Abstract
Interstitial cystitis/painful bladder syndrome is a disease seen mostly in women, and symptoms tend to be worse premenopausally or during ovulation. The four cardinal symptoms of interstitial cystitis/painful bladder syndrome are bladder pain, urgency, frequency, and nocturia. Estrogen has been implicated in the etiology of this disease, but the role of the two estrogen receptors (ER), ERα and ERβ, has not been investigated. We found that, in the bladders of WT mice, ERβ is expressed in the basal cell layer of the urothelium. Bladders of male ERβ−/− mice were intact and morphologically indistinguishable from those of their WT littermates. However, in female ERβ−/− mice, there was ulceration and atrophy of bladder urothelium concomitant with infiltration of γδ T cells concentrated in the areas of atrophy and shedding of urothelium. The data support the idea that activated γδ T cells are causing the damage to the urothelium. The hyperactivity of T cells may be because of an imbalance between ERα and ERβ signaling in female ERβ−/− mice. Our data suggest that reduced ERβ signaling might have a role in the pathogenesis of interstitial cystitis, and ERβ could be a candidate for a target of medical therapy.
Keywords: γδ T cells, urothelium, painful bladder syndrome
The etiology of interstitial cystitis (IC) is unknown, and available treatment options are limited and mostly palliative. There is a clear role for the immune system in this disease: increased numbers of T lymphocytes, particularly γδ T cells, abnormal urothelial HLA, and overexpression of HLA class II (1) in the urothelium and lamina propria in patients with IC strongly suggest a role for autoimmunity in development of IC (2). Because IC is almost exclusively a disease of young women, and symptoms tend to worsen premenopausally or during ovulation, the role of estrogen receptors (ERs) is likely to be important.
The bladder is one of those tissues that were long thought to be nonestrogen-responsive on the basis of the lack of ERα expression. The discovery of the second ER, ERβ, provided an explanation for the effects of estrogen in several ERα-negative tissues such as the prostate, intestine, and lung, where ERβ serves as a modulator of terminal differentiation of epithelium (3–7). ERβ and ERα knockout mice (ERβ−/− and ERα−/−) have been invaluable in analyzing and dissecting the functions of ERβ and ERα (8, 9).
The predominant ER in the bladder is ERβ, with very little ERα expression (10, 11). Reduced ERβ expression levels have been reported in the bladders of rats with chemically induced cystitis (12). In the present study, with the use of ERβ−/− mice, we have investigated the role of ERβ in the bladder urothelium. We report that, in female ERβ−/− but not in ERα−/− mice, there is autoimmune destruction of urothelium resembling IC.
Results
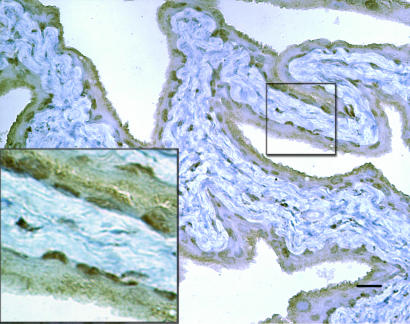
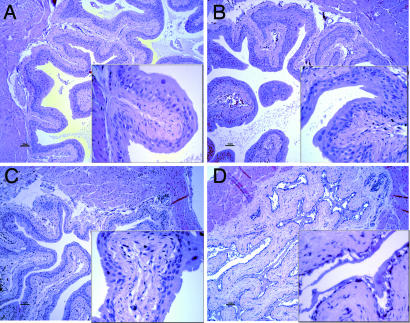
Immunohistochemical staining of WT mouse bladder sections showed that ERβ is expressed in bladder urothelium and localized in the basal cell layer (Fig. 1). In the bladders of female ERβ−/− mice, there were specific morphological changes, i.e., ulceration and atrophy of bladder urothelium. A comparison of the differences in morphology between ERβ−/− mice and their WT littermates is shown in Fig. 2. At 8 months of age, there are epithelial atrophy, massive ulcerations of the bladder, and invasion of immune cells in the stroma and epithelium in ERβ−/− mice. Bladders of male ERβ−/− mice were intact and morphologically indistinguishable from their WT littermates. The morphological changes became evident when mice were 6 weeks of age, which corresponds to the time of puberty in mice. There was progressive destruction of the urotheluim with age.
Fig. 1.
ERβ expression in female bladder section. Note positive nuclei in basal layer of cells. (Magnifications: main image, ×20; Inset, ×60.)
Fig. 2.
Hematoxylin/eosin staining of male and female bladders sections of ERβ−/− and WT mice. (A) WT male. (B) ERβ−/− male. (C) WT female. (D) ERβ−/− female. Note massive ulceration of urothelium. (Magnifications: A–D, ×20; Insets, ×60.)
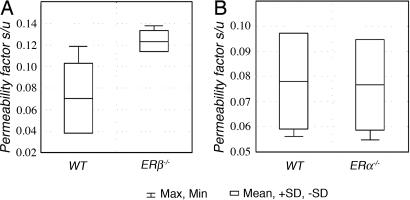
Bladder permeability assessed by measuring the serum/urine concentration coefficient of sodium fluorescein after it had been noninvasively instilled into the bladder revealed a significantly higher bladder permeability in female ERβ−/− mice (0.12 ± 0.009) than in WT littermates (0.07 ± 0.03; P = 0.01). Bladder permeability in ERα−/− female mice was not different from that of their WT littermates (Fig. 3).
Fig. 3.
Permeability of female bladders for sodium fluorescein. (A) WT vs. ERβ−/−mice. (B) WT vs. ERα−/− mice.
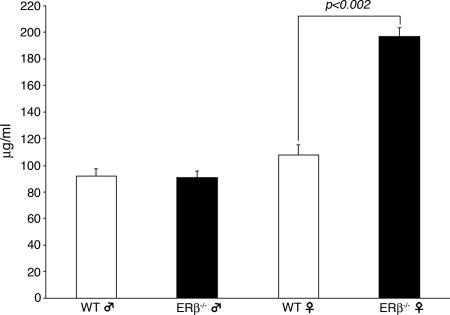
There was no difference between ERβ−/− and WT male mice in the total glucosaminoglycan (GAG) content of urine (male ERβ−/− mice, 90.7 ± 5.11 μg/ml vs. WT littermates, 92.1 ± 5.48 μg/ml). However, in female ERβ−/− mice, total GAG content in urine was significantly higher (197 ± 6.7 μg/ml) than in WT littermates (108 ± 7.3 μg/ml, P < 0.002) (Fig. 4).
Fig. 4.
Total urinary GAG concentration in male and female ERβ−/− (filled bars) and WT (open bars) mice.
Expression of p63, a marker for basal urotheliocytes, and Ck20, a marker for terminally differentiated umbrella cells, were similar in ERβ−/− and WT female mice (data not shown), and no differences in morphology or distribution of GAG layer on the apical side of urotheliocytes were found by electron microscopy (data not shown).
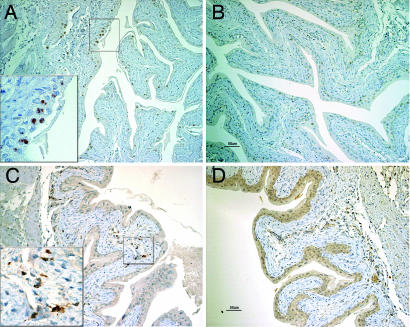
However, upon immunohistochemistry with antibodies GL-3 and F4/80, it became evident there was severe infiltration of γδ T cells and macrophages in the areas of atrophy and shredding of bladder urothelium in ERβ−/− compared with WT mice (Fig. 5 A and B). The difference between ERβ−/− and WT mice was observed in both 3.5- (data not shown) and 7-month-old-mice (Fig. 5 A and B). High numbers of F4/80-positive cells (Fig. 5C) were found in the areas of bladder lamina propria in ERβ−/− 3.5-month-old mice, indicating macrophage infiltration. In 7-month-old mice, there was no difference in numbers of F4/80-positive cells (data not shown).
Fig. 5.
T cells and macrophage infiltration of ERβ−/− female bladders. Infiltration of urothelium by γδ T cells in ERβ−/− (A) and WT (B) mice. Bladder sections from 7-month-old ERβ−/− (A) and WT mice (B) were stained with γδ TCR and sections from 3.5-month-old ERβ−/− (C) and WT mice (D) were stained with F4/80 antibodies and counterstained with hematoxylin. (Magnifications: A–D, ×20; Insets, ×60.)
Discussion
In this study, we have demonstrated morphological changes in the bladders of female ERβ−/− mice, which bear a strong resemblance to bladders of patients with IC.
We based our project on the Parsons hypothesis of IC pathogenesis, where GAG layer protects the epithelium against the urine (13). Because ERβ has been shown to be necessary for terminal differentiation in the prostate, mammary gland and colon, we hypothesized that, in the absence of ERβ signaling, bladder urothelium could have an altered differentiation, which could cause alterations in GAG production and subsequently failure of the bladder wall to protect against the hostile environment of urine.
We found that in males, there was no difference between the genotypes in the total GAG content in bladders (Fig. 4). However, in females, the amount of GAG in urine was higher in ERβ−/− that in WT littermates. This finding was somewhat puzzling, because the concentration of GAG have been reported to be reduced in IC (14). Subsequent analysis of morphology of bladders of 8-month-old female ERβ−/− mice revealed atrophy and shredding of bladder urothelium (Fig. 2). This particular phenotype is reminiscent of a classical Hunner's ulcer seen in patients with severe forms of IC. The phenotype starts to develop at ≈6 weeks of age, which corresponds to mouse puberty.
In 8-month-old female ERβ−/− mice with manifested urothelial atrophy, bladder permeability is almost twice higher than in their WT littermates (Fig. 3). Bladder permeability was not affected by loss of ERα (in ERα−/− mice). No bladder permeability tests were done in male mice because of technical difficulties in performing bladder catheterization of males.
To evaluate the differentiation process of bladder urothelium, we used Ck20 as an established marker protein for terminally differentiated urothelium. In 5-week-old female mice, bladder expression of Ck20 was similar in ERβ−/− and WT mice. Thus, the differentiation of mouse urothelium, unlike prostatic epithelium, is not affected by the absence of ERβ (6). In contrast to the prostate, where ERβ is expressed in the differentiating epithelium, in the bladder, ERβ is predominantly expressed in the basal epitheliocytes. Its role in the bladder may not be regulation of differentiation. Because differentiation was not affected in ERβ−/− urothelium, GAG production was probably not reduced and could not account for the primary mechanism of urothelial destruction. The difference of GAG content in urine was perhaps attributed to the high amount of cellular debris in urine of ERβ−/− female mice.
In the bladders of 3.5-month-old mice, i.e., before the phenotype appeared, electron microscopic studies showed that the layer of GAGs on the apical side of urotheliocytes was even and equally well seen in ERβ−/− and WT mice. The number and distribution of high resistance tight junctions on the cell–cell membranes were similar in both genotypes. Thus the bladder epithelium in ERβ−/− female mice before the manifestation of phenotype appears to be well differentiated.
Because ERβ plays a role in the immune system, and one of the possible pathogenetic mechanisms of IC involves autoimmunity, we analyzed infiltrating immune cells in ERβ−/− and WT female bladders. The role of mast cells in IC has previously been investigated. Increased numbers of mast cells have been found in mucosa and lamina propria of the bladders of patients with IC, and mast cells have been suggested to contribute to the pathogenesis of IC (15, 16). However, using an experimental rat model of autoimmune IC, Luber-Narod et al. (17) found no statistical differences in the bladders between experimental animals and controls.
Human γδ T cells have also been demonstrated to be associated with IC (2). γδ T cells participate in the elimination of stressed or damaged cells. Killing of damaged cells by γδ T cells involves perforin/granzyme and Fas/Fas ligand-dependent pathways (18, 19). Evidence for a pathogenic role of γδ T cells in autoimmunity has been reported (20). Other studies suggest a regulatory role of γδ T cells in autoimmune responses. It has been shown that E2 administration enhances Ag-specific Th1 cell responses through ERα expression in hematopoietic cells (21). Most γδ T cells are biased toward Th1 responses (22). In our study, we observed that in ERβ−/− mice γδ T cells were concentrated in the areas of atrophy and shedding of urothelium (Fig. 5A). Our data suggest that γδ T cell are activated in ERβ−/− mice, and this response may be the cause of the damage to the urothelium. Our previous results indicated that ERβ is the predominant type of ER in lymphocytes (23), and negative effects of estrogen on the immune system may be mediated by ERβ (24). The disruption of the ERβ gene in ERβ−/− mice may cause abnormal activation of systemic and local regulatory immune response. Previous studies demonstrated that 28% of IC patients develop autoimmune exocrinopathy, Sjogren's syndrome (SS) (25). Our studies show that ArKO mice, which have complete loss of estrogen, spontaneously develop signs of lymphoproliferative autoimmunity, which particularly resembles SS (7).
The accumulation of activated macrophages is an important event in pathogenesis of autoimmune diseases and chronic infections. γδ T cells engage in regulatory interactions with macrophages and dendritic cells (26) and play a significant role in macrophage homeostasis. Up-regulation of macrophage cytokine production by γδ T cells during bacterial infection was recently demonstrated (27). In ERβ−/− mice, the increased recruitment of macrophages to lamina propria may be caused by activated γδ T cells. In our study, we have found high infiltration with both γδ T cells and macrophages in bladder of 3.5-month-old ERβ−/− female mice. It is possible that at this stage, γδ T cells and macrophages may act synergistically, damaging urothelium and lamina propria.
Several pieces of evidence indicate that IC, chronic pelvic pain, and abacterial prostatitis might share the same pathogenetic mechanism. It has been reported that up to 70% of men with chronic abacterial prostatitis have cystoscopic signs of IC (28, 29). There is a published hypothesis that links together prostatitis, IC, chronic pelvic pain, and urethral syndrome. In this report, the author postulates that these diseases share “dysfunctional urinary epithelium and potassium recycling” impairment (30). Furthermore, all these diseases have been reported to respond to phytoestrogens, such as sitosterols, quercetin, and genistein (31–33). Many phytoestrogens, like genistein, are better ligands for ERβ than ERα (34, 35). Because the morphological changes in female ERβ−/− bladders, described in the present study, resemble those seen in IC, one could speculate that ERβ might be a valuable target for pharmacological intervention in IC, chronic pelvic pain, and chronic abacterial prostatitis.
Materials and Methods
Animals were used in accordance with the guidelines for care and use of experimental animals issued by Stockholm's Södra Djurförsöksetiska Nämnd. Mice were fed a standard diet with ad libitum access to water. Mice were bred from heterozygous mice. Genotyping using PCR was performed on DNA isolated from the tails of 2-week-old mice, as described elsewhere. Mice were killed by cervical dislocation. For immunohistochemical studies, bladders were removed, rinsed in ice-cold phosphate-buffered saline (PBS), and fixed overnight in 4% buffered paraformaldehyde. The bladders were routinely embedded in paraffin, cut in 4-μm sections, and subsequently mounted on organosilane-coated slides.
Detection of GAG Content in Urine.
Animals were killed as described above, and the urine was collected by puncturing the bladder wall. The total GAG content of urine was obtained by using the Di Ferrante (36) method, modified by Goldberg and Cotlier (37) and Pennock et al. (38). The analysis procedure was done as described by Panin et al. (39).
One percent hexadecyltrimethylammonium bromide (CTAB) (Sigma, St. Louis, MO; no. H9151) was mixed with an equal volume of urine, incubated at room temperature for 2 h, and centrifuged at 20,000 × g for 20 min at room temperature. Supernatant was discarded and tubes drained upside down on a filter paper. A thin layer of CTAB-GAG was dissolved by 1 ml of 60% isopropanol. The remaining CTAB-GAG undissolved in isopropanol was taken by rinsing with 8 ml of ethanol, containing 1% natrium acetate. Two washings were pulled together, mixed thoroughly, and kept at 4°C overnight. The precipitate was centrifuged at 25,000 × g, 10 min at 0°C. Supernatant was discarded and the pellet washed with ethanol. The tubes were dried and pellets resuspended with 0.5 ml of distilled water. Reagent dye solution [dimethyl methylene-blue-chloride (DMB)] was prepared as follows: 16 mg of DMB (Serva, Heidelberg, Germany) 5 ml of 95% ethanol, 2 g of sodium formate, and 2 ml of formic acid were mixed and diluted to 1.5 l with deionized water. As standards, samples of chondroitin-4-sulfate A sodium salt (Sigma–Aldrich, Stockholm, Sweden; no. 27042) were used in sequential concentrations. The DMB solution was added to each sample, and the absorbance was read at 535 nm. The results were calculated as mg/ml of urine.
Bladder Permeability Measurement.
Mice were deeply anesthetized with avertin, bladders were catheterized by using modified soft ureteral catheter Ch. 3, the residual urine was drained out and 110 μl of sodium fluorescein (Chauvin Pharmaceuticals, Ltd., Kingston-upon-Thames, Surrey, U.K.) saline solution (0.8 mg/ml) was instilled into bladders. After 15-min exposure, animals were killed; blood was obtained from heart and centrifuged at 1,000 × g for 5 min to get clean serum. Bladder content was also collected. Fifty microliters of serum was mixed with 100 μl of 0.2 M acetate buffer (pH 5.1) containing glucurase (Sigma, no. G4882) (5,000 units/ml), and incubated for 30 min. Ten microliters of urine was mixed with 20 μl of acetate buffer with glucurase and incubated for 30 min. To serum sample 1.15 ml and to urine sample 220 μl of carbonate buffer 0.2 M, pH 9.15, was added. The sodium fluorescein concentration was determined by using a fluorometer set at 494-nm excitation and 516-nm emission wavelengths. The permeability index was calculated as relation of urine concentration to serum concentration multiplied by 100.
Immunohistochemistry.
Antigen retrieval was performed by microwaving sections in 0.01 M citrate buffer, pH 6.0. The sections were permeabilized with 0.1% Nonidet P-40 in PBS for 15 min at room temperature. Endogenous peroxidase was blocked by incubation for 30 min with a solution of 0.5% hydrogen peroxide. Tissue sections were incubated for 2 h at 4°C with 1% BSA in PBS. Primary antibodies were diluted in PBS containing 1% BSA; 1:50 dilution was used for both biotin-conjugated antibodies to γδ TCR (GL-3; BD PharMingen, San Jose, CA) and antibodies to F4/80 (BD PharMingen); 1:75 dilution was used in staining for ERβ (chicken ERβ503 antibody, produced in our laboratory). Bladder sections were incubated with primary antibodies overnight at 4°C. The ABC method was used to visualize the signal according to the manual provided by the manufacturer (Vector Laboratories, Burlingame, CA). Sections were developed with 3,3′-diaminobenzidine tetrahydrochloride (Dako, Carpenteria, CA), counterstained with Mayer's hematoxylin, and dehydrated through ethanol series, followed by exposure to xylene and mounting.
Acknowledgments
We thank Prof. Gerd Fricker (Institut für Pharmazie und Biotechnologie, Heidelberg, Germany) for valuable advice in interpreting permeabilization data. We also thank Dr. Jose Inzunza and Annemarie Witte for helping with laboratory animals. This study was supported by grants from the Swedish Cancer Fund and KaroBio AB.
Abbreviations
- ER
estrogen receptor
- GAG
glucosaminoglycan
- IC
interstitial cystitis.
Footnotes
Conflict of interest: J.-Å.G. is a shareholder and consultant of KaroBio AB.
References
- 1.Christmas TJ, Bottazzo GF. Clin Exp Immunol. 1992;87:450–454. doi: 10.1111/j.1365-2249.1992.tb03018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christmas TJ. Br J Urol. 1994;73:508–515. doi: 10.1111/j.1464-410x.1994.tb07635.x. [DOI] [PubMed] [Google Scholar]
- 3.Forster C, Makela S, Warri A, Kietz S, Becker D, Hultenby K, Warner M, Gustafsson J-Å. Proc Natl Acad Sci USA. 2002;99:15578–15583. doi: 10.1073/pnas.192561299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wada-Hiraike O, Imamov O, Hiraike H, Hultenby K, Schwend T, Omoto Y, Warner M, Gustafsson J-Å. Proc Natl Acad Sci USA. 2006;103:2959–2964. doi: 10.1073/pnas.0511271103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morani A, Barros RP, Imamov O, Hultenby K, Arner A, Warner M, Gustafsson J-Å. Proc Natl Acad Sci USA. 2006;103:7165–7169. doi: 10.1073/pnas.0602194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imamov O, Morani A, Shim GJ, Omoto Y, Thulin-Andersson C, Warner M, Gustafsson J-Å. Proc Natl Acad Sci USA. 2004;101:9375–9380. doi: 10.1073/pnas.0403041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shim GJ, Warner M, Kim HJ, Andersson S, Liu L, Ekman J, Imamov O, Jones ME, Simpson ER, Gustafsson J-Å. Proc Natl Acad Sci USA. 2004;101:12628–12633. doi: 10.1073/pnas.0405099101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson J-Å, Smithies O. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders PT, Maguire SM, Gaughan J, Millar MR. J Endocrinol. 1997;154:R13–6. doi: 10.1677/joe.0.154r013. [DOI] [PubMed] [Google Scholar]
- 11.Taylor AH, Al-Azzawi F. J Mol Endocrinol. 2000;24:145–155. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- 12.Acar D, Cayan S, Aktas S, Tek M, Akbay E. Neurourol Urodyn. 2006;26:309–316. doi: 10.1002/nau.20247. [DOI] [PubMed] [Google Scholar]
- 13.Parsons CL, Lilly JD, Stein P. J Urol. 1991;145:732–735. doi: 10.1016/s0022-5347(17)38437-9. [DOI] [PubMed] [Google Scholar]
- 14.Hurst RE, Roy JB, Min KW, Veltri RW, Marley G, Patton K, Shackelford DL, Stein P, Parsons CL. Urology. 1996;48:817–821. doi: 10.1016/S0090-4295(96)00322-6. [DOI] [PubMed] [Google Scholar]
- 15.Pang X, Sant G, Theoharides TC. Urology. 1998;51:939–944. doi: 10.1016/s0090-4295(98)00032-6. [DOI] [PubMed] [Google Scholar]
- 16.Yamada T, Murayama T, Mita H, Akiyama K. Int J Urol. 2000;7:292–297. doi: 10.1046/j.1442-2042.2000.00197.x. [DOI] [PubMed] [Google Scholar]
- 17.Luber-Narod J, Austin-Ritchie T, Banner B, Hollins C, III, Maramag C, Price H, Menon M. Urol Res. 1996;24:367–373. doi: 10.1007/BF00389795. [DOI] [PubMed] [Google Scholar]
- 18.Nakata M, Kawasaki A, Azuma M, Tsuji K, Matsuda H, Shinkai Y, Yagita H, Okumura K. Int Immunol. 1992;4:1049–1054. doi: 10.1093/intimm/4.9.1049. [DOI] [PubMed] [Google Scholar]
- 19.Huber S, Shi C, Budd RC. J Virol. 2002;76:6487–6494. doi: 10.1128/JVI.76.13.6487-6494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olive C. Immunol Cell Biol. 1997;75:102–106. doi: 10.1038/icb.1997.14. [DOI] [PubMed] [Google Scholar]
- 21.Maret A, Coudert JD, Garidou L, Foucras G, Gourdy P, Krust A, Dupont S, Chambon P, Druet P, Bayard F, Guery JC. Eur J Immunol. 2003;33:512–521. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- 22.Yin Z, Chen C, Szabo SJ, Glimcher LH, Ray A, Craft J. J Immunol. 2002;168:1566–1571. doi: 10.4049/jimmunol.168.4.1566. [DOI] [PubMed] [Google Scholar]
- 23.Shim GJ, Gherman D, Kim HJ, Omoto Y, Iwase H, Bouton D, Kis LL, Andersson CT, Warner M, Gustafsson J-Å. J Pathol. 2006;208:408–414. doi: 10.1002/path.1883. [DOI] [PubMed] [Google Scholar]
- 24.Shim GJ, Wang L, Andersson S, Nagy N, Kis LL, Zhang Q, Makela S, Warner M, Gustafsson J-Å. Proc Natl Acad Sci USA. 2003;100:6694–6699. doi: 10.1073/pnas.0731830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Merwe JP, Yamada T, Sakamoto Y. Int J Urol. 2003;10(Suppl):S35–S38. doi: 10.1046/j.1442-2042.10.s1.10.x. [DOI] [PubMed] [Google Scholar]
- 26.Born WK, Reardon CL, O'Brien RL. Curr Opin Immunol. 2006;18:31–38. doi: 10.1016/j.coi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Tramonti D, Andrew EM, Rhodes K, Newton DJ, Carding SR. Eur J Immunol. 2006;36:1729–1738. doi: 10.1002/eji.200635959. [DOI] [PubMed] [Google Scholar]
- 28.Miller JL, Rothman I, Bavendam TG, Berger RE. Urology. 1995;45:587–590. doi: 10.1016/S0090-4295(99)80048-X. [DOI] [PubMed] [Google Scholar]
- 29.Berger RE, Miller JE, Rothman I, Krieger JN, Muller CH. J Urol. 1998;159:83–85. doi: 10.1016/s0022-5347(01)64018-7. [DOI] [PubMed] [Google Scholar]
- 30.Parsons CL, Greene RA, Chung M, Stanford EJ, Singh G. J Urol. 2005;173:1182–1185. doi: 10.1097/01.ju.0000148361.82074.77. [DOI] [PubMed] [Google Scholar]
- 31.Bouic PJ, Etsebeth S, Liebenberg RW, Albrecht CF, Pegel K, Van Jaarsveld PP. Int J Immunopharmacol. 1996;18:693–700. doi: 10.1016/s0192-0561(97)85551-8. [DOI] [PubMed] [Google Scholar]
- 32.Shoskes DA, Zeitlin SI, Shahed A, Rajfer J. Urology. 1999;54:960–963. doi: 10.1016/s0090-4295(99)00358-1. [DOI] [PubMed] [Google Scholar]
- 33.Sun Y, Chen M, Lowentritt BH, Van Zijl PS, Koch KR, Keay S, Simard JM, Chai TC. Am J Physiol. 2007;292:C106–C114. doi: 10.1152/ajpcell.00209.2006. [DOI] [PubMed] [Google Scholar]
- 34.Miller CP, Collini MD, Harris HA. Bioorg Med Chem Lett. 2003;13:2399–2403. doi: 10.1016/s0960-894x(03)00394-9. [DOI] [PubMed] [Google Scholar]
- 35.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Ferrante NM. Anal Biochem. 1967;21:98–106. doi: 10.1016/0003-2697(67)90087-5. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg JM, Cotlier E. Clin Chim Acta. 1972;41:19–27. doi: 10.1016/0009-8981(72)90491-3. [DOI] [PubMed] [Google Scholar]
- 38.Pennock CA, White F, Murphy D, Charles RG, Kerr H. Acta Paediatr Scand. 1973;62:481–491. doi: 10.1111/j.1651-2227.1973.tb08141.x. [DOI] [PubMed] [Google Scholar]
- 39.Panin G, Naia S, Dall'Amico R, Chiandetti L, Zachello F, Catassi C, Felici L, Coppa GV. Clin Chem. 1986;32:2073–2076. [PubMed] [Google Scholar]