Abstract
The Fc receptor-like protein 5 (FCRL5) on B cells has both an immunoreceptor tyrosine-based activation motif (ITAM)-like sequence and two consensus immunoreceptor tyrosine-based inhibitory motifs (ITIM) in its cytoplasmic region. To evaluate its signaling potential, we expressed constructs for chimeric molecules composed of the cytoplasmic region of FCRL5 and the extracellular and transmembrane regions of the IgG Fc receptor FcγRIIB in a B cell line lacking an endogenous Fc receptor. Coligation of this fusion protein with the B cell receptor (BCR) inhibited BCR-mediated calcium mobilization, intracellular tyrosine phosphorylation, and Erk kinase activation. Our mutational analysis indicated that, whereas tyrosines in both the inhibitory and activation motifs are phosphorylated after ligation, only those in ITIMs influence BCR-mediated signaling. This FCRL5 inhibitory effect was mediated through dual ITIM recruitment of the SH2-containing protein tyrosine phosphatase, SHP-1, which in turn dephosphorylates the ITAM-based tyrosines in BCR Igα/Igβ heterodimers. An FCRL5 inhibitory effect on BCR signaling was likewise demonstrable for primary B cells. Although its ligand is presently unknown, we conclude that FCRL5 has the functional potential to serve as an inhibitory coreceptor on mature B cells in humans.
Keywords: B cell receptor, Fc receptor-like protein 5, inhibitory, SH2 domain-containing tyrosine phosphatase 1
B cell receptor (BCR) engagement initiates signaling cascades that lead to activation of the Ras-MAPK pathway, phosphatidylinositol-3-kinase, and phospholipase C γ (PLCγ) (1, 2). The BCR triggering ultimately induces gene expression patterns that can promote cell activation, apoptosis, or anergy, depending upon the balance of enhancing and inhibitory influences that vary according to the stage in B cell differentiation (3). Costimulatory or inhibitory coreceptors on B cells modulate BCR signaling to either enhance or attenuate downstream signaling cascades (4). Inhibitory coreceptors may dampen BCR signaling via an immunoreceptor tyrosine-based inhibition motif (ITIM) in their cytoplasmic region. When tyrosine phosphorylated, the ITIMs recruit protein tyrosine phosphatases and lipid phosphatases via Src homology 2 (SH2) domain binding to achieve down-regulation or neutralization of BCR-induced activation (5). Conversely, the costimulatory receptors may have their own cytoplasmic immunoreceptor tyrosine-based activation motifs (ITAM) or they may pair with a transmembrane protein containing one or more ITAMs, whose tyrosines are phosphorylated by src family kinases to allow the recruitment of signaling molecules that promote cellular activation (6). The balance between activating and inhibitory receptor influences can be complicated by their coexpression on individual B cells. Moreover, individual cell surface receptors may possess both activating and inhibitory motifs; their differential engagement, according to ligand specificity and affinity, may trigger inhibitory and/or activating signaling pathways to calibrate effector cell responses (7).
The recently recognized Fc receptor-like (FCRL) family includes five members that are preferentially expressed by B lineage cells, possess variable numbers of Ig domains, and have either ITIMs, ITAMs, or both in their cytoplasmic tails (8–11). FCRL5 is the largest of the FCRL transmembrane proteins. In addition to its nine Ig-like extracellular domains and a transmembrane region, FCRL5 has a noncanonical ITAM-like consensus sequence and two canonical ITIMs in the cytoplasmic domain (8). Alternate isoforms have been identified, which include two secreted isoforms lacking transmembrane and cytoplasmic domains and a putative glycosylphosphatidylinositol-linked form, both of which lack transmembrane and cytoplasmic domains (9, 12). FCRL5 is found on most mature B cells with the highest levels being present on naïve and memory B cells and plasma cells (13). As for other FCRL family members, all of which have extracellular Ig domain sequences suggestive of Ig binding potential, an FCRL5 ligand has not yet been identified.
In this study, we assessed the signaling potential of FCRL5 by generating chimeric receptors encoding the intracellular domain of FCRL5 combined with the extracellular and transmembrane domains of FcγRIIb and evaluated their function in a B cell line. Our findings indicate that FCRL5 has the potential to inhibit BCR signaling through the recruitment of SH2 domain-containing tyrosine phosphatase 1 (SHP-1) after tyrosine phosphorylation of its two ITIMs. FCRL5 on primary memory B cells was also shown to have inhibitory potential for BCR signaling when the two receptors were coligated. These findings have implications to understanding the pathogenesis of B cell malignancies, in which aberrant FCRL5 expression is often seen.
Results
FCRL5 Signaling Inhibits BCR-Mediated Protein Tyrosine Phosphorylation.
For these experiments, chimeric fusion proteins consisting of the FcγRIIb extracellular and transmembrane domains and the FCRL5 intracellular domain were expressed in the IgG-expressing A20-IIA1.6 mouse B cell line variant that lacks endogenous FcγRIIb. The inhibitory or activating potential of the FCRL intracellular domain was then examined by coligating the BCR with the chimeric FcγRIIb/FCRL5 receptor by using intact anti-IgG antibodies. For comparison, BCR ligation was accomplished by treating the B cells with F(ab′)2 fragments of the anti-IgG antibodies. By using this model system, a panel of FcγRIIb/FCRL5 constructs with tyrosine to phenylalanine mutations of the ITAM-like and consensus ITIM sequences were expressed to determine their potential for modulating BCR-mediated signaling (Fig. 1). Transduced B cell populations expressing comparable levels of chimeric receptors were selected by fluorescence-activated cell sorting for use in these experiments [supporting information (SI) Fig. 7].
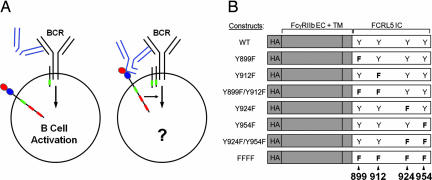
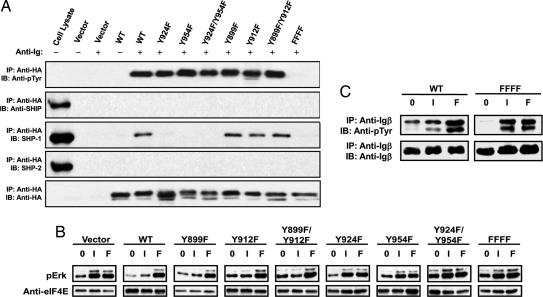
Fig. 1.
B cell stimulation models and constructs used to evaluate FCRL5 function. (A) Schematic illustration of exclusive BCR engagement using anti-IgG F(ab′)2 fragments results (Left) versus BCR coligation with FcγRIIb/FCRL5 chimeric receptor using intact anti-IgG antibodies (Right). ITAM(-like) sequences are represented in green. ITIM sequences are represented in red. (B) FcγRIIb/FCRL5 chimeric constructs. FcγRIIb extracellular and transmembrane domains are shaded in gray. Tyrosines 899 and 912 correspond to a noncanonical ITAM. Tyrosines 924 and 954 correspond to two canonical ITIMs. Tyrosine to phenylalanine mutations are indicated by the letter F (bold).
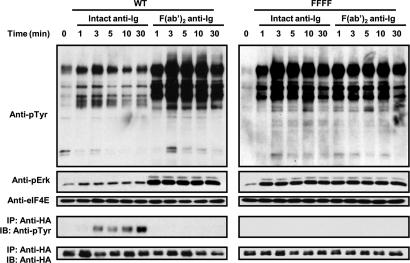
BCR ligation alone, using the F(ab′)2 anti-Ig antibodies, induced rapid tyrosine phosphorylation of multiple intracellular proteins (Fig. 2), whereas ligation of the WT FCRL5 fusion protein with an anti-HA antibody failed to induce tyrosine phosphorylation of the fusion protein itself or of other intracellular proteins (data not shown). However, when the BCR was coligated with the WT chimeric receptor, whole cell tyrosine phosphorylation was greatly reduced. Notably, Erk 1/2 activation was reduced relative to that seen after BCR ligation alone. After B cell stimulation with the intact anti-IgG antibodies, which bridge the BCR with FcγRIIb/FCRL5, the WT chimeric receptor was phosphorylated at increasing levels over a 30 min period, whereas ligation of the BCR alone with anti-IgG F(ab′)2 did not induce phosphorylation of the WT chimeric molecule (Fig. 2 Bottom). Longer chemiluminescent exposure of the Western blot membrane indicated FCRL5 tyrosine phosphorylation within the first minute after receptor ligation (data not shown). As anticipated, BCR coligation with the FFFF chimera, in which all of the cytoplasmic tyrosines were mutated, had no inhibitory effect on BCR-triggered protein tyrosine phosphorylation and Erk activation.
Fig. 2.
FcγRIIb/FCRL5 coligation inhibits BCR inhibited BCR-mediated protein tyrosine phosphorylation. WT or FFFF cells were treated with intact or F(ab′)2 fragments of anti-IgG antibodies, and whole cell tyrosine phosphorylation was gauged over time by means of Western blot analysis by using a phosphotyrosine antibody. Blots were reprobed with an anti-phospho Erk and anti-eIF4e antibodies to analyze Erk activation and verify equal protein loading. Immunoprecipitates of the chimeric receptor were analyzed by anti-phosphotyrosine and equal loading assured with an anti-HA control (Bottom).
FCRL5 Inhibits BCR-Induced Ca2+ Mobilization.
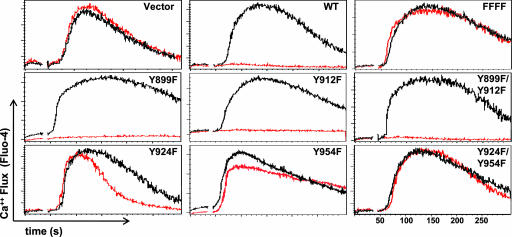
We next examined the effect of FCRL5 coengagement on calcium flux induced by BCR ligation on the A20-IIA1.6 B cells. Ligation of the BCR alone induced a characteristic wave of calcium mobilization, whereas BCR coligation with the WT FcγRIIb/FCRL5 chimeric receptor completely blocked BCR-induced calcium mobilization. When control B cells transduced with the “empty vector” were used, BCR stimulation with either the F(ab′)2 anti-IgG or the intact anti-IgG antibodies induced the same calcium mobilization responses as observed for the nonmanipulated cells (Fig. 3).
Fig. 3.
FcγRIIb/FCRL5 coligation inhibits BCR-triggered Ca2+ mobilization in B cells. Cells were preloaded with Fluo-4 NW for calcium flux evaluation, and the FcγRIIb/FCRL5 chimeras were coligated (red) or not (black) with BCR. Note that mutation of the ITIM tyrosines 924 and 954 to phenylalanine restored BCR-induced calcium mobilization.
A panel of chimeric receptors with tyrosine to phenylalanine mutations was used to identify the cytoplasmic tyrosine residues responsible for the inhibitory effect of FCRL5. Coligation of the BCR complex with either the Y899F or Y912F chimeric receptors failed to block inhibition of calcium mobilization. Coligation of the BCR with the Y899F/Y912F double mutant chimera also did not inhibit calcium mobilization, suggesting that neither of the tyrosines in this ITAM-like motif affect the inhibitory activity observed for FCRL5 in this B cell model. In contrast, the Y924F tyrosine mutant affected the duration of the calcium flux, truncating the response with little effect on maximal peak intensity in comparison with BCR-only induced calcium mobilization, whereas the Y954F mutant dampened the maximal peak intensity but not the duration of the calcium flux (Fig. 3). The Y924F/Y954F double mutant restored the normal pattern of BCR-induced calcium mobilization, as was observed for the “empty vector” control and the FFFF quadruple mutant. Notably, the tyrosine to phenylalanine mutations in both of these consensus ITIM motifs did not result in increased calcium flux relative to the BCR-only induced flux. Collectively, these results indicate that the two ITIMs contribute to the FCRL5 inhibition of BCR-mediated calcium mobilization. Moreover, the ITAM-like (Y899/Y912) consensus region has no obvious impact on the ability of FCRL5 to modulate the BCR-mediated calcium flux.
SHP-1 Binds a Tyrosine Phosphorylated Peptide Corresponding to the Membrane Distal ITIM of FCRL5.
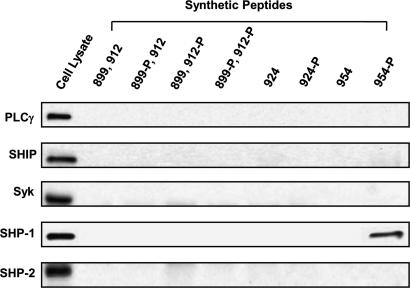
Having demonstrated the FCRL5 inhibitory effect on BCR-induced intracellular tyrosine phosphorylation, Erk activation, and calcium mobilization, we sought to identify the inhibitory effector molecules. In these experiments, we used biotinylated synthetic phosphopeptides corresponding to the FCRL5 ITAM-like and two ITIM sequences as affinity reagents to probe A20-IIA1.6 cell lysates (Fig. 4). After their tyrosine phosphorylation, ITIMs are known to serve as docking sites for molecules containing SH2 domains. Candidate inhibitory signaling molecules include the protein tyrosine phosphatases SHP-1 and SHP-2; and SHIP, an inositol polyphosphate phosphatase. When the tyrosines within ITAMs are phosphorylated by src family kinases, they may associate with Syk, a tyrosine kinase that resembles the classic src family kinases but which lacks an SH3 domain, or a signaling intermediate containing both SH2 and SH3 domains such as PLCγ2. Of these downstream effector molecules, only SHP-1 was found to bind to the most membrane-distal ITIM peptide with phosphorylated tyrosine residue 954 (Fig. 4). None of the inhibitory candidates were found to bind the unphosphorylated synthetic peptides.
Fig. 4.
A phosphopeptide mimic of the membrane distal ITIM of FCRL5 binds SHP-1. The indicated phosphopeptides corresponding to each ITAM-like and ITIM region of FCRL were incubated with the B cell lysates, and peptide precipitates were blotted with the indicated antibodies.
SHP-1 Binds to the FcγRIIb/FCRL5 Chimeric Receptor After BCR Coligation.
The phosphopeptide binding experiment and the kinetics of the calcium flux assay suggested the inhibitory effect of FCRL5 could be mediated by SHP-1. To determine whether SHP-1 can interact with the tyrosine-phosphorylated intracellular domain of the FcγRIIb/FCRL5 chimeric receptor after BCR coligation, we stimulated cells expressing each of the different mutants of FCRL5 with intact anti-IgG antibodies. After BCR coligation, we immunoprecipitated the FCRL5 chimeric receptors and probed Western blots of the immunoprecipitates with anti-phosphotyrosine antibodies and with antibodies to SHP-1, SHP-2, Syk, PLCγ2, and SH2-containing inositol phosphatase (SHIP). Interestingly, all of the chimeric receptors were tyrosine phosphorylated after coligation with the BCR, with the sole exception of the FFFF mutant which lacks intracellular tyrosines (Fig. 5A). Moreover, the level of phosphorylation did not vary greatly between the mutant chimeric receptors. This analysis suggests that both the ITAM-like and ITIM regions of FCRL5 were phosphorylated during BCR coligation. When the immunoprecipitates were probed with antibodies against the candidate proteins, SHP-1 was found to be coprecipitated with the WT FcγRIIb/FCRL5, the Y899F and Y912F single mutants, and the Y899F/Y912F double mutant, but not with the Y924F, Y954F, Y924F/Y954F, or FFFF mutants. In contrast, the SHP-2 and SHIP phosphatases failed to associate with any of the FcγRIIb/FCRL5 chimeric receptors. Association of the activating kinases Syk and PLCγ2 also was not detected (data not shown). In summary, the ability of phosphorylated tyrosines 924 and 954 to promote SHP-1 binding suggests that FCRL5 associates with SHP-1 by means of an interaction with both ITIMs to achieve its inhibitory effect on intracellular tyrosine phosphorylation and calcium mobilization. Whereas tyrosines 899 and 912 in the noncanonical ITAM are also phosphorylated after BCR coligation, they seem not to influence the FCRL5 modulating effect on BCR signaling.
Fig. 5.
SHP-1 binding to the FcγRIIb/FCRL5 chimeric receptor after BCR coligation. (A) After stimulation of A20-IIA1.6 cells expressing the chimeric constructs, cell lysate immunoprecipitates by a HA tag antibody were analyzed for FcγRIIb/FCRL5 interaction with candidate signaling components by Western blotting. The blots were reprobed with anti-phosphotyrosine to assess phosphorylation of the individual receptors and also with anti-HA as gel loading control. (B) Evaluation of Erk activation. Cell lysates for the individual mutants were from cells that were untreated (0), stimulated with intact anti-IgG antibodies (I), or treated with F(ab′)2 fragments of anti-IgG (F). The membranes were probed with anti-phospho Erk antibodies and with anti-eIF4e to assess equivalent protein loading. (C) Attenuation of Igα/Igβ phosphorylation. Cell lysates from either WT or FFFF mutant FCRL5 were immunoprecipitated by anti-Igβ mAb and analyzed for tyrosine phosphorylation by Western blotting. The stripped blot was reprobed with anti-Igβ as a loading control.
Inhibition of Erk Activation Correlates with SHP-1 Association and Inhibition of BCR-Induced Calcium Flux.
The results shown in Fig. 2 indicated that Erk phosphorylation is inhibited after BCR coligation with the WT FcγRIIb/FCRL5 chimeric receptor. We found that the Y899F, Y912F, and Y899F/Y912F mutants were capable of inhibiting Erk phosphorylation induced by BCR ligation, in keeping with their association with SHP-1 (Fig. 5B). In contrast, the non-SHP-1-associating Y924F, Y954F, Y924F/Y954F, and FFFF mutants were incapable of attenuating Erk activation upon coligation with the BCR. The differential ability of WT and the different FcγRIIb/FCRL5 mutants to alter Erk activation therefore parallels the effect on BCR-induced calcium mobilization and SHP-1 association. Because the tyrosines in the ITAM motifs of the Igα/Igβ components of the BCR complex are phosphorylated to trigger downstream signaling pathways that are activated by BCR ligation (14), they are logical targets for the FCRL5 associated SHP-1 tyrosine phosphatase. Accordingly, whereas BCR cross-linkage alone induced Igα and Igβ phosphorylation, WT FCRL5 coligation attenuated the Igα and Igβ tyrosine phosphorylation, and the FFFF mutant had no effect (Fig. 5C).
FCRL5 Coligation Attenuates BCR-Mediated Signaling in Primary Memory B Cells.
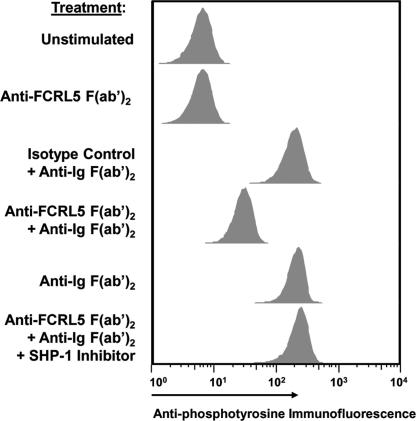
Having demonstrated in a model B cell line that the FcγRIIb/FCRL5 receptor chimeras can inhibit tyrosine phosphorylation by recruiting SHP-1, we wished to see whether the native FCRL5 receptor may function similarly in primary B cells. For these studies, memory B cells (CD19+, IgD−, CD38−) were isolated by fluorescence-activated cell sorting of tonsil samples. After confirming that these cells express FCRL5 (SI Fig. 8), we then subjected the isolated memory B cells to stimulatory conditions in which the BCR alone was cross-linked, the FCRL5 receptor alone was cross-linked, neither was cross-linked, or the BCR was coligated with the native FCRL5 receptor. The treated cells were then fixed and permeabilized before staining with a fluorochrome-labeled anti-phosphotyrosine antibody. BCR cross-linkage alone with anti-Ig F(ab′)2 fragments, or BCR cross-linkage coupled with treatment by a control irrelevant antibody led to a 10-fold shift in tyrosine phosphorylation (Fig. 6B). In contrast, cells stimulated with anti-FCRL5 F(ab′)2 fragments gave a signal comparable with that observed for the unstimulated population. However, tyrosine phosphorylation was significantly inhibited when FCRL5 was coligated with the BCR. When the memory B cells were pretreated with α-bromo-4-hydroxyacetophenone 4-hydroxyphenacyl Br, a potent SHP-1 inhibitor, the inhibition of tyrosine phosphorylation by FCRL5/BCR coligation was abrogated. With the caveat that receptor coligation was achieved by nonphysiological means, these results indicate that native FCRL5 receptors can engage SHP-1 to attenuate BCR triggered tyrosine phosphorylation after coligation of the two receptors.
Fig. 6.
FCRL5 attenuates tyrosine phosphorylation in tonsillar memory B cells after coligation with the BCR. Tonsillar memory B cells were treated as indicated for 10 min or preincubated with a SHP-1 inhibitor before treatment. Fixed cells were permeabilized, and the level of intracellular tyrosine phosphorylation was measured by flow cytometric analysis by using a fluorescein-conjugated phosphotyrosine antibody.
Discussion
This analysis of FCRL5 signaling capability establishes its inhibitory potential for modulating the BCR-mediated activation of B cells. A dominant role is shown for the two FCLR5 ITIMs, whereas no functional activity is revealed for the ITAM-like motif. By expressing wild-type and mutated versions of FcγRIIb/FCRL5 chimeric receptors in B cells lacking endogenous FcγRIIb, we could show that the tyrosine residues in the two ITIMs are essential for the inhibitory function of FCRL5. After its coligation with the BCR, WT FcγRIIb/FCRL5 fusion protein inhibited calcium mobilization, whole cell tyrosine phosphorylation, and Erk activation. Conversion of tyrosines in the noncanonical ITAM-like motif to phenylalanine had no demonstrable effect on B cell activation, and mutation of the ITIM tyrosines eliminated the inhibitory effect of the FcγRIIb/FCRL5 protein. The inhibitory function of FCRL5 involved SHP-1 tyrosine phosphatase recruitment and a reduction in tyrosine phosphorylation of Igα/Igβ ITAMs after FCRL5 and BCR coligation. These results accord with previous studies of SHP-1 function (14) to implicate the BCR Igα/β signaling units as important substrates for the FCRL5/SHP-1 mediated inhibition of BCR signaling in this model system.
Our mutational analysis of the intracellular tyrosines in the FCRL5 signaling domain indicates that both ITIM tyrosines, residues 924 and 954, contribute to the attenuation of BCR signaling. Whereas a synthetic phosphopeptide mimic of the most membrane-distal ITIM could be shown to bind SHP-1, an immunoprecipitation analysis of B cell lysates suggested that both ITIMs are needed for optimal SHP-1 binding, in that mutation of the tyrosine in either of the ITIMs eliminated detectable SHP-1 association. In comparison with the calcium mobilization pattern observed after BCR ligation alone, we observed a truncated response in B cell transductants wherein tyrosine 924 was mutated and a diminished calcium flux response when tyrosine 954 in the second ITIM was mutated. The cellular tyrosine phosphorylation response was also attenuated in primary B cells after coligation of the BCR with FCRL5, and this inhibitory effect was abrogated by pretreatment with a SHP-1 inhibitor.
Whereas these findings unambiguously indicate the FCRL5 potential for inhibiting B cell activation by BCR-mediated signaling, our studies notably used artificial means to coligate the two receptors. Under physiological conditions, IgG antibodies could possibly bridge antigen-bound BCR with FCRL5, given that the two membrane distal Ig domains of FCRL5 share sequence similarity with classical Fcγ receptors (8) and preliminary evidence has been reported for FCRL5 binding of heat-aggregated IgG (9). However, the absence of confirmatory evidence for an Fc receptor function suggests either a relatively low IgG binding affinity or that FCRL5 has other natural ligands. An unambiguous definition of a FCRL5 ligand(s) thus remains an important goal.
Enhanced FCRL5 expression has been observed in B lineage malignancies and in B cells infected with Epstein–Barr virus (EBV) (9, 12, 15, 16). Interestingly, FCRL5 was identified initially as an Ig superfamily gene located near the breakpoint of a chromosome 1q21 translocation event in a myeloma cell line (9). Moreover, up-regulated FCRL5 expression is frequently associated with a 1q21 translocation abnormality in both B cell non-Hodgkins lymphomas (B-NHL) and multiple myelomas (MM) (17–19). Patients with MM, chronic lymphocytic leukemia, and mantle cell lymphoma have been shown to have elevated levels of the soluble FCRL5 isoform. Their serum levels correspond with the tumor burden (12), and the tumor cells express transmembrane FCRL5 as well. Cell surface FCRL5 expression is also up-regulated in EBV-infected B cells (15). This effect is due to the formation of CBF1/RBPJκ heterodimers, which dock onto binding sites in the promoter regions of FCRL5 and other target genes. In EBV-infected cells, the EBNA2 protein replaces endogenous NOTCH as the transactivator unit for CBF1 heterodimers. Whereas these clues suggest that FCRL5 over-expression contributes to the pathogenesis of B cell malignancies, our results pose the question of how heightened expression of an inhibitory receptor can foster lymphomagenesis. It would be desirable to have a mouse model to address this issue, but mice have only two FCRL1–5 gene family homologs, Fcrl1 and Fcrl5, and neither shares high sequence homology with its human counterpart. Notably, mouse FCRL5 has only one consensus ITIM (20). Mouse models thus are unlikely to be very helpful in discerning the roles of the FCRL5 isoforms in lymphomagenesis.
FCRL5 is expressed at highest levels on mature B cells, memory B cells, and plasma cells, none of which are in a proliferative mode, and it is down-regulated on the proliferative B cells in germinal centers. The inhibitory FCRL5 on the resting B cells could participate in the delicate balance that checks cell cycle progression while allowing the basal level of constitutive BCR signaling needed for B cell survival (3). However, transcripts are expressed for multiple FCRL5 isoforms, including transmembrane, secreted, and glycosylphosphatidylinositol-anchored versions, by both normal B cells and B cell lines (9, 16) as another complicating feature in deducing the biological roles of FCRL5. The function of the soluble FCRL5 isoform, which is elevated in patients with MM, chronic lymphocytic leukemia, and mantle cell lymphoma (12), is presently unknown. Theoretically, the soluble isoform could compete with transmembrane FCRL5 for a natural ligand thereby abrogating its inhibitory function to allow unimpeded BCR mediated proliferation and cell survival. The soluble isoform of FCRL5 potentially could also modulate a ligand-bearing immunocompetent cell needed for tumor detection and clearance. In a similar scenario, soluble FCRL5 could play a role in EBV infection by serving either to block the ligand for the transmembrane FCRL5 isoform or as a modulating factor for cytotoxic T cells that eliminate EBV infected B cells. The speculative nature of these considerations further emphasizes the need to determine the natural FCRL5 ligand(s) to understand its function in normal B cell physiology, malignancies and EBV infection.
In conclusion, our studies show that FCRL5, like FCRL4 (21, 22), has potent inhibitory potential for BCR-mediated signaling. However, whereas FCRL4 expression is confined to a tissue-based subpopulation of memory B cells (22), FCRL5 is expressed throughout B cell differentiation and therefore could have a broader influence on B cell responses to both endogenous and exogenous antigens.
Methods
Cells and Antibodies.
A20-IIA1.6 B cells and BW5147 T cells were maintained in RPMI medium 1640 supplemented with 10% FCS, 25 mM Hepes, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5.0% CO2. BOSC23 cells were grown in DMEM supplemented with 10% FCS, 2 mM l-glutamine, and 100 units/ml penicillin and 100 μg/ml streptomycin. Anti-HA antibody 12CA5 was obtained from Roche Diagnostics (Mannheim, Germany), anti-PLCγ2, anti-SHP-1, anti-SHP-2, anti-SHIP, anti-Syk, anti-pErk (Tyr 204), and anti-eIF4E from Santa Cruz Biotechnology (Santa Cruz, CA), horseradish peroxidase-coupled anti-phosphotyrosine antibodies 4G10 from Upstate Biotechnologies (Lake Placid, NY), whole Ig and F(ab′)2 fragments against Ig from Zymed (Carlsbad, CA), anti-phosphotyrosine PY20 R-PE-conjugated for phosphospecific flow cytometry and anti-IgD-PE and anti-CD38-FITC from BD Biosciences (San Jose, CA), and anti-Igβ was produced in our lab.
Production of Monoclonal Anti-FCRL5 Antibodies.
Hybridoma clones producing monoclonal anti-FCRL5 antibodies were generated by hyperimmunizing BALB/c mice with BW5147 cells expressing full-length FCRL5 and extracellular FCRL5-Fc fusion protein (10 μg per injection) before fusion of regional lymph node cells with the Ag8.653 plasmacytoma cell line (23). Hybridoma supernatants were screened by ELISA for FCRL5 antibody (2A9) activity, the specificity of which was determined by cell surface immunofluorescence reactivity with BW5147 cell lines expressing FCRL1–5. Hybridomas producing anti-FCRL5 antibody were subcloned by limiting dilution, and the antibody isotype was determined by an indirect capture ELISA (Zymed). F(ab′)2 fragments were prepared by using the ImmunoPure F(ab′)2 Preparation Kit (Pierce, Rockford, IL).
Generation of Chimeric FcγRIIb/FCRL5 Constructs.
The wild-type and mutant chimera proteins were generated by fusing the extracellular and transmembrane domains of FcγRIIb to the intracellular domain of FCRL5 as described (21, 24). Site-directed mutagenesis was performed according to standard protocols. Wild-type and mutated cDNAs encoding the intracellular domain of FCRL5 were subcloned into pBluescript and verified by DNA sequencing. Clones were fused with cDNAs encoding the extracellular and transmembrane domains of HA-tagged murine FcγRIIb and cloned into pMX-PIE, a retroviral expression vector which expresses the gene of interest upstream of an internal ribosomal entry site and the enhanced green fluorescent protein (EGFP) (25).
Transfection of BOSC23 Cells and Generation of A20-IIA1.6 Cells Expressing Chimeric Receptors.
BOSC23 cells were transfected with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to manufacturer's protocol. The virus containing supernatant was passed through a 0.2 μm filter, mixed with polybrene to a final concentration of 5 μg/ml and added to 2 × 106 A20-IIA1.6 cells as described (21). Transduced A20-IIA1.6 cells were selected in medium containing puromycin (1.5 μg/ml) for 4 days followed by fluorescence-activated cell sorting (FACS; Becton Dickinson) to enrich for EGFP/chimeric receptor expressing cells.
Cellular Activation, Western Blotting, Affinity Precipitation, and Immunoprecipitation.
To examine the effects of the chimeric receptor on BCR-induced signaling, 5 × 106 cell aliquots were washed twice with PBS and incubated for 2 h in medium lacking FCS and supplemented with 20 mM Hepes (pH 7.2) before stimulation with intact anti-IgG antibodies (25 μg/ml) or anti-IgG F(ab′)2 fragments (16.6 μg/ml). Western blotting and immunoprecipitations were performed following standard protocols. Briefly, samples were lysed with M-PER cellular lysis buffer (Pierce) supplemented with a Protease Inhibitor Mixture (Roche Applied Sciences), and phosphatase inhibitors Na3VO4 (0.2 mM), Na2MoO4 (1 mM), and β-glycero-phosphate (5 mM). Proteins in cell lysates were quantified by using the bicinchoninic acid solution (BCA) reagent (Pierce). Whole cell lysates were treated with 20 μl of 50% slurry of anti-HA Sepharose beads (Roche Applied Sciences) and incubated for 1 h at 4°C. After brief centrifugation, the supernatants were removed and the beads were washed 5 times with 1 ml of M-PER buffer and boiled before being subjected to SDS/PAGE followed by transfer to nitrocellulose membranes (MSI, Westboro, MA) which were probed with the indicated antibodies, and the proteins were visualized by using the ECL reagent (Amersham Pharmacia Biosciences, Piscataway, NJ).
Calcium Mobilization Assay.
Cells (5 × 106) were washed twice in Hanks' balanced salt solution (HBSS) (with Ca2+ and Mg2+), then resuspended in 500 μl of Fluo-4 NW assay buffer (Invitrogen) and incubated at 37°C for 30 min followed by 30 min at room temperature. Two hundred fifty-microliter aliquots of the cells were analyzed by flow cytometry after addition of 25 μg/ml intact anti-IgG or 16.6 μg/ml F(ab′)2 fragments of anti-IgG. Data analysis was carried out by using the Flowjo software package (Treestar, Ashland, OR).
Phosphopeptide Immunoprecipitation Assay.
A20-IIA1.6 cells (5 × 106) were lysed with M-PER lysis buffer. Biotinylated phosphopeptides or control peptides (Alpha Diagnostic International, San Antonio, TX) were added to these lysates at a final concentration of 5 μg and incubated at 4°C for 30 min. Peptide-protein complexes were recovered by using streptavidin-conjugated beads. (Amersham Pharmacia Biosciences). The precipitates were then washed five times with M-PER lysis buffer and resuspended in SDS/PAGE sample buffer. After boiling, the precipitated proteins were separated by SDS/PAGE and transferred to nitrocellulose membranes. The blots were probed with the indicated antibodies and the proteins were visualized by using the ECL reagent.
Phosphospecific Flow Cytometric Analysis of Primary Tonsillar Cells.
Phosphospecific flow cytometry was performed by using a modified, previously reported protocol (26). Tonsillar memory B cells (CD19+, CD38−, IgD−) purified by fluorescence-activated cell sorting (27, 28) were incubated at 37°C in a CO2 incubator for 2 h before stimulation. The cells (1 × 106 aliquots) were then treated for 15 min on ice with anti-FCRL5 (5C3) F(ab′)2 fragments before stimulation with rabbit anti-Ig F(ab′)2 fragments (Zymed) for 10 min at 37°C in a CO2 incubator. Cells were also treated with 200 μM α-bromo-4-hydroxyacetophenone 4-Hydroxyphenacyl Br (Calbiochem, San Diego, CA), a cell-permeable, protein tyrosine phosphatase inhibitor (29). Finally, the treated cells were fixed with 50 μl of BD Phosflow Fix Buffer I (BD Biosciences) at 37°C for 10 min, permeabilized with BD Phosflow Perm Buffer II (BD Biosciences) for 30 min at 4°C, and then stained with R-PE-conjugated anti-phosphotyrosine PY20 before FACS analysis.
Supplementary Material
Acknowledgments
We thank Dr. Larry Gartland for help with FACS analysis and Dr. Peter Burrows for reading the manuscript. This work was supported by National Institutes of Health Grants AI39816 and AI007051 from the National Institute of Allergy and Infectious Diseases, the Dana Foundation Program in Human Immunology, and the V Foundation for Cancer Research.
Abbreviations
- BCR
B cell receptor
- PLC
phospholipase C
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- ITAM
immunoreceptor tyrosine-based inhibitory motif
- SH2
Src homology 2
- SHP
SH2 domain-containing tyrosine phosphatase
- FCRL5
Fc receptor-like protein 5
- MM
multiple myeloma.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703354104/DC1.
References
- 1.Kurosaki T. Int J Mol Med. 1998;1:515–527. doi: 10.3892/ijmm.1.3.515. [DOI] [PubMed] [Google Scholar]
- 2.Kurosaki T. Annu Rev Immunol. 1999;17:555–592. doi: 10.1146/annurev.immunol.17.1.555. [DOI] [PubMed] [Google Scholar]
- 3.Healy JI, Goodnow CC. Annu Rev Immunol. 1998;16:645–670. doi: 10.1146/annurev.immunol.16.1.645. [DOI] [PubMed] [Google Scholar]
- 4.Gold MR. Trends Pharmacol Sci. 2002;23:316–324. doi: 10.1016/s0165-6147(02)02045-x. [DOI] [PubMed] [Google Scholar]
- 5.Bolland S, Ravetch J. Adv Immunol. 1999;72:149–177. doi: 10.1016/s0065-2776(08)60019-x. [DOI] [PubMed] [Google Scholar]
- 6.Monroe JG. Nat Rev Immunol. 2006;6:283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 7.Gergely J, Pecht I, Sarmay G. Immunol Lett. 1999;68:3–15. doi: 10.1016/s0165-2478(99)00024-3. [DOI] [PubMed] [Google Scholar]
- 8.Davis RS, Wang Y.-H., Kubagawa H, Cooper MD. Proc Natl Acad Sci USA. 2001;98:9772–9777. doi: 10.1073/pnas.171308498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatzivassiliou G, Miller I, Takizawa J, Palanisamy N, Rao PH, Iida S, Tagawa S, Taniwaki M, Russo J, Neri A, et al. Immunity. 2001;14:277–289. doi: 10.1016/s1074-7613(01)00109-1. [DOI] [PubMed] [Google Scholar]
- 10.Davis RS. Annu Rev Immunol. 2007;25:525–569. doi: 10.1146/annurev.immunol.25.022106.141541. [DOI] [PubMed] [Google Scholar]
- 11.Miller I, Hatzivassiliou G, Cattoretti G, Mendelsohn C, Dalla-Favera R. Blood. 2002;99:2662–2669. doi: 10.1182/blood.v99.8.2662. [DOI] [PubMed] [Google Scholar]
- 12.Ise T, Nagata S, Kreitman RJ, Wilson WH, Wayne AS, Stetler-Stevenson M, Bishop MR, Scheinberg DA, Rassenti L, Kipps TJ, et al. Leukemia. 2007;21:169–174. doi: 10.1038/sj.leu.2404445. [DOI] [PubMed] [Google Scholar]
- 13.Polson AG, Zheng B, Elkins K, Chang W, Du C, Dowd P, Yen L, Tan C, Hongo J.-A., Koeppen H, et al. Int Immunol. 2006;18:1363–1373. doi: 10.1093/intimm/dxl069. [DOI] [PubMed] [Google Scholar]
- 14.Adachi T, Wienands J, Wakabayashi C, Yakura H, Reth M, Tsubata T. J Biol Chem. 2001;276:26648–26655. doi: 10.1074/jbc.M100997200. [DOI] [PubMed] [Google Scholar]
- 15.Mohan J, Dement-Brown J, Maier S, Ise T, Kempkes B, Tolnay M. Blood. 2006;107:4433–4439. doi: 10.1182/blood-2005-09-3815. [DOI] [PubMed] [Google Scholar]
- 16.Ise T, Maeda H, Santora K, Xiang L, Kreitman RJ, Pastan I, Nagata S. Clin Cancer Res. 2005;11:87–96. [PubMed] [Google Scholar]
- 17.Offit K, Wong G, Filippa DA, Tao Y, Chaganti RS. Blood. 1991;77:1508–1515. [PubMed] [Google Scholar]
- 18.Sawyer JR, Tricot G, Mattox S, Jagannath S, Barlogie B. Blood. 1998;91:1732–1741. [PubMed] [Google Scholar]
- 19.Whang-Peng J, Knutsen T, Jaffe ES, Steinberg SM, Raffeld M, Zhao WP, Duffey P, Condron K, Yano T, Longo DL. Blood. 1995;85:203–216. [PubMed] [Google Scholar]
- 20.Won WJ, Foote JB, Odom MR, Pan J, Kearney JF, Davis RS. J Immunol. 2006;177:6815–6823. doi: 10.4049/jimmunol.177.10.6815. [DOI] [PubMed] [Google Scholar]
- 21.Ehrhardt GRA, Davis RS, Hsu JT, Leu C.-M., Ehrhardt A, Cooper MD. Proc Natl Acad Sci USA. 2003;100:13489–13494. doi: 10.1073/pnas.1935944100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrhardt GRA, Hsu JT, Gartland L, Leu C.-M., Zhang S, Davis RS, Cooper MD. J Exp Med. 2005;202:783–791. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kearney J, Radbruch A, Liesegang B, Rajewsky K. J Immunol. 1979;123:1548–1550. [PubMed] [Google Scholar]
- 24.Blery M, Kubagawa H, Chen CC, Vely F, Cooper MD, Vivier E. Proc Natl Acad Sci USA. 1998;95:2446–2451. doi: 10.1073/pnas.95.5.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onishi M, Kinoshita S, Morikawa Y, Shibuya A, Phillips J, Lanier L, Gorman D, Nolan G, Miyajima A, Kitamura T. Exp Hematol. 1996;24:324–329. [PubMed] [Google Scholar]
- 26.Irish JM, Czerwinski DK, Nolan GP, Levy R. J Immunol. 2006;177:1581–1589. doi: 10.4049/jimmunol.177.3.1581. [DOI] [PubMed] [Google Scholar]
- 27.Pascual V, Liu YJ, Magalski A, de Bouteiller O, Banchereau J, Capra JD. J Exp Med. 1994;180:329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjorgan MB, Thoen JE, Natvig JB, Thompson KM. J Immunol. 2001;167:3610–3618. doi: 10.4049/jimmunol.167.7.3610. [DOI] [PubMed] [Google Scholar]
- 29.Arabaci G, Guo XC, Beebe KD, Coggeshall KM, Pei DH. J Am Chem Soc. 1999;121:5085–5086. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.