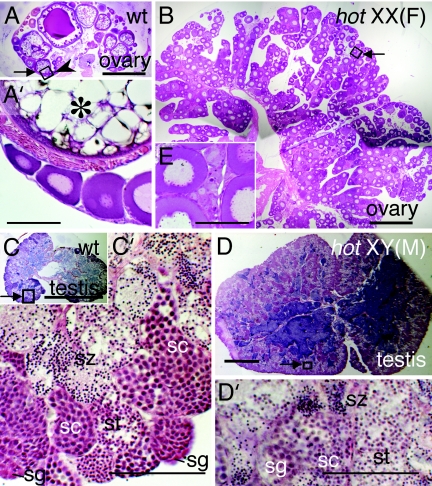
Wild-type mature ovaries during the spawning period of the medaka contain follicles at various developmental stages, from previtellogenic to fully grown (Fig. 2 A and A′). In contrast to wild-type, the hypertrophic ovaries of XX and XY female hot homozygotes are filled with small follicles (Fig. 2 B and B′). Thus, in hot homozygotes the germ cells continue to increase, whereas follicular development is arrested during early vitellogenesis, and these features can be observed even at 6 months after hatching. In wild-type testes, spermatogonia are located in the most peripheral regions of the lobule structures, and spermatogenesis proceeds synchronously within the cysts in these lobules (Fig. 2 C and C′). In the male-type XY hot mutants, hypertrophic testes develop that are filled with germ cells and sperm at various developmental stages (Fig. 2 D and D′). In a minor fraction of XY hot homozygotes, the gonads contained both testicular and ovarian components (see Fig. 6E).
Fig. 2.
Histological sections of gonads from wild-type and hot-homozygous fish at 6 months after hatching. The specimens are sections of the tissues shown in Fig. 1K stained with hematoxylin-eosin. (A) Wild-type ovary. (B) XX hot-homozygous ovary. The arrowhead in A (enlarged in A′) indicates previtellogenic follicles, with which the immature follicles abundant in B share a similar appearance (enlarged in B′). An oocyte at the yolk formation stage is indicated by an asterisk in A′. (C) Wild-type testis. (C′) Enlargement of the boxed area in C (arrow), where spermatogonia (sg), spermatocytes (sc), spermatids (st), and spermatozoa (sz) are labeled according to the morphological criteria (39). (D′) Enlargement of the boxed area in D (arrow), showing sp, sc, st and sz. [Scale bars, 0.5 mm (A–D); 0.1 mm (A′–D′).]
Fig. 6.
Expression of aromatase transcripts in both wild-type and homozygous hot medaka. Wild-type (A and D) and hot-homozygous (B, C, and E) larvae at 20 dph were hybridized with an aromatase probe. Aromatase signals are detectable in the wild-type ovary (A) and in the ovarian-type gonads of the hot-homozygous mutant (B). (A′ and B′) Shown are sections of the gonads in A and B, respectively, demonstrating expression of aromatase in the somatic cells near the ocytes (arrows) in both wild type (A′) and hot homozygotes (B′). Aromatase signals are not detected in the gonads of XY hot homozygotes with testicular development (C) or XY wild-type (D). In XY hot-homozygous gonads possessing both ovarian and testicular components (E), aromatase signals are detected only in the region of somatic cells that surround the oocytes (arrows). (F) RT-PCR analysis of aromatase in the gonads and vitellogenin in the liver in adult fish (6 months old). Both vitellogenin and aromatase are expressed in the phenotypically female fish (i.e., wild-type XX, XX hot-homozygotes, and XY hot-homozygotes, showing the development of ovaries), but not in phenotypically male fish. [Scale bars, 0.5 mm (A–C); 0.1 mm (A′–C′, D, and E).]
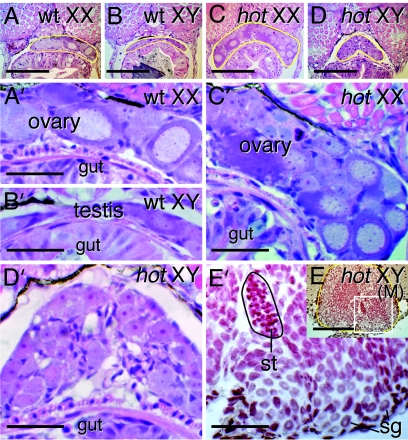
In hot homozygotes, the germ cells had already increased their number at 20 days post hatching (dph), analogous to normal ovary, and regardless of ovarian or testicular development, hypertrophy of the gonad occur during the posthatching period. The increasing number of germ cells is illustrated by Fig. 3 A–E, which show gonads of hot-homozygous larvae at 20 dph that contain more abundant germ cells compared with wild type, regardless of the XX or XY genotypes. Even in the XY hot fish, the germ cells within the gonads rapidly increase their number and start meiosis by 20 dph regardless of the sex of the organs (Fig. 3 D and E). This contrasts markedly with wild-type testis, where meiosis does not commence until ≈4 weeks after hatching. Furthermore, in the wild-type medaka, the germ cells are maintained in a thin layer in the gonads (Fig. 3 A and B). In contrast, the gonads of both XX and XY hot homozygotes were expanded in all dimensions, with more abundant and disorganized germ cells.
Fig. 3.
Histology of wild-type and hot-homozygous mutant larvae. (A–E) Sections of 20-dph larvae. The gonads are encircled by a yellow line. (A) Wild-type XX. (B) Wild-type XY. (C) XX hot/hot. (D) An XX hot/hot, showing hyperplasic and undifferentiated gonad. (E) An XY hot/hot gonad showing exclusively testicular development. (Scale bars, 0.1 mm.) (A′–E′) Enlargement of the gonadal area in A–E. (Scale bars, 0.05 mm.) In XY hot testis (E′, showing enlargement of the boxed area in E), the germ cells have prematurely initiated meiosis at this larval stage. The area encircled by a black line indicates a cluster of spermatid-like cells. sg, spermatogonia-like cells. Hematoxylin-eosin staining is shown in A–D, and neutral red staining is shown in E.
Identification of hot as an amhrII Gene Mutation by Positional Cloning.
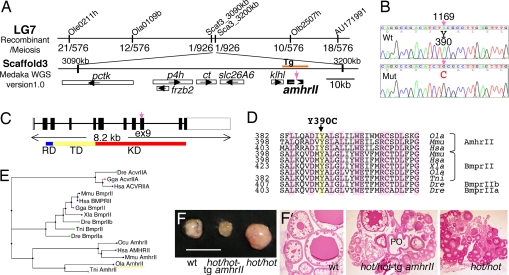
We performed positional cloning to characterize the specific gene that is mutated in the hot strains of medaka. This mutation was mapped to linkage group 7, based on its cosegregation with the Cab-line (southern strain)-associated polymorphic markers against Kaga (northern strain), within the 4 cM interval flanked by the EST markers Ola0109b and Olb2507h (Fig. 4A). Chromosomal walking was carried out by using these markers, and the position of the hot mutation was narrowed down to scaffold 3 [Medaka Whole-Genome Shotgun (WGS) sequencing project (http://medaka.utgenome.org), version 1.0]. Possible candidate genes for the hot mutation on this scaffold were then examined by sequencing, and this subsequently identified an A-to-G mutation in exon 9 of the recently identified amhrII gene (25) (Fig. 4 B and C). Cloning of the full-length amhrII cDNA confirmed that this gene consists of 11 exons and encodes a 492-aa-long Ser/Thr receptor kinase (Fig. 4C). The hot mutation causes a Cys substitution at the Tyr residue at position 390 in the kinase domain, which is a highly conserved amino acid among the type II receptors (Fig. 4 D and E).
Fig. 4.
Characterization of the hot mutation. (A) Genetic mapping of the hot mutation using polymorphic markers. Genetic map of medaka linkage group 7, indicating the recombination frequency of the hot mutation with markers that place this mutation in Scaffold 3 [Medaka Whole-Genome Shotgun (WGS) sequencing project, version 1.0]. Genes: pctk, PCTAIRE-motif protein kinase; p4h, prolyl-4-hydroxylase; ct, creatine transporter; slc26A6, solute carrier family 26, member 6; klhl, kelch-like protein. (B) A missense mutation (TAC to TGC) has occurred in exon 9 of the amhrII gene, causing an amino acid substitution (Tyr to Cys) in the intracellular kinase domain. (C) Schematic representation of the amhrII gene and the position of the hot mutation. RD, activin types I and II receptor domain; TD, transmembrane domain; KD, serine/threonine protein kinase domain. The hot mutation in amhrII gene exon 9 is indicated by a vertical arrow. (D) Comparisons of the deduced amino acid sequences of the medaka and mammalian AmhrII, and of the BmprII proteins of various vertebrate species. Note that the mutated Tyr residue in hot is conserved among the type II receptors and across the species shown. (E) Phylogenetic relationship between the medaka AmhrII with other type II receptors of the TGF-β superfamily. Dre, Danio relio (zebrafish); Gga, Gallus gallus (chicken); Hsa, Homo sapiens (human); Mmu, Mus musculus (mouse); Xla, Xenopus laevis; Tni, Tetraodon nigroviridis (pufferfish); Ocu, Oryctolagus cuniculus (rabbit); Ola, O. latipes (medaka). (F) Rescue of the hot-homozygous phenotype by expression of a wild-type amhrII transgene. The hyperproliferation of germ cells in the ovary was inhibited in the transgenic fish at 3 months of age. (Left) Wild-type ovary. (Center) hot/hot XX fish harboring the amhrII transgene. (Right) hot/hot XX fish. (Scale bar, 10 mm.) (F′) Sections of the ovaries shown in F. In the hot/hot XX fish carrying the amhrII transgene, the number of growing follicles is reduced compared with the hot-homozygous ovary, and postovulatory follicle is also observed (PO).
To confirm that the Y390C mutation in exon 9 of the amhrII gene is responsible for the hot phenotypes, a 12-kb region derived from HNI (northern inbred line) genome (nucleotide positions 3172–3184 kb in scaffold 3; represented in Fig. 4A as Tg) was used as a transgene and injected into fertilized eggs of hot heterozygous parents. Among the progeny of the F1 generation, two fish were identified as hot-homozygous females harboring this amhrII transgene. In both of these transgenic homozygotes, hypertrophy of the ovary was suppressed at 3 months after hatching (Fig. 4F). The GSI (gonad weight/body weight percentage) was calculated as 2.3% and 5.0% in these transgenic fish, whereas it was measured as 25.6% (20.3–30.0%) and 3.3% (0.6–6.1%) in a hot-homozygous and wild-type female, respectively (Fig. 1L). Furthermore, histological analysis indicated that the follicular development had been largely recovered in the transgenic fish (Fig. 4F′). These data further confirm that hot corresponds to a mutation in the amhrII gene.
Gonad-Restricted Expression of the amhrII Gene.
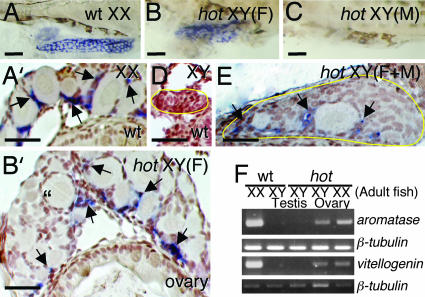
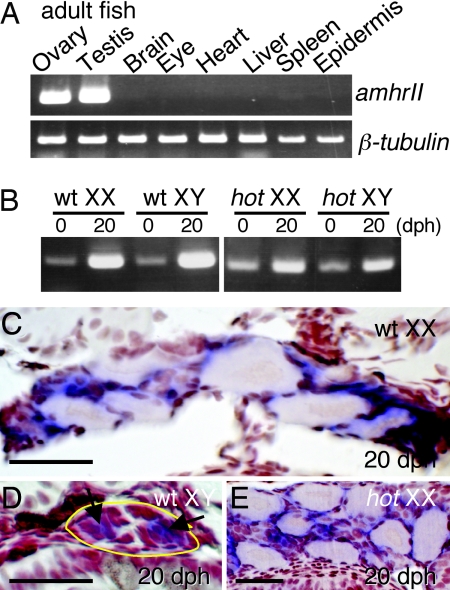
To correlate the defect of AmhrII with the hot-homozygous phenotype, the tissue specificity of the expression of amhrII was investigated by RT-PCR and in situ hybridization. In adult fish, amhrII was found to be specifically expressed in the ovary and testis (Fig. 5A). In larvae, amhrII is also expressed in the gonads of both wild-type and hot homozygotes, regardless of their sex (Fig. 5B). In situ hybridization further indicated that amhrII is expressed in the somatic cells around the germ cells (Fig. 5 C–E). In addition, the amh gene, which encodes the ligand for AmhrII, has been reported to be expressed in the cells around the germ cells of wild-type fish (25, 29), which is also true for hot homozygotes, regardless of their sex genotype (data not shown).
Fig. 5.
Expression of amhrII in adult and developing larvae. (A) RT-PCR analysis of the tissue distribution of amhrII expression in adult medaka, using β-tubulin as control, showing restricted expression of amhrII in the ovary and testis. (B) Expression of amhrII in wild-type and hot-homozygous larvae, analyzed by RT-PCR. Regardless of the genetic sex, amhrII is expressed in both wild-type and hot homozygotes during the period between 0 and 20 dph. (C–E) In situ hybridization of the amhrII transcripts in cross sections of the gonads in 20-dph larvae. AmhrII expression is detectable in the somatic cells (arrows) surrounding the germ cells in the gonads of either phenotypic sex. The gonadal regions are enclosed with a yellow line in D. (C) Wild-type XX. (D) Wild-type XY. (E) XX hot/hot. (Scale bars, 0.1 mm.)
Phenotypic Sex and Expression of Sex-Related Genes in hot-Homozygous Mutants.
Because a significant fraction of the hot-homozygous XY fish examined in our experiments show reversal of their secondary sex characteristics, the expression of the estrogen-synthesizing aromatase gene was examined in these animals. In wild-type larvae, aromatase is highly expressed in the female XX ovary (Fig. 6 A and A′), but not in XY male testis (Fig. 6D) at 20 dph. In hot-homozygous larvae, the expression of aromatase is detectable in both XX and XY ovary-type gonads (Fig. 6 B and B′, and data not shown), whereas no signal can be observed in XY testis-type gonads by using whole mount specimens (Fig. 6C) or cross sections (data not shown). In the XY hot, gonads of either female-type development or female/male mixed development, aromatase was expressed only in the somatic cells around the oocytes (Fig. 6 B and E).
vitellogenin gene was also found to be expressed in the liver of phenotypically female adult fish, even those with XY chromosomes (Fig. 6F). Continuous incorporation of vitellogenin from the circulating serum results in the growth of large follicles in the normal ovary. However, in the adult hot-homozygous ovary, most follicles do not progress beyond the early stages of their development and do not accumulate vitellogenin, which indicates that the defects in Amh signaling cause multiple abnormalities associated with the functions of follicular cells in addition to the dysregulation of germ cell proliferation.
Discussion
hotei homozygous medaka mutants show remarkable phenotypes, including (i) excessive proliferation of germ cells that initiates soon after hatching regardless of the phenotypic sex of the fish; (ii) initiation of premature meiosis in phenotypically male hot homozygotes; (iii) sex reversal in 50% of the hot-homozygous XY fish, which accompanies the expression of aromatase in the somatic cells of the gonad; and (iv) growth arrest at an early stage of follicular development, despite vitellogenin expression in the liver. We also demonstrate in our current study that the hot mutation is a critical amino acid substitution (Y390C) in the amhrII gene, and that all of the phenotypes that arise in the corresponding mutant fish can be ascribed to the consequences of this defect on Amh/AmhrII signaling. Recessive Mendelian transmission of the mutation and the rescue of the mutant phenotype by the amhrII transgene suggest that the hot mutation caused loss of function of AmhrII.
The germ cells in normal female gonads initiate successive proliferative events to produce cystic clusters at the time of hatching, which results in the abundance of germ cells in females. Some populations of these clusters subsequently enter meiosis (D.S. and M.T., unpublished data). By contrast, in the normal wild-type male medaka, such successive proliferation does not occur until 4 weeks after hatching. It is possible, therefore, that Amh/AmhrII signaling regulates these successive proliferations and consequently that mutation of the amhrII gene causes unrestrained proliferation in males with a similar timing to that of the females. In this context, the phenotypically female hot homozygotes also show uncontrolled proliferation of germ cells and large increases in the germ cell number.
Of interest are the downstream effects of the disrupted Amh signaling in the hot homozygotes. The follicular cells in hot mutants express the gene for aromatase that catalyzes the synthesis of estrogen in the somatic cells around the developing oocytes, regardless of its genetic sex (Fig. 6). It had been demonstrated that estrogen promotes the hepatic synthesis of vitellogenin, and we subsequently confirmed vitellogenin expression in the liver of female hot homozygotes. In hot mutants, the growth of follicles is arrested, despite the occurrence of aromatase-dependent vitellogenin synthesis, suggesting that the vitellogenin uptake by the follicular cells (30) is affected.
Although the “anti-Müllerian duct” effects of Amh have been primarily emphasized in higher vertebrates, this protein is a phylogenetically conserved signaling molecule and has been identified in many teleost fish species that lack a Müllerian duct (18–20, 25, 29). The phenotypes of the hot homozygotes indicate that there are conserved functions of Amh/AmhrII signaling that are distinct from its role in Müllerian duct regression. Indeed, Amh was initially reported as a spermatogenesis preventing substance (eSRS21), because it shows inhibitory activity toward the initiation of this process in cocultures of germ cells and somatic cells (18). A possibly related observation is that mammalian Amh also inhibits the proliferation of both Leydig cells (12, 13) and ovarian cancer cells (31). Amh has also been implicated in male sex differentiation, female follicular development, and also steroidogenesis in both sexes (9–13). In mammals, the lack of AMH signaling as manifested in Amh knockout mice and human patients causes precocious folliculogenesis (32, 33), in contrast to the defect of folliculogenesis in hot homozygotes described here. The mechanism underlying this difference is not clear at this point; however, the mouse model with the disrupted amhrII gene demonstrates male internal pseudohermaphroditism, having both male and female reproductive organs (34), again indicating analogy with the cases of hot-homozygous XY fish.
Because one half of the XY hot homozygotes develop sperm in their gonads, Amh/AmhrII signaling may not be a component of the major sex differentiation pathways in medaka. It is also possible that, in the hot mutants, signals that are derived from the somatic cells under the control of DMY/dmrt1bY and that masculinize the germ cells are not transmitted efficiently to the proliferating germ cells. In the gonad of female-dominant mixed phenotype XY hot homozygotes, aromatase is expressed only in the region of gonadal somatic cells where germ cells undergo folliculogenesis (Fig. 6E). The feminization of germ cells may then release the somatic cells from the repression of aromatase expression that is exerted by DMY/dmrt1bY. In zebrafish (35) and chicken (36), amh expression commences earlier than that of other genes, such as sox9, and its sexual dimorphic expression is not apparent during this early stage. Sexually dimorphic expression of the amh gene may therefore be a consequence of the sexual differentiation of the gonad that takes place first. How such species-dependent variations in the association of the Amh/AmhrII system with male development are coupled with sex-differentiation mechanisms is an interesting issue that is deserving of further study.
In conclusion, the multifaceted gonad- and sex-associated phenotypes of the hotei medaka mutant have further elucidated the central functions of the Amh/AmhrII system during gonadal development. This also underscores the value of focused mutant screening in vertebrates and demonstrates the advantage of the medaka as a model system in genetic studies.
Materials and Methods
Genetic Mapping and Gene Cloning.
The hotei mutation was induced in the Cab-Kyoto line (called “tot” in refs. 27 and 28). The Kaga-Kyoto line (28) was used for polymorphic marker-based genetic mapping.
Genetic mapping and chromosome walking were performed essentially as described by Geisler (37), using restriction fragment length polymorphism markers [medaka linkage map database (ML Base), http://mbase.bioweb.ne.jp/∼dclust/ml_base.html] and markers designed based on information from the Tetraodon Genome Browser (www.genoscope.cns.fr/externe/tetranew) and Medaka University of Tokyo Genome Browser (http://medaka.utgenome.org) databases. To identify the mutation, genomic DNA was amplified by PCR, using primers designed from the information in the University of Tokyo Genome Browser. This database was also used to predict introns and exons. The central fragment of the amhrII cDNA was isolated by RT-PCR from adult testes, and full-length cDNA was obtained by 5′ RACE and 3′ RACE. Phylogenic distance was calculated by using BLAST Tree View Widget (National Center for Biotechnology Information).
Transgene Rescue of the hot-Homozygous Phenotype.
The genomic fragment containing the entire amhrII gene plus klhl 3′ UTR was excised from BAC clone Mn0073N15 derived from the HNI line, and placed in the pCRII–TOPO vector (Invitrogen, Carlsbad, CA). The klhl sequence in the hot mutants contained no mutation. Fertilized eggs of hot heterozygous parents were injected with the DNA (6) and reared (28). Three hot-homozygous and two heterozygous fish carried the HNI-derived amhrII transgene. These G0 fish were mated with hot heterozygotes, and two progeny fish identified as the transgene-carrying hot homozygotes were examined for the phenotype.
RT-PCR and In-Whole Mount in Situ Hybridization.
RT-PCRwas performed by using the following primers: amhrII forward 5′-GTTGTGGGACAAGGACACTTTGCC-3′ and reverse 5′-CAGGATCTCAGGAGACATGTAGTGGG-3′; Aromatase forward 5′-CTGTGCTCATTGTTGCTTG-3′ and reverse 5′-CGTAAGACAAACTGCTCAGG-3′; Vitellogenin forward 5′-TCTGAGAGGTTCAACATCATACAG-3′ and reverse 5′-GCGTATCTCTGTACTTGCTCC-3′. The RT-PCR-derived sequences were also used as a probe for in situ hybridization (27, 38). The amhrII sequence, corresponding to to exons 6–9, is highly diversified from other TGFβ-family receptors (the closest being bmprII with 55% sequence identity), ruling out cross-hybridization with other type II receptor sequences.
Supplementary Material
Acknowledgments
We thank the Kondoh Team members, in particular Y. Osada and T. Sasado, for their support. This study was carried out as a part of the Exploratory Research for Advanced Technology/Solution Oriented Research for Science and Technology program of the Japan Science and Technology Agency. This work was supported by Grants-in-Aid for Scientific Research 17052027 and 17370083 (to M.T.) and 8570064 (to D.S.) and a National Institute for Basic Biology collaborative research program.
Abbreviations
- Amh
anti-Müllerian hormone
- Amhr
anti-Müllerian hormone receptor
- dph
days post hatching.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. A.M. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611379104/DC1.
References
- 1.Wittbrodt J, Shima A, Schartl M. Nat Rev Genet. 2002;3:53–64. doi: 10.1038/nrg704. [DOI] [PubMed] [Google Scholar]
- 2.Aida T. Genetics. 1921;6:554–573. doi: 10.1093/genetics/6.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, et al. Nature. 2002;417:559–563. doi: 10.1038/nature751. [DOI] [PubMed] [Google Scholar]
- 4.Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z, Haaf T, Shimizu N, Shima A, et al. Proc Natl Acad Sci USA. 2002;99:11778–11783. doi: 10.1073/pnas.182314699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanamori A, Nagahama Y, Egami N. Zoolog Sci. 1985;2:695–706. [Google Scholar]
- 6.Tanaka M, Kinoshita M, Kobayashi D, Nagahama Y. Proc Natl Acad Sci USA. 2001;98:2544–2549. doi: 10.1073/pnas.041315498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilmann C, Capel B. Recent Prog Horm Res. 2002;57:1–18. doi: 10.1210/rp.57.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Park SY, Jameson JL. Endocrinology. 2005;146:1035–1042. doi: 10.1210/en.2004-1454. [DOI] [PubMed] [Google Scholar]
- 9.Jost A. Recent Prog Horm Res. 1953;8:379–418. [Google Scholar]
- 10.Behringer RR, Cate RL, Froelick GJ, Palmiter RD, Brinster RL. Nature. 1990;345:167–170. doi: 10.1038/345167a0. [DOI] [PubMed] [Google Scholar]
- 11.Lee MM, Donahoe PK. Endocr Rev. 1993;14:152–164. doi: 10.1210/edrv-14-2-152. [DOI] [PubMed] [Google Scholar]
- 12.Behringer RR, Finegold MJ, Cate RL. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 13.Josso N, Racine C, di Clemente N, Rey R, Xavier F. Mol Cell Endocrinol. 1998;145:3–7. doi: 10.1016/s0303-7207(98)00186-5. [DOI] [PubMed] [Google Scholar]
- 14.Mishina Y, Whitworth DJ, Racine C, Behringer RR. Endocrinology. 1999;140:2084–2088. doi: 10.1210/endo.140.5.6705. [DOI] [PubMed] [Google Scholar]
- 15.Josso N, di Clemente N, Gouedard L. Mol Cell Endocrinol. 2001;179:25–32. doi: 10.1016/s0303-7207(01)00467-1. [DOI] [PubMed] [Google Scholar]
- 16.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 17.Visser JA. Mol Cell Endocrinol. 2003;211:65–73. doi: 10.1016/j.mce.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Miura T, Miura C, Konda Y, Yamauchi K. Development (Cambridge, UK) 2002;129:2689–2697. doi: 10.1242/dev.129.11.2689. [DOI] [PubMed] [Google Scholar]
- 19.Yoshinaga N, Shiraishi E, Yamamoto T, Iguchi T, Abe S, Kitano T. Biochem Biophys Res Commun. 2004;322:508–513. doi: 10.1016/j.bbrc.2004.07.162. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Mari A, Yan YL, Bremiller RA, Wilson C, Canestro C, Postlethwait JH. Gene Expr Patterns. 2005;5:655–667. doi: 10.1016/j.modgep.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Simpson ER, Davis SR. Endocrinology. 2001;142:4589–4594. doi: 10.1210/endo.142.11.8547. [DOI] [PubMed] [Google Scholar]
- 22.Vaillant S, Magre S, Dorizzi M, Pieau C, Richard-Mercier N. Dev Dyn. 2001;222:228–237. doi: 10.1002/dvdy.1190. [DOI] [PubMed] [Google Scholar]
- 23.di Clemente N, Ghaffari S, Pepinsky RB, Pieau C, Josso N, Cate RL, Vigier B. Development (Cambridge, UK) 1992;114:721–727. doi: 10.1242/dev.114.3.721. [DOI] [PubMed] [Google Scholar]
- 24.Nishikimi H, Kansaku N, Saito N, Usami M, Ohno Y, Shimada K. Mol Reprod Dev. 2000;55:20–30. doi: 10.1002/(SICI)1098-2795(200001)55:1<20::AID-MRD4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 25.Klüver N, Pfennig F, Pala I, Storch K, Schlieder M, Froschauer A, Gutzeit HO, Schartl M. Dev Dyn. 2007;236:271–281. doi: 10.1002/dvdy.20997. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki A, Shibata N. Zoolog Sci. 2004;21:397–406. doi: 10.2108/zsj.21.397. [DOI] [PubMed] [Google Scholar]
- 27.Morinaga C, Tomonaga T, Sasado T, Suwa H, Niwa K, Yasuoka A, Henrich T, Watanabe T, Deguchi T, Yoda H, et al. Mech Dev. 2004;121:829–839. doi: 10.1016/j.mod.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 28.Furutani-Seiki M, Sasado T, Morinaga C, Suwa H, Niwa K, Yoda H, Deguchi T, Hirose Y, Yasuoka A, Henrich T, et al. Mech Dev. 2004;121:647–658. doi: 10.1016/j.mod.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura S, Kobayashi D, Aoki Y, Yokoi H, Ebe Y, Wittbrodt J, Tanaka M. Dev Biol. 2006;295:678–688. doi: 10.1016/j.ydbio.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 30.Stifani S, Barber DL, Nimpf J, Schneider WJ. Proc Natl Acad Sci USA. 1990;87:1955–1959. doi: 10.1073/pnas.87.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ha TU, Segev DL, Barbie D, Masiakos PT, Tran TT, Dombkowski D, Glander M, Clarke TR, Lorenzo HK, Donahoe PK, Maheswaran S. J Biol Chem. 2000;275:37101–37109. doi: 10.1074/jbc.M005701200. [DOI] [PubMed] [Google Scholar]
- 32.Durlinger AL, Visser JA, Themmen AP. Reproduction. 2002;124:601–609. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 33.Visser JA, de Jong FH, Laven JS, Themmen AP. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 34.Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, Behringer RR. Genes Dev. 1996;10:2577–2587. doi: 10.1101/gad.10.20.2577. [DOI] [PubMed] [Google Scholar]
- 35.von Hofsten J, Larsson A, Olsson PE. Dev Dyn. 2005;233:595–604. doi: 10.1002/dvdy.20335. [DOI] [PubMed] [Google Scholar]
- 36.Oreal E, Pieau C, Mattei MG, Josso N, Picard JY, Carre-Eusebe D, Magre S. Dev Dyn. 1998;212:522–532. doi: 10.1002/(SICI)1097-0177(199808)212:4<522::AID-AJA5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 37.Geisler R. Zebrafish: A Practical Approach (The Practical Approach Series, 261) New York: Oxford Univ Press; 2002. pp. 175–212. [Google Scholar]
- 38.Oliver G, Loosli F, Koster R, Wittbrodt J, Gruss P. Mech Dev. 2002;60:233–239. doi: 10.1016/s0925-4773(96)00632-6. [DOI] [PubMed] [Google Scholar]
- 39.Schulz RW, Menting S, Bogerd J, Franca LR, Vilela DA, Godinho HP. Biol Reprod. 2005;73:891–898. doi: 10.1095/biolreprod.105.039891. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.