Abstract
To elucidate the intracellular pathways that mediate early B cell development, we directed expression of activated Ras to the B cell lineage in the context of the recombination-activating gene 1 (RAG1)-deficient background (referred to as Ras–RAG). Similar to the effects of an immunoglobulin (Ig) μ heavy chain (HC) transgene, activated Ras caused progression of RAG1–deficient progenitor (pro)-B cells to cells that shared many characteristics with precursor (pre)-B cells, including downregulation of surface CD43 expression plus expression of λ5, RAG2, and germline κ locus transcripts. However, these Ras–RAG pre-B cells also upregulated surface markers characteristic of more mature B cell stages and populated peripheral lymphoid tissues, with an overall phenotype reminiscent of B lineage cells generated in a RAG- deficient background as a result of expression of an Ig μ HC together with a Bcl-2 transgene. Taken together, these findings suggest that activated Ras signaling in pro-B cells induces developmental progression by activating both differentiation and survival signals.
Keywords: B cell development, pre-B cell receptor, signal transduction, Ras, recombinase-activating gene 2–deficient blastocyst complementation
Blymphocyte development proceeds through a series of stages defined by the expression of surface markers and by the status of Ig gene rearrangement (1). In this developmental program, upon productive rearrangement and expression of Ig μ heavy chain (HC)1 genes, B220+CD43+ pro-B cells progress to B220+CD43− pre-B cells. This transition requires expression of the pre-B cell receptor (pre-BCR) complex consisting of μ HC associated with the invariant surrogate light chain proteins λ5 and VpreB, most likely on the cell surface (2). Consistent with this notion, targeted germline deletion of the μ membrane exon arrested murine B cell development at the pro-B cell stage (3). Moreover, germline inactivation in mice of either the recombinase-activating gene (RAG)1 or RAG2 genes, which encode components of the V(D)J recombinase required for initiation of antigen receptor gene rearrangement, again resulted in a block in B lymphocyte development at the pro-B cell stage (4, 5). However, expression of a rearranged μ HC transgene in the RAG-deficient background partially rescued this developmental block in the B lineage, leading to the generation of B220+CD43− pre-B cells and demonstrating that μ chain expression was sufficient to drive this developmental transition (6, 7).
Because the pre-BCR, like the mature BCR, has no known intrinsic enzymatic functions, it must rely upon associated proteins to provide a functional linkage with intracellular signaling pathways. The mature and pre-BCR– associated Igα and Igβ contain immunoreceptor tyrosine-based activation motifs (ITAMs), which are targets for phosphorylation by tyrosine kinases (8); these proteins are required for normal B cell development (9, 10). Furthermore, the importance of an ITAM-associated tyrosine kinase activity during early B lymphopoiesis was demonstrated in mice deficient in the syk tyrosine kinase, in which an incomplete block in development was observed at the B220+CD43+ pro-B cell stage (11, 12). Although several downstream signaling pathways can be induced in B cell lines (13), the identity of the targets downstream of the nonreceptor tyrosine kinases that are activated by the pre-BCR complex has remained unclear. In this context, the Ras family of GTPases (14) represents an attractive candidate. In numerous vertebrate systems, Ras proteins have been implicated in linking tyrosine kinase-mediated signal transduction to downstream effectors (15). In the T cell lineage, constitutive expression of activated Ras in a RAG-deficient background has been shown to drive the expansion and differentiation of double negative thymocytes to the CD4+ CD8+ (double positive, or DP) stage (16). Moreover, Ras-dependent signaling after cross-linking of the mature BCR has also been observed in lymphocyte cell lines (17, 18). We hypothesized that if activation of endogenous Ras represents a necessary event in pre-BCR signaling, then introduction of constitutively activated Ras into RAG-deficient pro-B cells could mimic signaling by the pre-BCR and result in developmental progression.
Materials and Methods
DNA Constructs.
The plasmid pEμ was constructed through ligation of a 1,042-bp fragment containing the Ig HC enhancer (Eμ) linked to a variable region promoter (19) into the SmaI site of Bluescript II SK. A BamHI/PstI fragment containing two exons of the human β-globin gene was introduced at the KpnI site of Bluescript II SK to provide splice sites and a polyadenylation signal. pEμ was digested with SalI, treated with Klenow fragment and alkaline phosphatase, and ligated to the c-Ha-ras V12 cDNA (20) to complete pEμRasV12.
Embryonic Stem Cell Transfection and RAG2-deficient Chimera Generation.
Cotransfection of RAG1−/− (16) CCE embryonic stem (ES) cells was carried out with 10 μg NotI-linearized pEμRasV12 together with 1 μg linearized PGK-puro (a gift of P.W. Laird, University of Southern California School of Medicine, Los Angeles, CA). DNA was added to 107 ES cells, and transfection was via electroporation at 300 V, 70 μF twice. Cells were selected in 0.5 μg/ml puromycin (Sigma Chemical Co.). Drug-resistant ES colonies were picked and subcloned for injection into RAG2-deficient blastocysts as previously described (21).
Analysis of RAG2-deficient Chimeras.
RAG2-deficient chimeras were maintained in a pathogen-free environment, and were analyzed at 4–6 wk of age. FACS® analyses of bone marrow, spleen, and lymph nodes were carried out as previously described (22). Antibodies were purchased from PharMingen and were: Cy-Chrome conjugated B220/CD45R (clone RA3-6B2); FITC-conjugated CD21/35 (CR2/CR1) (7G6), CD22.2 (Cy34.1), IgM (II/41), Ly 9.1 (30C7), B220/CD45R, and CD43 (S7); and PE-conjugated CD43 (S7), CD2 (RM2-5), CD23 (B3B4), and CD22.2. Analysis of stained samples was performed on a Becton Dickinson FACSCalibur®, and sorting of B220+ Ly 9.1+ B lineage cells was carried out on an Ortho Cytofluorograf II or Becton Dickinson FACScan®; dot plots were generated using Cell Quest software (Becton Dickinson).
Western Blot Analysis.
After red blood cell lysis in ammonium chloride, single-cell splenocyte or lymph node suspensions were treated with RIPA lysis solution (0.15 mM NaCl, 0.05 mM Tris-HCl, pH 7.2, 1% Triton X-100, 1% sodium deoxycholate, and 0.1% SDS) at 108 cells per ml, and postnuclear supernatants were prepared following standard procedures. Proteins were resolved using SDS/10% PAGE (loading 2 × 106 cell equivalents of lysate per lane), transferred to Immobilon-P membranes (Millipore), and probed with an anti-Ha-ras monoclonal antibody (clone F235; Calbiochem), followed by a horseradish peroxidase–linked F(ab′)2 sheep anti–mouse Ig (Boehringer Mannheim). For detection we used the ECL system (Amersham), and Ponceau-S staining was employed to verify equivalent protein loading.
Reverse Transcription PCR Analysis.
RNA was isolated from 106 sorted B220+Ly 9.1+ cells derived from Ras–RAG1−/− chimera or wild-type lymph node and spleen, together with sorted B220+CD43−IgM− wild-type pre-B cells, and B220+CD43+ RAG2-deficient pro-B cells using the TRIZOL reagent (GIBCO BRL) and the manufacturer's protocol. 2 μg of RNA was used for first-strand cDNA synthesis using SuperScript II reverse transcriptase (RT) (GIBCO BRL), following conditions recommended by the manufacturer. 1% of each cDNA synthesis reaction was used for PCR amplification, together with two serial fivefold dilutions. 5- and 25-fold cDNA dilution samples were mixed with cDNA derived from J1 ES cells to equalize template amount in reactions amplifying lymphoid-specific λ5 and RAG2; because Bcl-2 and Bcl-xL were both expressed in ES cDNA (data not shown), ES cell cDNA was not diluted into these reactions. The ES cell cDNA dilution was determined through amplification of β-actin. 25 μl reactions contained: 1× PCR buffer, 200 μM dNTPs, 2 μM of both sense and antisense oligonucleotide primers, and 1 U Taq polymerase (Qiagen). Primers for λ5, RAG2, Bcl-2, and β-actin were as previously described (23), except that the 5′ λ5 oligonucleotide primer was 5′ CTTGAGGGTCAATGAAGCTCAGA 3′. Primers for Bcl-xL were as previously described (24). All primers contained sequences spanning at least two exons, allowing clear distinction between RNA and genomic DNA signals. PCR amplification conditions were as previously described (23). Expected sizes of amplified products were: λ5, 337 bp; RAG2, 515 bp; Bcl-2, 315 bp, Bcl-xL, 557 bp; β-actin, 623 bp. For analysis of germline κ transcripts, a primer 5′ of Jκ1 (5′CCACGCATGCTTGGAGAGGGGGTT3′) and a 3′ primer within the coding sequence of Jκ2 were used (25). RNA samples were treated with 5 U of RNase-free DNase (Boehringer Mannheim) in 1× RT buffer for 30 min at 37°C. The DNase was inactivated at 75°C for 10 min, followed by reverse transcription as above. Since germline κ transcripts could not be distinguished from contaminating genomic DNA, samples not treated with RT were subjected to PCR analysis. Conditions for amplification were 95°C for 2 min, then 24 cycles at 95°C for 30 s, 60°C for 1 min, and 72°C for 1.5 min. PCR products were resolved on 1.5% agarose gels, blotted onto Zetaprobe GT (Bio-Rad), and probed with 32P-labeled cDNAs for λ5 (6), with cloned PCR fragments for RAG2, β-actin, Bcl-2, and Bcl-xL, or with a HindIII fragment containing the germline Jκ region (26).
Results
To direct expression of activated Ras to B lineage cells, we used an expression construct containing a c-Ha-rasV12 cDNA and Ig HC regulatory sequences (Fig. 1 A). This expression construct was transfected into RAG1-deficient ES cells (16), and the resulting ES clones were tested for their ability to generate B lineage cells in the RAG2-deficient blastocyst complementation assay (21). Flow cytometry analysis of bone marrow cells from Ras–RAG chimeras revealed low numbers of IgM−B220+CD43− B lineage cells (which are absent in RAG-deficient mice); however, these cells were also found in the spleen and lymph nodes in numbers approaching those of normal, Ig-positive B cells in wild-type mice (Fig. 2 and data not shown). To verify that B220+ cells were derived from ES cells, the clonotypic marker Ly 9.1 was used (data not shown). Furthermore, expression of the Ha-ras protein in these chimeric mice was confirmed by Western analysis of spleen and lymph node cell lysates using an Ha-ras-specific monoclonal antibody (expression of endogenous Ras in lymphocytes is limited to N-ras and K-ras; reference 27) (Fig. 1 B). Therefore, activated Ras expression results in the generation of B lineage cell populations that substantially populate the peripheral lymphoid tissues of RAG1–deficient mice.
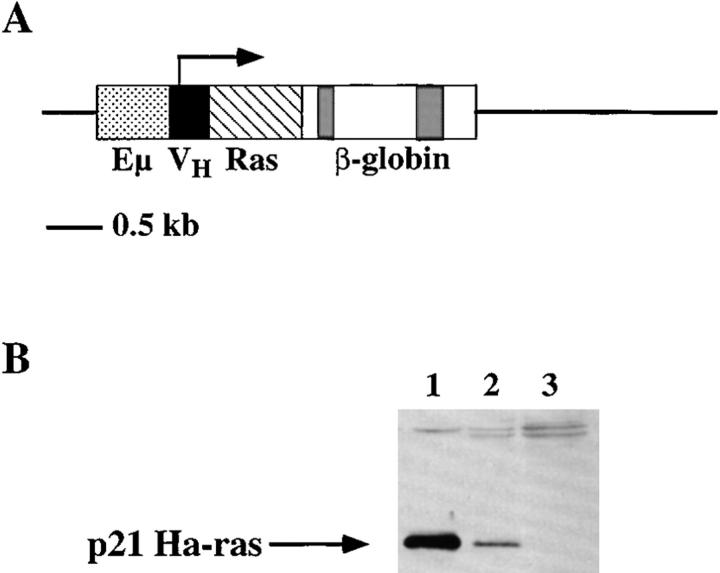
Figure 1.
Activated c-Ha-Ras expression vector and Western blot analysis of c-Ha-Ras expression. (A) Diagram of the pEμRasV12 expression vector. Eμ, Ig HC enhancer; VH, HC variable region promoter; Ras, c-Ha-ras V12 cDNA; β-globin, β-globin exons 2 and 3 with polyadenylation signal. (B) Western blot analysis of total spleen or lymph node lysates from a Ras–RAG1−/−, RAG2-deficient chimera and RAG2-deficient mouse, probed with a monoclonal antibody against Ha-ras. The position of p21 Ha-ras is indicated by the arrow. Ponceau-S staining was employed to verify equivalent loading of protein. Lane 1, Ras–RAG1−/− total lymph node; lane 2, Ras–RAG1−/− total spleen; lane 3, RAG2−/− total spleen.
Figure 2.
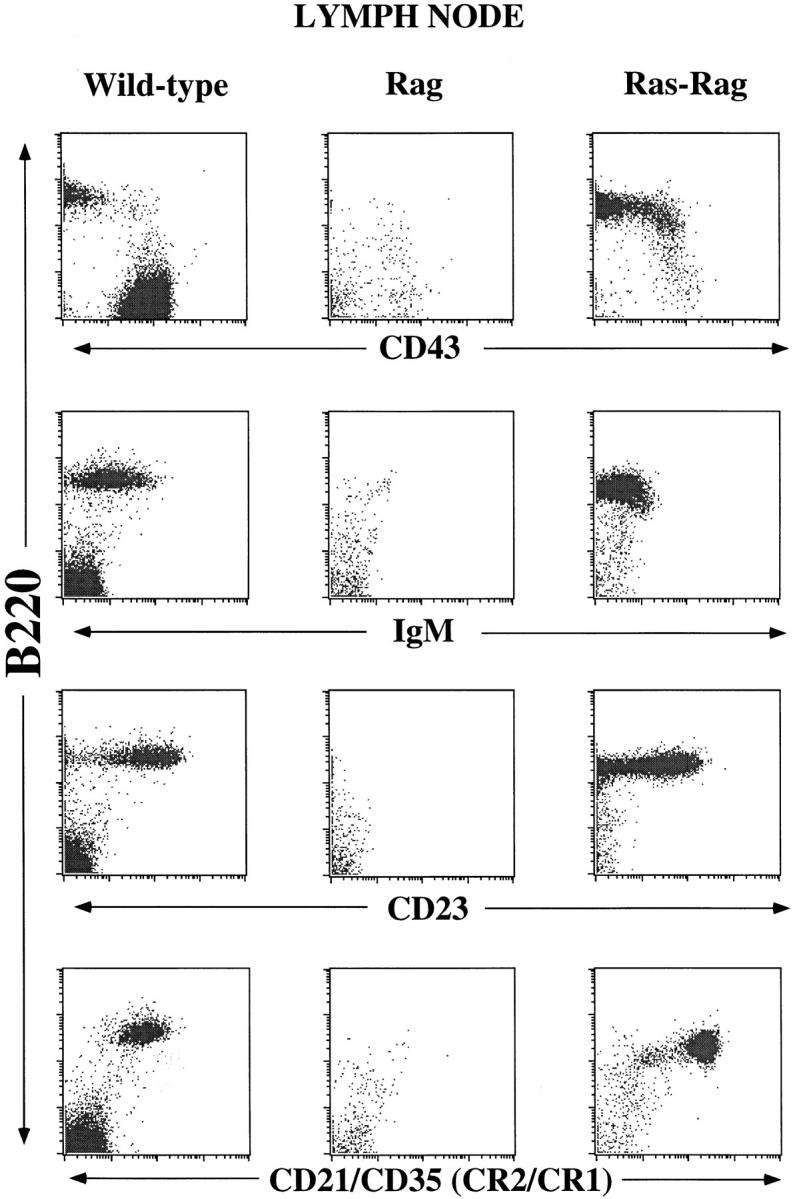
Surface phenotype of Ras–RAG B lineage cells. Lymphocytes isolated from lymph node of Ras–RAG1−/−, RAG2-deficient chimera, RAG2−/−, and wild-type (129 Sv/Ev) mice were subjected to FACS® analyses. Depicted are log-scale dot plots of B220 versus CD43, IgM, CD23, and CD21/CD35. The plots reflect 20,000 collected events, with dead cells excluded by forward and side scatter. Similar results were found in the spleen. Results shown are representative of those obtained in the analysis of six chimeric mice derived from two independent transfected ES clones. The number of B220+Ly9.1+ cells in these RAG2-deficient chimeras ranged from 5 × 105 to 1.6 × 107 in lymph node and 2 × 106 to 7 × 106 in spleen. Chimeric animals analyzed for evidence of B cell developmental progression showed no gross evidence of malignancy. Older chimeric mice do tend to develop B lineage lymphomas, but such transformed cells are significantly larger in cell size and have markedly altered surface antigen staining characteristics (data not shown). Ras-Rag, activated Ras-complemented-RAG1–/–, RAG2-deficient chimera; Rag, RAG2−/− mouse.
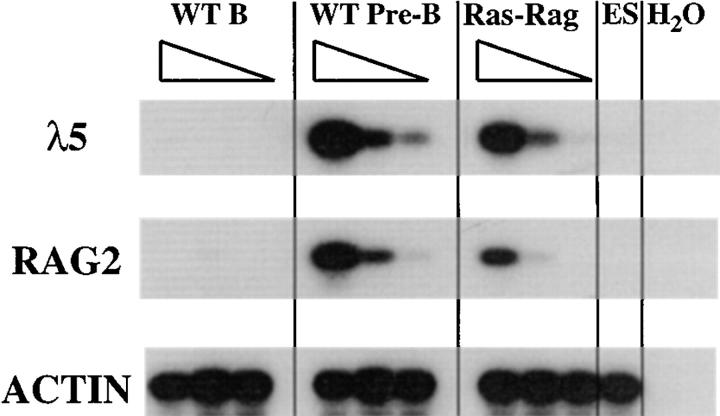
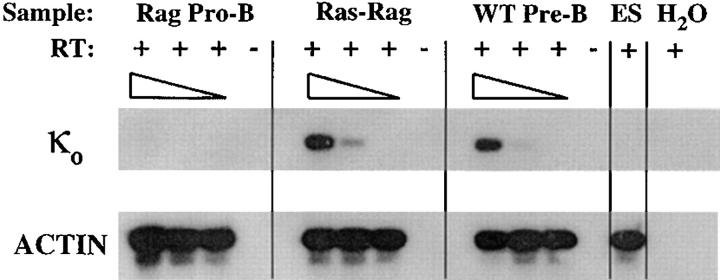
To further delineate the stage of maturation of B lineage cells in Ras–RAG mice, we assayed for expression of various genes used to define stages of B cell differentiation. RT-PCR assays demonstrated that populations of splenic or lymph node Ras–RAG B lineage cells expressed substantial levels of λ5 and RAG2 transcripts, which are normally transcribed in the pro-B and pre-B cells but generally are absent during later stages of development (23, 28). On the basis of semiquantitative RT-PCR analyses, we determined that Ras–RAG cell populations expressed λ5 and RAG2 at levels comparable to those in purified wild-type pre-B cells (Fig. 3). In normal mice, productive rearrangement and expression of μ HC genes in developing B cell progenitors leads to the transcriptional activation and rearrangement of κ light chain genes (29–33). To study if signaling by activated Ras could mimic the induction of κ germline transcription normally induced by expression of HC in pro-B cells, we determined the levels of germline κ transcripts in Ras–RAG B lineage cells. We observed that such transcripts were present in Ras–RAG B lineage cells at levels similar to those in wild-type pre-B cells (Fig. 4). These results suggest that activated Ras signaling in RAG-deficient B lineage cells promotes transcriptional activation of the κ light chain gene locus.
Figure 3.
Analysis of λ5 and RAG expression. RNA isolated from Ras–RAG1−/− (Ras-Rag), wild-type mature B (WT B), and wild-type pre-B (WT Pre-B) lymphocytes purified from lymph node and spleen was reverse transcribed, and first-strand cDNA was subjected to PCR analysis using λ5, RAG2, and β-actin primer pairs (see Materials and Methods). For each B lineage cell type, serial fivefold dilutions are shown. ES cell cDNA was added to the 5- and 25-fold dilutions of each B lineage cDNA sample to equalize template quantity. Single lanes containing no cDNA (H2O) or ES cDNA alone are shown for each primer pair.
Figure 4.
κ light chain locus germline transcripts are upregulated in Ras–RAG B lineage cells. RNAs from sorted B220+ Ly 9.1+Ras– RAG1−/− (Ras-Rag) B lineage cells, from B220+ CD43−IgM− wild-type pre-B cells (WT Pre-B), and from B220+CD43+ RAG2-deficient pro-B cells (Rag Pro-B) were subjected to RT-PCR analysis for detection of germline κ transcripts. Serial fivefold dilutions are shown, and ES cell cDNA was diluted into 5- and 25-fold dilutions. Samples with (+) and without (–) reverse transcriptase are indicated.
Given the large number of peripheral B lineage cells in Ras–RAG mice, we further assayed for staining of more mature B cell surface markers. On the basis of these assays, we also found that the Ras–RAG B lineage cells in both the bone marrow and periphery expressed surface antigens usually associated with later stages of B cell development, such as the low affinity IgE Fc receptor CD23 (34), the BCR coreceptor CD22 (35), and complement receptor CD21/CD35 (36) (Fig. 2 and data not shown). Thus, our data suggest that expression of activated Ras results in the development of RAG-deficient B lineage cells that retain major properties of pre-B lymphocytes while also expressing cell surface markers usually found only in mature B cell stages. These Ras–RAG B lineage cells are distinct from those generated in the RAG-deficient background via expression of an Ig μ HC transgene, which only promotes differentiation to cells that show pre-B cell characteristics and remain primarily in the bone marrow (6, 7), but are similar in patterns of gene expression and tissue distribution to those observed in μ HC/Bcl-2 double transgenic, RAG-deficient mice (22, 37).
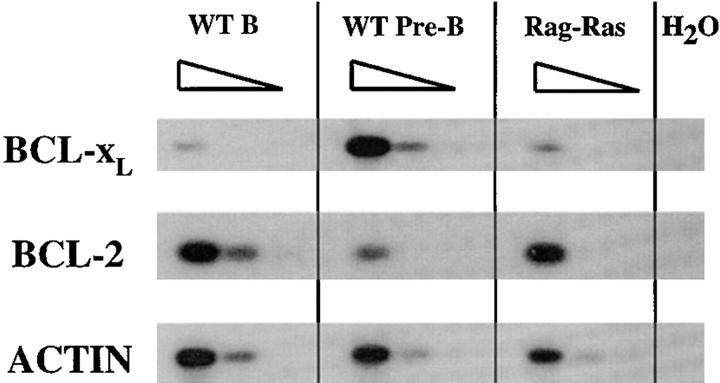
The similarity of the Ras–RAG phenotype to that promoted by μ HC plus Bcl-2 transgenes suggested to us that activated Ras may signal both differentiative and cell survival processes. During normal B cell development, the antiapoptotic gene Bcl-2 is expressed at the pro-B stage, but is downregulated in pre-B cells and later upregulated in mature B lymphocytes (23, 38). In contrast, the cell survival gene Bcl-xL displays a reciprocal pattern of expression, with high levels in pre-B cells that are downregulated in mature B cells (39). To assay for Bcl-2 and Bcl-xL expression in sorted Ras–RAG peripheral B lineage cells, we used RT-PCR analysis and determined that the expression levels of Bcl-2 and Bcl-xL in Ras–RAG B cells were more comparable with those in wild-type mature B cells with substantial levels of Bcl-2 expression (Fig. 5).
Figure 5.
Analysis of Bcl-2 and Bcl-xL expression. RNAs from sorted Ras–RAG1−/− (Ras-Rag), mature wild-type B (WT B), and wild-type pre-B (WT Pre-B) lymphocytes were subjected to RT-PCR analysis, using β-actin expression as a standard. Serial fivefold dilutions are shown for each B lineage cell type.
Discussion
Several studies have implicated Ras as an intermediate in signal transduction downstream of the BCR (17, 18). Our studies of Ras–RAG mice demonstrate that activated Ras can induce differentiation of pro-B cells in the absence of μ HC. B lineage cells that develop in Ras–RAG mice acquired characteristics shared with normal pre-B cells, including expression of RAG and λ5, and the induction of germline κ light chain locus transcripts. However, these cells also expressed surface markers characteristic of more mature stages of B lymphopoiesis. Thus, we believe it is most likely that Ras–RAG B cells have progressed in their differentiation beyond the pre-B cell stage, at least to the pre-B–B cell junction. It also remains possible, given their peripheral location, that Ras–RAG B cells may be developmentally similar to the recently described subset of germinal center B cells that reinitiate light chain gene rearrangement as a result of antigenic challenge (40–42). Although such cells, unlike Ras–RAG B cells, lack CD23 expression (43), both cell types demonstrate the concurrent expression of λ5 and RAG genes with mature B cell surface markers. The accumulation of the Ras–RAG B lineage cells in the periphery could be due to several potential effects of activated Ras expression, including acquisition of surface markers necessary for transit from the bone marrow or prolonged survival allowing exit from the marrow and accumulation in the periphery.
Previous work has demonstrated that the introduction of a rearranged μ transgene into RAG-deficient pro-B cells induced their differentiation to pre-B cells (6, 7). Although such cells remained predominantly within the bone marrow and did not express surface markers characteristic of more mature B cells, the expression of a Bcl-2 transgene in the B lineage of μ-RAG mice resulted in the appearance of cells in the marrow and periphery with a phenotype that resembles B lineage cells found in Ras–RAG mice (22, 37). These findings suggested that survival signals provided by Bcl-2 may advance B lymphopoiesis beyond the stage achieved by μ HC alone. In this regard, we found that Ras–RAG cells expressed significantly higher levels of endogenous Bcl-2 than normal pre-B cells; in fact, the observed Bcl-2 expression levels approached those in mature B cells. At present, we do not know whether this upregulation of Bcl-2 expression in Ras–RAG cells is directly induced by activated Ras, or, alternatively, occurs as a result of developmental progression to a more mature stage. Nevertheless, the phenotypic similarities between μ HC/Bcl-2/RAG and Ras–RAG B cells suggest that introduction of activated Ras may induce and/or enable both differentiation and survival signals in RAG-deficient and, presumably, normal progenitor B lineage cells.
Our finding that B cells in Ras–RAG mice develop to a stage beyond that of B cells in μ-RAG mice indicates that signaling events triggered by constitutively activated Ras may surpass or differ from those initiated upon HC-mediated activation of endogenous Ras. In this context, in other experimental systems the effects of activated Ras on cultured cells varied depending on the level and duration of Ras expression (44). It is also possible that the expression of activated Ras in B lineage cells mimics signaling from other surface receptors, in addition to the pre-BCR, which normally trigger endogenous Ras. Numerous Ras effector pathways have been identified to date, including a well-characterized mitogen-activated protein kinase cascade and a growing number of stress-activated protein kinase cascades (45). Ras has also been shown to induce phosphatidylinositol-3 kinase (46, 47), as well as the Rho family of GTPases which regulate the actin cytoskeleton (48). It remains to be established which of these (or other) Ras-effector pathways are involved in mediating the developmental progression of pro-B cells. Selective engagement of Ras effectors using activated mutant alleles may facilitate further elucidation of these issues.
Recent data demonstrate that Ras signaling is used during several stages of B and T lymphocyte development. For example, a dominant negative Ras transgene was shown to cause an incomplete block in B cell development at the earliest known B cell precursor stage before B220+CD43+ pro-B cells (49). In T lineage cells, several studies with dominant negative alleles implicated the Ras/Raf/Mitogen-activated protein kinase pathway in the development of CD4+CD8+ (DP) thymocytes and mature T cells (50– 52). Notably, a complete reconstitution of DP thymocytes was induced by activated Ras in RAG-deficient mice; however, in these mice no developmental progression beyond the DP stage was observed, and no T cells were detected in the peripheral lymphoid organs (16). These results suggested that additional signals are required for the T cell positive selection process that normally results from signaling events accompanying ligation of the T cell receptor with self-MHC ligands of specific avidity (53). Such a requirement for additional signaling events, independent of Ras, in the development of T cells beyond the DP stage suggests that an important distinction may exist in the signals required to effect further development of precursor B versus precursor T lineage cells.
Acknowledgments
We thank Juanita Campos-Torres for invaluable cell sorting assistance, and Drs. Yansong Gu, Timo Breit, and Nienke van der Stoep for helpful advice and discussions.
This work was supported in part by National Institutes of Health grants AI20047 (to F.W. Alt) and AI01532-01 (to A.C. Shaw). A.C. Shaw was a recipient of a Howard Hughes Medical Institute Postdoctoral Research Fellowship for Physicians. W. Swat is a recipient of the Arthritis Foundation Hulda Irene Duggan Investigator Award. F.W. Alt is an investigator of the Howard Hughes Medical Institute.
Abbreviations used in this paper
- BCR
B cell receptor
- ES
embryonic stem
- DP
double positive (CD4+ CD8+)
- HC
immunoglobulin heavy chain
- RAG
recombinase-activating gene
- RT
reverse transcriptase
References
- 1.Willerford DM, Swat W, Alt FW. Developmental regulation of V(D)J recombination and lymphocyte differentiation. Curr Opin Genet Dev. 1996;6:603–609. doi: 10.1016/s0959-437x(96)80090-6. [DOI] [PubMed] [Google Scholar]
- 2.Karasuyama H, Rolink A, Melchers F. Surrogate light chain in B cell development. Adv Immunol. 1996;63:1–41. doi: 10.1016/s0065-2776(08)60853-6. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 4.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall A, Alt FW. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 5.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 6.Young F, Ardman B, Shinkai Y, Lansford R, Blackwell TK, Mendelsohn M, Rolink A, Melchers F, Alt FW. Influence of immunoglobulin heavy- and light-chain expression on B-cell differentiation. Genes Dev. 1994;8:1043–1057. doi: 10.1101/gad.8.9.1043. [DOI] [PubMed] [Google Scholar]
- 7.Spanopoulou E, Roman CA, Corcoran LM, Schlissel MS, Silver DP, Nemazee D, Nussenzweig MC, Shinton SA, Hardy RR, Baltimore D. Functional immunoglobulin transgenes guide ordered B-cell differentiation in Rag-1-deficient mice. Genes Dev. 1994;8:1030–1042. doi: 10.1101/gad.8.9.1030. [DOI] [PubMed] [Google Scholar]
- 8.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 9.Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Igβ. Science. 1996;272:411–414. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- 10.Torres RM, Flaswinkel H, Reth M, Rajewsky K. Aberrant B cell development and immune response in mice with a compromised BCR complex. Science. 1996;272:1804–1808. doi: 10.1126/science.272.5269.1804. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 12.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VLJ. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 13.DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 14.Boguski MS, McCormick F. Proteins regulating Ras and its relatives. Nature. 1993;366:643–654. doi: 10.1038/366643a0. [DOI] [PubMed] [Google Scholar]
- 15.Kazlauskas A. Receptor tyrosine kinases and their targets. Curr Opin Genet Dev. 1994;4:5–14. doi: 10.1016/0959-437x(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 16.Swat W, Shinkai Y, Cheng H-L, Davidson L, Alt FW. Activated Ras signals differentiation and expansion of CD4+8+thymocytes. Proc Natl Acad Sci USA. 1996;93:4683–4687. doi: 10.1073/pnas.93.10.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarus AH, Kawauchi K, Rapoport MJ, Delovitch TJ. Antigen-induced B lymphocyte activation involves the p21rasand ras GAP signaling pathway. J Exp Med. 1993;178:1765–1769. doi: 10.1084/jem.178.5.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harwood AE, Cambier JC. B cell antigen receptor cross-linking triggers rapid protein kinase C independent activation of p21ras1 . J Immunol. 1993;151:4513–4522. [PubMed] [Google Scholar]
- 19.Blankenstein T, Winter E, Muller W. A retroviral expression vector containing murine immunoglobulin heavy chain promoter/enhancer. Nucleic Acids Res. 1988;16:10939. doi: 10.1093/nar/16.22.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibuya EK, Polverino AJ, Chang E, Wigler M, Ruderman JV. Oncogenic Ras triggers the activation of 42-kDa mitogen-activated protein kinase in extracts of quiescent Xenopus oocytes. Proc Natl Acad Sci USA. 1992;89:9831–9835. doi: 10.1073/pnas.89.20.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Lansford R, Stewart V, Young F, Alt FW. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci USA. 1993;90:4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young F, Mizoguchi E, Bhan AK, Alt FW. Constitutive Bcl-2 expression during immunoglobulin heavy chain-promoted B cell differentiation expands novel precursor B cells. Immunity. 1997;6:23–33. doi: 10.1016/s1074-7613(00)80239-3. [DOI] [PubMed] [Google Scholar]
- 23.Li YS, Hayakawa K, Hardy RR. The regulated expression of B lineage associated genes during B cell differentiation in bone marrow and fetal liver. J Exp Med. 1993;178:951–960. doi: 10.1084/jem.178.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider TJ, Grillot D, Foote LC, Nunez GE, Rothstein TL. Bcl-x protects primary B cells against Fas-mediated apoptosis. J Immunol. 1997;159:4834–4839. [PubMed] [Google Scholar]
- 25.Schlissel MS, Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989;58:1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- 26.Gorman JR, van der Stoep N, Monroe R, Cogne M, Davidson L, Alt FW. The Igκ 3′ enhancer influences the ratio of Igκ versus Igλ B lymphocytes. Immunity. 1996;5:241–252. doi: 10.1016/s1074-7613(00)80319-2. [DOI] [PubMed] [Google Scholar]
- 27.Leon J, Guerrero I, Pellicer A. Differential expression of the ras gene family in mice. Mol Cell Biol. 1987;7:1535–1540. doi: 10.1128/mcb.7.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grawunder U, Leu TMJ, Schatz DG, Werner A, Rolink AG, Melchers F, Winkler TH. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–608. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 29.Alt F, Rosenberg N, Lewis S, Thomas E, Baltimore D. Organization and reorganization of immunoglobulin genes in A-MuLV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981;27:381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- 30.Ritchie KA, Brinster RL, Storb U. Allelic exclusion and control of endogenous immunoglobulin gene rearrangement in κ transgenic mice. Nature. 1984;312:517–520. doi: 10.1038/312517a0. [DOI] [PubMed] [Google Scholar]
- 31.Reth MG, Ammirati P, Jackson S, Alt FW. Regulated progression of a cultured pre-B cell line to the B cell stage. Nature. 1985;317:353–355. doi: 10.1038/317353a0. [DOI] [PubMed] [Google Scholar]
- 32.Reth M, Petrac E, Wiese P, Lobel L, Alt FW. Activation of V kappa gene rearrangement in pre-B cells follows the expression of membrane-bound immunoglobulin heavy chains. EMBO (Eur Mol Biol Organ) J. 1987;6:3299–3305. doi: 10.1002/j.1460-2075.1987.tb02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nussenzweig MC, Shaw AC, Sinn E, Danner DB, Holmes KL, Morse HC, Leder P. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin μ. Science. 1987;236:816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- 34.Wells, S.M., A.M. Stall, A.B. Kantor, and L.A. Herzenberg. 1995. Development of B-cell subsets. In Immunoglobulin Genes, 2nd Edition. T. Honjo and F.W. Alt, editors. Academic Press, San Diego. 83–101.
- 35.Tedder TF, Tuscano J, Sato S, Kehrl JH. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu Rev Immunol. 1997;15:481–504. doi: 10.1146/annurev.immunol.15.1.481. [DOI] [PubMed] [Google Scholar]
- 36.Tedder TF, Inaoki M, Sato S. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6:107–118. doi: 10.1016/s1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- 37.Tarlinton DM, Corcoran LM, Strasser A. Continued differentiation during B lymphopoiesis requires signals in addition to cell survival. Int Immunol. 1997;9:1481–1494. doi: 10.1093/intimm/9.10.1481. [DOI] [PubMed] [Google Scholar]
- 38.Merino R, Ding L, Veis DJ, Korsmeyer SJ, Nunez G. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO (Eur Mol Biol Organ) J. 1994;13:683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi MSK, Holman M, Atkins CJ, Klaus GGB. Expression of bcl-x during mouse B cell differentiation and following activation by various stimuli. Eur J Immunol. 1996;26:676–682. doi: 10.1002/eji.1830260325. [DOI] [PubMed] [Google Scholar]
- 40.Hikida M, Mori M, Takai T, Tomochika K-I, Hamatani K, Ohmori H. Reexpression of RAG-1 and RAG-2 genes in activated mature mouse B cells. Science. 1996;274:2092–2094. doi: 10.1126/science.274.5295.2092. [DOI] [PubMed] [Google Scholar]
- 41.Han S, Zheng B, Schatz DG, Spanopoulou E, Kelsoe G. Neoteny in lymphocytes: Rag1 and Rag2 expression in germinal center B cells. Science. 1996;274:2094–2097. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- 42.Han S, Dillon SR, Zheng B, Shimoda M, Schlissel MS, Kelsoe G. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–305. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- 43.Kelsoe G. In situ studies of the germinal center reaction. Adv Immunol. 1995;60:267–288. doi: 10.1016/s0065-2776(08)60587-8. [DOI] [PubMed] [Google Scholar]
- 44.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 45.Su B, Karin M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr Opin Immunol. 1996;8:402–411. doi: 10.1016/s0952-7915(96)80131-2. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH-kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Viciana P, Warne PH, Vanhaesebroeck B, Waterfield MD, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO (Eur Mol Biol Organ) J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]
- 48.Reif K, Cantrell DA. Networking Rho family GTPases in lymphocytes. Immunity. 1998;8:395–401. doi: 10.1016/s1074-7613(00)80545-2. [DOI] [PubMed] [Google Scholar]
- 49.Iritani BM, Forbush KA, Farrar MA, Perlmutter RM. Control of B cell development by Ras-mediated activation of Raf. EMBO (Eur Mol Biol Organ) J. 1997;16:7019–7031. doi: 10.1093/emboj/16.23.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crompton T, Gilmour KC, Owen MJ. The MAP kinase pathway controls differentiation from double-negative to double-positive thymocyte. Cell. 1996;86:243–251. doi: 10.1016/s0092-8674(00)80096-3. [DOI] [PubMed] [Google Scholar]
- 51.Swan KA, Alberola-Ila J, Gross JA, Appleby MW, Forbush KA, Thomas JF, Perlmutter RM. Involvement of p21ras distinguishes positive and negative selection in thymocytes. EMBO (Eur Mol Biol Organ) J. 1995;14:276–285. doi: 10.1002/j.1460-2075.1995.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alberola-Ila J, Forbush KA, Seger R, Krebs EG, Perlmutter RM. Selective requirement for MAP kinase activation in thymocyte differentiation. Nature. 1995;373:620–623. doi: 10.1038/373620a0. [DOI] [PubMed] [Google Scholar]
- 53.Kisielow P, von Boehmer H. Development and selection of T cells: facts and puzzles. Adv Immunol. 1995;58:87–209. doi: 10.1016/s0065-2776(08)60620-3. [DOI] [PubMed] [Google Scholar]