Abstract
To investigate the influence of endogenous peptides on the developmental processes that occur during thymocyte selection, we have used monoclonal antibodies that preferentially recognize the major histocompatibility complex (MHC) molecule I-Ek when it is bound to the moth cytochrome c peptide (88–103). One of these antibodies (G35) specifically blocks the positive selection of transgenic thymocytes expressing a T cell receptor that is reactive to this peptide– MHC complex. Furthermore, G35 does not block the differentiation of transgenic T cells bearing receptors for a different I-Ek–peptide complex. This antibody recognizes a subset of endogenous I-Ek–peptide complexes found on a significant fraction of thymic antigen-presenting cells, including cortical and medullary epithelial cells. The sensitivity of G35 to minor alterations in peptide sequence suggests that the thymic peptide–MHC complexes that mediate the positive selection of a particular class II MHC–restricted thymocyte are structurally related to the complexes that can activate it in the periphery.
Keywords: thymic selection, peptides, CD4+ T cell, thymocyte, monoclonal antibodies
Tcell maturation depends on the specificity of thymocyte TCRs for peptide–MHC complexes that are expressed in the thymus (1–3). Thymocytes that fail to transduce a signal in response to thymic MHC do not mature and instead die in the thymus. The surviving T cells that may eventually emigrate to the periphery are said to be positively selected. The result of this positive selection is a repertoire of thymocytes bearing TCRs that are reactive to self-MHC when it is bound to both foreign and endogenous peptides. Before leaving the thymus cells that react strongly with thymic peptide–MHC complexes are eliminated by activation-induced cell death or negative selection (1). Together, these processes select a peripheral TCR repertoire composed of cells that preferentially recognize self-MHC molecules bound to foreign peptides.
The molecular basis by which the same MHC molecule can mediate these different developmental decisions remains unclear (for review see reference 4). Several factors may influence the fate of a given thymocyte. These include its developmental stage at the time it receives a signal, the characteristics of the APCs that interact with it, and the nature of the peptide–MHC complexes that are displayed by the APCs. Here we employ TCR transgenic mice in which all T cells express the same TCR to examine the influence of peptides on the positive selection of a particular TCR specificity.
Peptides are clearly required for the positive selection of a complete T cell repertoire, yet the precise role of peptide sequence in these processes is not well understood (for review see reference 4). Positive selection by both class I and class II MHC molecules is inhibited by mutations that diminish peptide loading and/or surface expression of MHC molecules (5–10). Addition of certain peptides to fetal thymic organ cultures restores the positive selection of class I–restricted TCR transgenic thymocytes (11–15). Although the peptides that can positively select a given TCR are varied, many resemble (or in one case are identical to) the peptides that can stimulate the TCR transgenic T cells in the periphery. More recently, endogenous peptides that induce the positive selection of CD8+ class I–restricted T cells have been identified. Surprisingly, these self-peptides bear little resemblance (in sequence) to the peptides that are known to activate the mature TCR transgenic cells (16, 17).
Because in vitro rescue of positive selection has not been possible for CD4+ T cells, studies of class II–restricted T cells have examined class II–mediated positive selection in vivo. Mice that have been genetically modified so that they express class II MHC molecules bound to a single peptide generate a large peripheral CD4+ T cell population and an unperturbed repertoire of TCR β chains (8–10, 18, 19). However, these “single-complex mice” do not select all TCR specificities and fail to support the development of several specific transgenic TCRs (20–22). Thus, although a peptide's sequence and/or concentration may direct an individual thymocyte toward either positive or negative selection, current models still fail to predict the selective properties of an individual peptide.
To refine these models, several questions remain. In particular, what are the endogenous peptides that normally induce positive selection of a particular class II–restricted TCR specificity? How are these peptides related to the nominal peptide(s) to which a mature T cell responds? And finally, what is the expression level and tissue distribution of these endogenously expressed peptides? Without such information, it is difficult to assess how other components of thymocyte recognition, such as affinity and coreceptor stimulation, contribute to the distinction between positive and negative selection.
To investigate these aspects of the role of peptide in the class II system, we have used mAbs that recognize the class II MHC molecule I-Ek only when it is bound to peptides that resemble the COOH-terminal portion of moth cytochrome c (MCC 88–103).1 One of these antibodies specifically blocks positive selection of TCR transgenic thymocytes that bear a receptor specific for this same peptide–MHC combination. The distribution of this antibody epitope in the thymus indicates that this endogenous complex is present in both the thymic cortex and the medulla. Thus, not only are a subset of endogenous I-Ek–associated peptides essential for positive selection, but the sequence or structure of these endogenous peptides must be related to the peptides that can stimulate the selected T cell in the periphery.
Materials and Methods
Antibodies
mAbs were generated by immunizing mice with soluble MCC–I-Ek complexes and purified with a 1:1 mixture of protein A– and protein G–sepharose (Pharmacia) from tissue culture supernatants derived from bulk culture of hybridomas grown in roller bottles, or on a CellPharm (Unisyn Technologies) using RPMI supplemented with penicillin-streptomycin and 10% fetal calf serum (Gemini). Purified antibodies were concentrated by ultrafiltration, and yields were quantified by absorbance. Where necessary, antibodies were conjugated to FITC (Molecular Probes), biotin (Pierce), or digoxigenin (Boehringer Mannheim) using standard protocols.
Mice
Specific pathogen-free B10.Br, C57BL/6, and AKR/J mice were obtained from The Jackson Laboratory and crossed to mice transgenic for the TCR derived from the T cell clone 5C.C7 (23) or the T cell hybridoma A18 (24). All mice were 4–8 wk of age or 1–3-d-old neonates. Mouse handling and experimental procedures were conducted in accordance with institutional guidelines for animal care and use.
Antibody Administration In Vivo
Male 5C.C7 TCR transgenic mice were crossed under specific pathogen-free conditions with B10.Br females (The Jackson Laboratory). Pregnant mice were removed on the day after impregnation (day 0), and were injected intraperitoneally with 1 mg per day of the indicated antibodies in 100 μl sterile PBS.
Preparation of Stromal Cells
Thymuses were digested for 45 min at 37°C with collagenase type IV (Sigma Chemical Co.) and dissociated by vigorous pipetting. Stromal cell cultures were then grown for 5–8 d in selective media (25) and either incubated with thymocytes for the in vitro positive selection assays or removed from the plate by a combination of collagenase treatment and vigorous pipetting for FACS® analysis.
In Vitro Positive Selection Assays
5C.C7 Only Assays.
Unsorted 5C.C7 cells from 5C.C7/ C57BL/6 TCR transgenic, 4-wk-old females were isolated by passing the cells through nylon mesh and washing. These cells were then incubated with thymic stromal cell cultures at 37°C for the indicated times.
A18/5C.C7 Assays.
Purified unselected CD4+8+ thymocytes were isolated from positively selecting strains by four-color flow cytometry. Dead thymocytes and non-T cells were excluded by gating out dead cells staining positive for propidium iodide or thymic APCs that were positive for class II MHC or B220. 2 × 106 CD4lo/+CD8lo/+CD69− cells were sorted from each transgenic mouse. 2 × 105 immature thymocytes per well were incubated with 5–8-d-old stromal cell cultures for 2.5 d. Cells were removed by incubation at 4°C in PBS/2% FCS/2.5 mM EDTA followed by vigorous pipetting. Thymocytes were subsequently stained for the transgenic α or β chain, CD4, CD8, and class II MHC/B220.
FACS® Analyses
In Vivo Positive Selection Assay.
Cells from neonatal thymuses were isolated by pushing whole thymuses through metal screens. Cells were stained with CD4-PE, CD8-APC (Caltag), KJ25, followed by anti–hamster Texas Red, and rabbit anti-5C.C7 Vα followed by anti–rabbit-FITC. Dead cells were excluded from analyses by size and failure to exclude propidium iodide.
In Vitro Positive Selection Assay.
Cells were stained with anti-Vα11–FITC (clone RR8.1; PharMingen) or anti Vβ8 (clone F23.1-FITC; gift from Dr. Irving Weissman, Stanford University, Stanford, CA), anti–I-Ak/b-biotin, B220-biotin followed by streptavidin-PE (all from PharMingen), CD4-APC (clone GK1.5; Caltag Labs.), and CD8-Texas Red (Elizabeth Kerr, Stanford University) before analysis on a modified FACS® II dual laser flow cytometer (Becton Dickinson) and analyzed using FACS® Desk software (Beckman Center Shared FACS Facility, Stanford, CA). Where necessary, stromal cells and dead cells were excluded from analysis by gating out propidium iodide– and class II MHC/ B220–positive cells. Stromal cells were stained with anti I-Ek (14.4.4S-biotin) followed by avidin–Texas Red, B220-APC (clone RA3-6B2), CD3-PE (clone 145-2C11) (all PharMingen, San Diego, CA), and G35 or D4 directly conjugated to FITC.
ELISA
Plates were coated with 10 μg/ml of the anti–I-Ek antibody G14 (Reay, P.A., unpublished data). Soluble propidium iodide– linked I-Ek (26) was incubated for 3 d at 37°C in citrate phosphate buffer, pH 5.0, in the presence of a 50-fold molar excess of each of the 220 single amino acid substituted peptide variants (27). After neutralization, 50 μl of a 10 μM solution of each variant-complex was captured in the presence of 2% BSA. After extensive washing, biotinylated G35 was titrated on each complex, and the amount bound was assessed using alkaline phosphatase– conjugated avidin (Biomeda). The amount of G35 resulting in one-half maximal signal was determined by curve fitting and extrapolation.
Immunohistochemistry
Light and electron microscopic immunohistochemical studies were performed as described previously (28, 29). Digoxigenin-conjugated mAbs were detected with horseradish peroxidase– conjugated Fab fragments of goat anti-digoxigenin antibodies (Boehringer Mannheim). Light microscopic studies were done with fresh frozen sections fixed by immersion in cold acetone. Ultrastructural immunohistochemistry was performed on thin slices of thymus tissue previously fixed by vascular perfusion with 4% paraformaldehyde. After demonstration of peroxidase activity with hydrogen peroxide and 3,3′ diaminobenzidine, these tissue sections were treated with OsO4, and then processed for conventional electron microscopy.
Purification of Thymic I-Ek
Thymuses were removed from 327 B10.Br mice and placed on ice in PBS. Thymuses were dissociated by dounce homogenization and lysed in PBS containing protease inhibitors (2 mM PMSF, 100 μM iodoacetamide, 5 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin A, 3 ng/ml EDTA, and 0.2% sodium azide) and 2% Mega-9 detergent (Sigma Chemical Co.) for 1 h. The detergent lysate was spun at 100,000 g for 1 h to remove nuclei and insoluble protein. The lysate was then cleared by filtration through 0.22-μm filters (Corning) and run serially over five cyanogen bromide–conjugated antibody columns in the following order: CN-Br conjugated to total mouse IgG, CN-Br D4, CN-BR-G35A, CN-Br G35 B, and CN-Br 14.4.4. Each column was washed extensively with detergent containing Tris, pH 8.0, plus 150 mM NaCl, then Tris, pH 8.0, plus 150 mM NaCl, Tris, pH 8.0, plus 0.5 M NaCl, Tris, pH 8.0, and finally eluted with acetic acid, pH 2.7, containing 10 ng/ml leupeptin as a carrier. Fractions were acidified to 2.1 with glacial acetic acid and boiled for 10 min to release peptides. Fractions were then separated on a Millipore Ultrafree CL device with a 5,000 dalton cut-off (Millipore UFC4LCC25).
Western Blotting Analysis/SDS-PAGE
SDS-PAGE was performed according to standard methods. Samples were collected from the top (>5 kD) portion of Millipore Ultrafree CL devices and resuspended in running buffer containing SDS and the reducing agent β-ME. The 12% gels with the 14.4.4 and G35 samples were prepared and run in parallel. Proteins were transferred overnight at 4°C onto Hybond filter paper that was then blocked with 2% dehydrated milk/PBS/0.1% Tween solution. The blots were incubated with polyclonal rabbit anti-I-Ek serum in blocking solution, washed, and incubated with goat anti–rabbit HRP (Biomeda). Horseradish peroxidase activity was visualized with the ECL detection kit (Amersham).
Microbore HPLC Analysis of Peptides
Fractions were concentrated by lyophilization and peptides from equivalent amounts of total I-Ek were separated by reverse-phase HPLC. Samples were separated on a C18 column (1 × 150 mM; Reliosil) with a linear water/acetonitrile gradient in 0.1% TFA (0–45% acetonitrile, 30 min) at a flow rate of 75 μl/min. Chromatograms are displayed as absorbance at 209 nm versus retention time in minutes.
Results
G35 Blocks Positive Selection of I-Ek/MCC–reactive T Cells In Vivo.
We have generated several mAbs that specifically recognize the class II MHC molecule I-Ek bound to a peptide derived from MCC 88–103. Two of these antibodies (D4 and G35) bind to the same region of this complex as T cells and inhibit the activation of T cells that recognize this complex (Reay, P.A., unpublished data). We wondered if these antibodies might also inhibit the activation events involved in thymic selection.
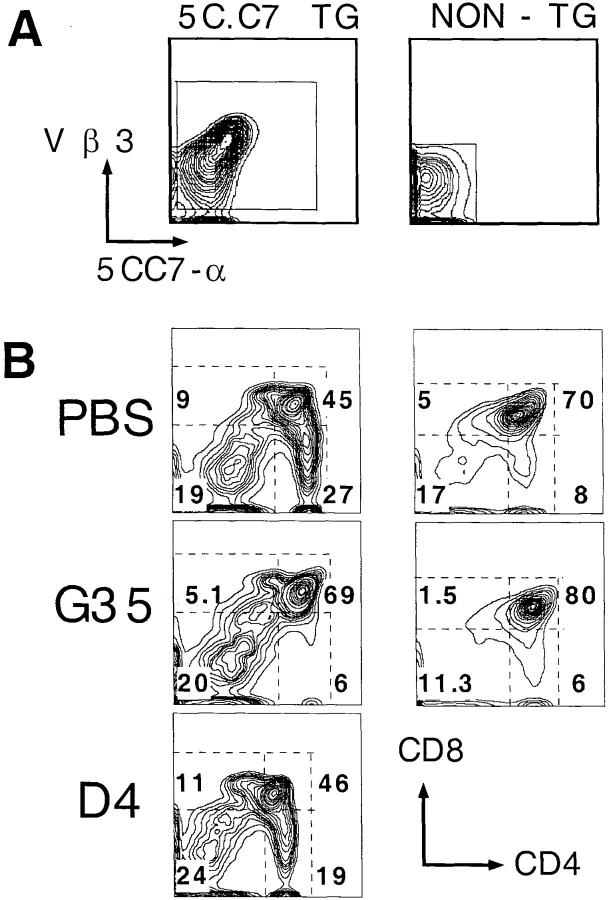
To address this question, we injected mice with G35, D4, and PBS and analyzed the effects of these antibodies on the development of I-Ek–MCC-specific thymocytes that express the TCR transgene 5C.C7. To provide nontransgenic internal controls, 5C.C7 TCR transgenic males were crossed to nontransgenic females to generate litters in which 50% of the progeny were transgenic. The pregnant females were injected intraperitoneally each day (from day 15 of gestation until birth) with either 1 mg of antibody or PBS. On day 21, thymuses were harvested from the neonates and four-color flow cytometry was used to determine the transgenic status and CD4 versus CD8 profile of each mouse (Fig. 1 A). In transgenic pups, the percentage of identified transgene-positive cells was ∼80% in all treatment groups; the rest of the cells were presumably immature cells that lacked detectable TCR expression or thymic APCs.
Figure 1.
Effect of G35 on the selection of 5C.C7 transgenic thymocytes. (A) Distribution of 5C.C7-α chain and KJ25 (Vβ3) determinants on thymocytes derived from transgenic (left) or nontransgenic (right) pups. (B) Distribution of CD4 and CD8 on separate populations of cells derived from transgenic or nontransgenic pups exposed in utero to G35, PBS, or D4 as indicated. Numbers refer to the percentage of total live transgenic or nontransgenic thymocytes that fall within each boxed population.
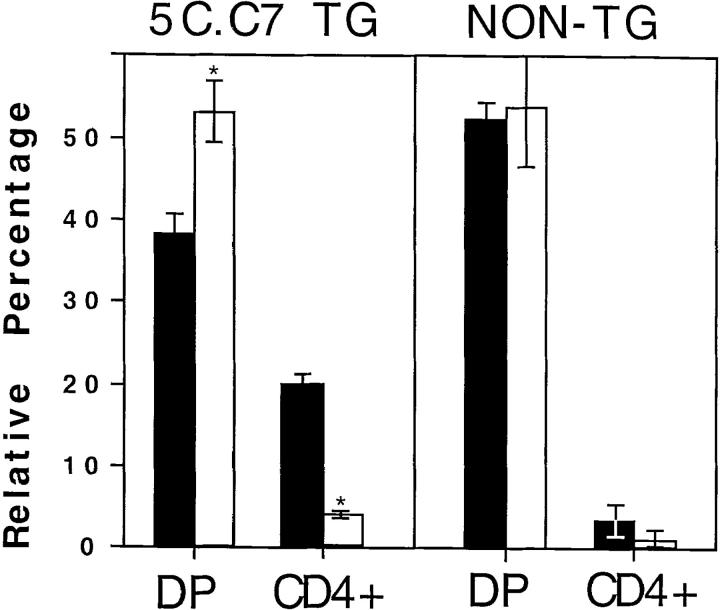
Analysis of several pups from each mother showed that the G35-treated transgenic mice are almost devoid of CD4+8− cells, whereas the nontransgenic pups produce normal numbers of CD4+8− cells. Representative plots of CD4 versus CD8 from one nontransgenic and one transgenic neonate from mothers that were injected with PBS or the relevant mAb are displayed in Fig. 1 B. Because even identically treated littermates often exhibit substantial variation in the percentage of cells in each thymic subset, the average percentage of total live cells that fell into the CD4+ or CD4+8+ gates in each treatment group was computed and plotted (Fig. 2). G35 treatment of transgenic mice causes a fourfold reduction in the percentage of positively selected CD4+8− thymocytes present at day 21, while modestly increasing the relative proportion of CD4+8+ thymocytes. Notably, treatment with G35 antibody has no reproducible or significant effect on the thymocyte profiles of the nontransgenic littermates of the transgenic mice. Similarly, injections with PBS or the isotype-matched control antibody, D4, did not perturb thymic development in transgenic or nontransgenic mice. All transgenic and nontransgenic pups had equivalent circulating levels of G35 (45–68 μg/ml) and D4 (88–113 μg/ml).
Figure 2.
Effects of antibody treatment on thymocyte subpopulations. The figure shows the average percentage of total live cells with the indicated CD4/ CD8 expression patterns from three to five transgenic or nontransgenic neonates born to mothers treated with G35 (white bars) or PBS (black bars). Error bars represent 1 SD.
G35 Blocks Positive Selection of I-Ek/MCC–reactive T Cells In Vitro.
In previous studies, injection of anti–class II MHC mAbs that are peptide independent inhibited positive selection of CD4+ T cells (30, 31). This effect may have resulted from a simple blockade of TCR access to MHC or, alternatively, long-term antibody treatment may have caused general downregulation of MHC or killed the MHC-bearing cells required for positive selection. Because we were interested in peptide-specific effects of antibody treatment we wanted to exclude the possibility of complete ablation of positive selection. Thus, we examined the effect of anti-MHC antibodies in vitro by using short-term thymic stromal cell explant cultures (25).
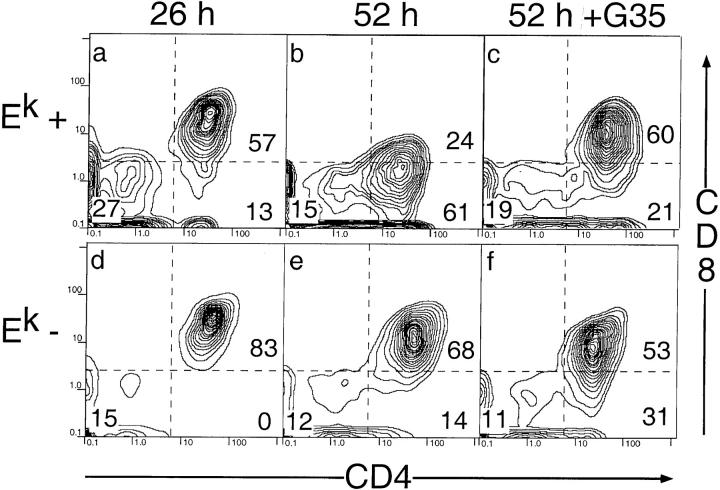
Thymic epithelial cells were isolated from thymuses taken from 3–4-wk-old mice and grown in media that selectively kills fibroblast-derived cells to enrich for thymic stromal epithelial cells (32). To provide unmanipulated immature CD4+8+ cells bearing the 5C.C7 receptor, TCR transgenic mice were crossed to a nonselecting background (C57BL/6). The majority of the immature thymocytes from these mice are immature CD4+8+CD69− TCRlow/intermediate cells that fail to initiate CD8 downregulation even after 52 h in culture (Baldwin, K.K., unpublished data and reference 33). In contrast, when these immature thymocytes are cocultured with thymic stromal cells that express I-Ek, they begin to downregulate CD8 after 26 h (Fig. 3, a and d). After 52 h, the thymocytes still attached to the I-Ek–positive stromal cells are almost entirely mature CD4+8− cells (Fig. 3, b and e). Additional FACS® analyses show that these cells express the high levels of CD69 and low levels of HSA that are characteristic of differentiated CD4+8− thymocytes (Baldwin, K.K., unpublished data).
Figure 3.
G35 blocks 5C.C7 transgenic T cell differentiation in vitro. Analysis of CD4/8 expression on thymocytes removed from stromal cell cultures derived from B10.Br (top) or C57BL/6 (bottom) mice after 26 (a and d) or 52 h (b, c, e, and f). G35 was added to a final concentration of 50 μg/ml in c and f. Numbers on plots refer to the percent of total live transgenic thymocytes that fall within each boxed population.
Importantly, as in the in vivo experiments, the presence of 50 μg/ml of G35 antibody inhibits the positive selection of the transgenic thymocytes but does not affect the thymocytes on nonselecting stromal cells (Fig. 3, c and f). The effects of G35 on selection do not increase at antibody concentrations of 100 μg/ml, are more modest at 33 μg/ml, and are undetectable at 10 μg/ml (data not shown). Furthermore, G35 treatment does not affect the viability or MHC expression levels of the cells in the culture (data not shown). We conclude that G35 inhibits positive selection of thymocytes in vitro by blocking their access to I-Ek– peptide complexes rather than by killing APCs or inducing MHC downregulation.
Blockade by G35 Is Specific to TCRs That Recognize MCC–I-Ek Complexes.
The fact that G35 does not influence the development of nontransgenic CD4 thymocytes suggests that it may recognize only the subset of thymic peptides that is responsible for selecting this particular range of TCR specificity. Alternatively, G35 may block selection by binding to all or most thymic I-Ek molecules regardless of their bound peptides. In this model, nontransgenic thymocytes would not be visibly affected because they could mature on non–I-Ek class II MHC molecules such as I-Ak. To distinguish between these models, we examined the influence of G35 on the development of thymocytes from mice transgenic for a different TCR that requires I-Ek for positive selection yet does not cross-react with MCC (24). These mice express a TCR derived from the T cell clone A18, which is specific for a peptide derived from the murine C5 complement protein that is present in the B10.Br strain. Since the C5 protein is deleted in the I-Ek–bearing strain AKR/J, A18+ thymocytes are positively selected in AKR/J backgrounds but negatively selected on B10.Br. Rather than crossing these mice to a nonselecting background (C57BL/6) that would also introduce a potential source of the negatively selecting C5 protein, we modified the in vitro positive selection assay.
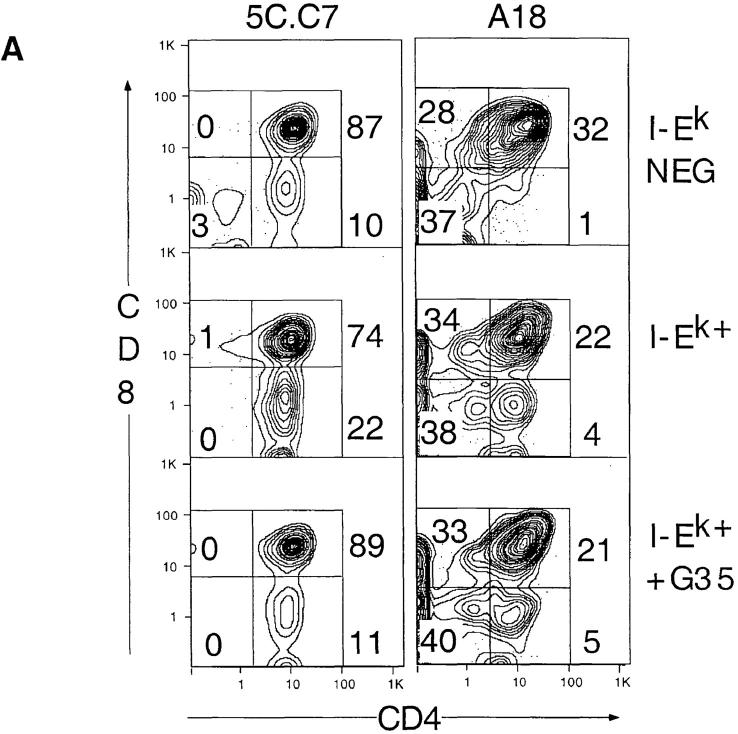
Instead of taking immature thymocytes from nonselecting strains, we isolated immature (CD4+8+) unactivated (CD69lo) thymocytes from positively selecting strains by FACS®. To standardize the results between the two transgenic mouse strains, and to test the validity of the modified in vitro system, this sorting procedure was performed on cells from both the 5C.C7 × B10.Br and A18 × AKR/J positively selecting lines. As shown in the previous experiments, the immature thymocytes from both strains downregulate CD8 only in the presence of I-Ek (Fig. 4 A). Furthermore, G35 efficiently blocks 5C.C7 thymocyte differentiation at the CD4+8+ stage. In contrast, although A18 thymocyte development depends on I-Ek expression, G35 does not influence A18 T cell development (Fig. 4 A).
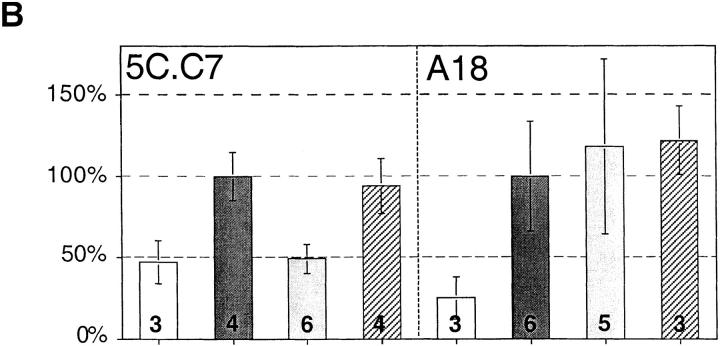
Figure 4.
Inhibition of positive selection by G35 is specific for the MCC-I-Ek–restricted TCR. (A) Immature thymocytes from 5C.C7/B10.Br or A18/AKR/J TCR transgenic mice were cultured with thymic stromal cells from C57BL/6 (top) B10.Br (middle) or AKR/J (bottom) mice. Numbers on plots refer to the percentage of total live transgenic thymocytes that fall within each boxed population. (B) Analysis of the effects of G35 on production of CD4+ cells. The selection index was generated for each treatment group by computing the average percentage of CD4+8−/CD4+8+ for untreated, I-Ek–positive stromal cells on each day and comparing the average percentage of CD4+8−/CD4+8+ from other samples to that number. Shown are the pooled results of two independent experiments, each with three to six replicates of identically treated stromal cells (the exact number is printed on each bar). Conditions: C57BL/6 stroma, white bars; B10.Br stroma, dark gray bars; B10.Br stroma plus G35 50 μg/ml, light gray bars; and B10.Br stroma plus D4 50 μg/ ml, hatched bars. Error bars represent 1 SD.
The results of three independent experiments are plotted in Fig. 4 B. Because the ability of the stroma to support differentiation varies among experiments, the data are presented as the percentage of live, transgene-positive, CD4+8− cells relative to CD4+8+ cells (termed selective index), normalized to the average CD4+8−/CD4+8+ ratio for the untreated I-Ek–positive samples. These data show that G35 blocks development in a TCR-specific manner.
G35 Stains a Subset of Class II MHC Positive Cells.
To conclude that the TCR-specific nature of the G35 blockade results from recognition of the self-peptide–MHC complexes that mediate positive selection of thymocytes bearing the 5C.C7 but not the A18 TCRs, it is necessary to show that G35 recognizes only a subset of thymic peptide–MHC complexes. To determine the localization and expression level of the G35 epitope, we examined the distribution of G35, D4, or 14.4.4S antibodies on frozen sections of thymus, spleen, and lymph node derived from B10.Br mice (Fig. 5 A). As shown in Fig. 5 A, a, 14.4.4S, which detects I-Ek in a peptide-independent manner, reacts homogeneously with both the cortical and medullary areas, although it exhibits a more confluent pattern within the medulla. In contrast, the determinant for G35 is clearly expressed at higher levels by a subset of cells within the thymic medulla, although a lower level of G35 staining of cortical stromal processes and the subcapsular region was routinely observed (Fig 5 A, b). The 14.4.4S antibody exhibits widespread reactivity in lymph node and spleen, labeling both B and T cell–dependent areas (Fig 5 A, d and g). Labeling of these tissues with G35 was more heterogeneous; staining was restricted to follicular structures in the lymph node cortex, and to B cell–dependent areas of splenic white pulp. Reactivity of G35 with T cell–dependent areas of lymph node or spleen was weak and restricted to a few scattered cells. B10.Br thymic tissue failed to react with the D4 mAb (Fig. 5 A, c) and no G35 reactivity with tissues from C57BL/6 mice was detected (data not shown).
Figure 5.

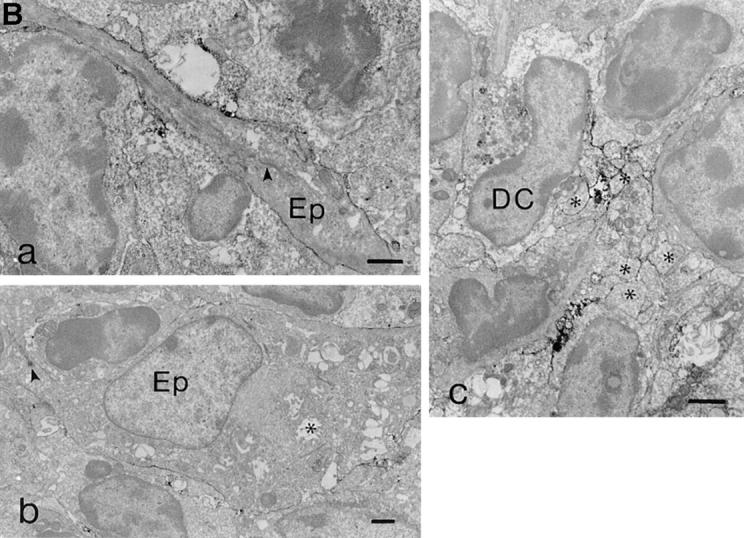
G35 reactivity with thymus and peripheral lymphoid tissues. (A) Frozen sections of thymus (a–c), lymph node (d–f), and spleen (g–i) were labeled with 14.4.4S (a, d, g), G35 (b, e, h), D4 (c), or an irrelevant mAb (f, i). PC, T cell–dependent paracortical area; RP, red pulp and white pulp. Original magnification: a–i, ×45–55. (B) Ultrastructural analysis of G35 reactivity with thymus tissue. Labeling was associated with (a) cortical epithelium, (b) medullary epithelium (desmosome indicated by arrowhead, characteristic cystic structures indicated by asterisk), and (c) dendritic cells (characteristic bullate processes indicated by asterisks). Original magnification: a, ×8,400; b, ×4,950; c, ×8,050.
G35 staining is more intense in some regions of the thymus than others, even when these areas express similar levels of I-Ek. Thus, all I-Ek–bearing cells do not bind equally well to G35. To determine which cell types express the G35-reactive peptide–MHC complexes, we performed ultrastructural analyses of thymic sections. These analyses demonstrated that G35 reactivity is associated with the membranes of all three morphologically-defined thymic APC types, including cortical epithelium, medullary epithelium, and dendritic cells (Fig. 5 B). Staining is most intense on dendritic cells, intermediate on cortical epithelium, and weak but detectable on medullary epithelium. Thus, although G35 staining is not restricted to a particular APC subset, expression of the relevant complexes may be enhanced in cortical epithelial cells as compared with medullary epithelial cells.
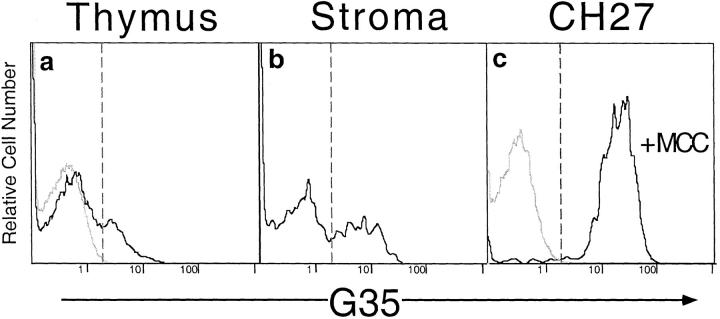
To further examine the heterogeneity of G35 reactivity among thymic stromal cells and to confirm that the G35 epitope was expressed by class II–positive cells in the thymus, we used four-color flow cytometry. Immortalized B cells from I-Ek–positive mice (CH27 cells), cells from whole thymuses, and thymic stromal cell cultures were analyzed for expression of the 14.4.4, CD3, B220, and G35 or D4 epitopes. Importantly, the I-Ek-expressing B cell line CH27, which expresses a diverse array of peptides, exhibits no G35 reactivity. Thus G35 cannot react with all endogenously produced I-Ek complexes (Fig. 6 a). In contrast, when these cells are incubated overnight with MCC peptide, all of the cells exhibit strong G35 reactivity (Fig. 6 a). These staining levels were used to assess the percentage of cells from other tissues that could be recognized by G35. Approximately 20% of freshly isolated class II–positive thymic cells from B10.Br but not C57BL/6 mice express the G35 determinant (Fig. 6 c). Close to 40% of I-Ek–positive cells from this particular thymic stromal cell culture bind specifically to the G35 antibody (Fig. 6 b). Neither G35 nor D4 labels CD3+ cells and D4 fails to stain any of the thymic stromal cells (data not shown). Thus, a subset of thymic and peripheral I-Ek–positive cells are preferentially labeled by G35. Because G35 stains only a subset of cells that all express equivalent levels of I-Ek, it must preferentially label a subset of peptide–MHC complexes that are unevenly distributed on different cells. In addition, the low affinity of this antibody for MCC–I-Ek complexes (with respect to the similar antibody D4) and the weak overall staining of these tissues suggest that nonspecific binding of G35 to all thymic I-Ek– peptide complexes is unlikely to be the mechanism for the TCR-specific blockade of thymic differentiation.
Figure 6.
G35 recognizes endogenous I-Ek–peptide complexes on a subset of thymic APCs. Levels of G35 expression on live, large CD3−14.4.4+ cells. (a) Cells from B10.Br (black line) or C57BL/6 (gray line) thymuses. (b) The staining of a stromal cell culture derived from B10.Br mice. (c) G35 expression on CH27 B cells pulsed overnight with 10 μM MCC 88–103 peptide (black line) or PBS (gray line). The percentage of cells with fluorescence greater than the intercept of the dashed lines was <2% in negative controls, 20% in a, 41% in b, and 97% in c.
Purification of I-Ek–bound Peptides.
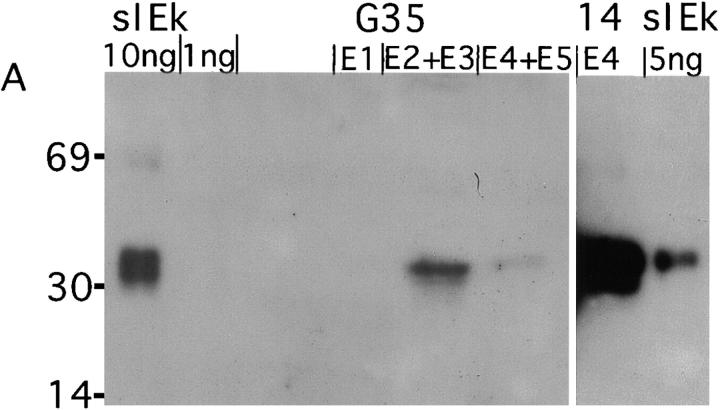
To directly compare the peptides recognized by G35 with those bound to all I-Ek molecules in the thymus, we isolated thymuses from 327 B10.Br and B10.A (a related I-Ek–positive congenic strain) mice. Using a modified version of established MHC purification protocols (34–36), we isolated thymic I-Ek molecules that were bound by G35 or by the peptide-nonspecific antibody 14.4.4. Two G35 columns were run in series and the unbound lysate was then passed over the 14.4.4 column. Western blotting was used to determine the amount of MHC present in each fraction. The first G35 column bound ∼670 ng (11 pmol) of I-Ek, whereas the second G35 column yielded no detectable MHC (Fig. 7 A). The pooled fractions from the 14.4.4 column bound to ∼7 μg of I-Ek (Fig. 7 A). Thus although both G35 and 14.4.4 can recognize the endogenous peptide–MHC complexes displayed on thymic APCs, G35 is less efficient at removing these complexes from thymic extracts. These data argue that G35 recognizes a finite and unique subset of thymic I-Ek–peptide complexes.
Figure 7.
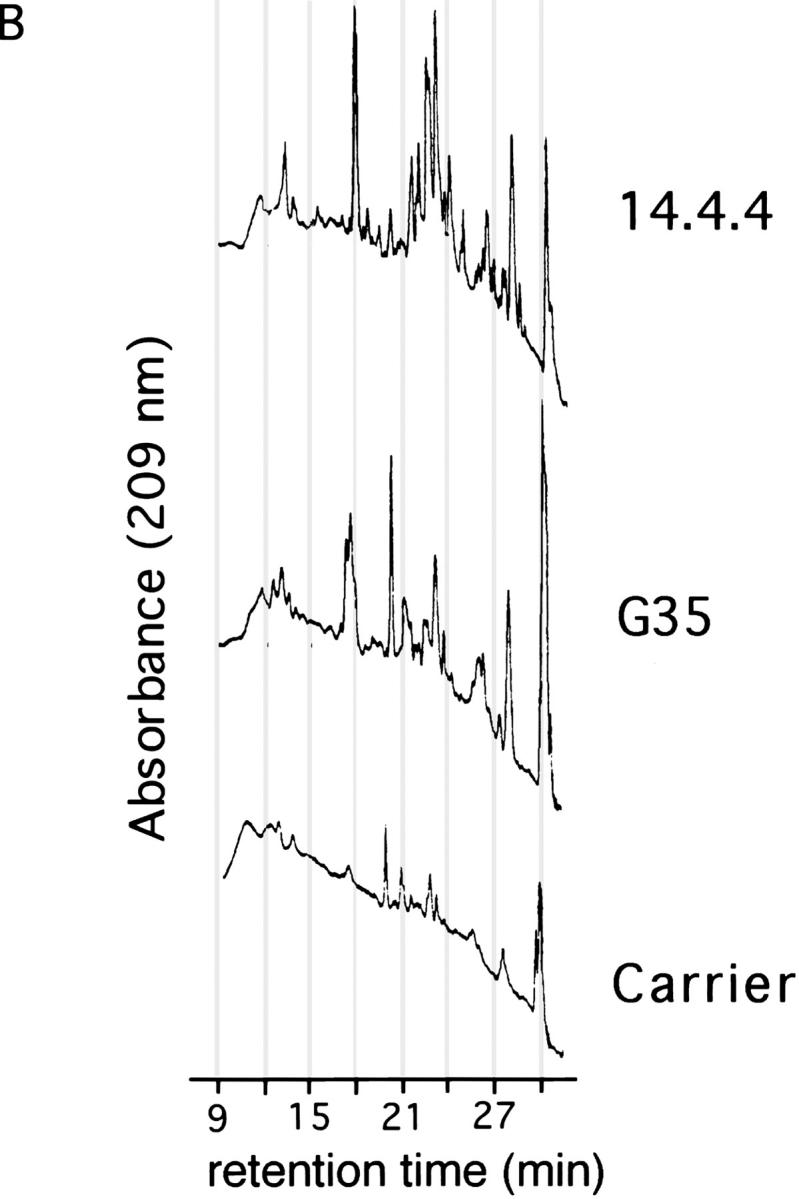
Purification of thymic I-Ek peptide complexes. (A) Western blot of 12% acrylamide gel with polyclonal rabbit anti– I-Ek antisera. 1/60 of each fraction (denoted E1–5) from the first G35 column, the 14.4.4. column or the indicated amount of soluble I-Ek (sIEk) was run in each lane as noted on the figure. (B) HPLC chromatograms of the second fraction of the peptide eluates (in carrier solution) from the G35 or 14.4.4 columns, as noted. Carrier is 10 ng/ml leupeptin in 0.2 N acetic acid.
To confirm that G35 recognizes a unique subset of thymic I-Ek–peptide complexes, and to estimate the peptide diversity in this preparation, we analyzed the peptides eluted from the thymic I-Ek by HPLC. Equivalent amounts of G35, 14.4.4, or a blank solution containing the carrier peptide and protease inhibitor leupeptin were separated on a C18 column with a linear water/acetonitrile gradient in 0.1% TFA. The portion of the gradient in which peptides are known to elute is displayed in Fig. 7 B. Comparison of the G35 and 14.4.4 chromatograms reveals the presence of several peaks that are unique to each sample. For example, at 17.37 min, a triplet peak unique to the G35 sample appears, whereas the 14.4.4 sample has a singlet at 18 min. The peptide-rich region between 20 and 25 min is extremely different in the G35 and 14.4.4 samples as well. The complexity of the 14.4.4 trace resembles the multipeak smears observed in other studies of 14.4.4-reactive I-Ek where sequencing of the peptides from these peaks reveals a diverse spectrum of peptide species (34, 36). In contrast, the number of peptide-like peaks in the G35 column is reduced, although more complex than would be expected for a single peptide species. Thus, G35 recognizes a unique, multipeptide subset of the thymic I-Ek–peptide complexes that is required for the positive selection of 5C.C7 CD4+ T cells.
Epitope Map of G35.
Because the binding of G35 to this subset of thymic MHC–peptide complexes blocks positive selection, the characteristics and degeneracy of G35 recognition are interesting with respect to the range of endogenous peptides that mediate the positive selection of this TCR specificity. To assess the tolerance of G35 to subtle changes in peptide sequence, we performed an ELISA to measure antibody reactivity with I-Ek bound to each of a complete replacement set of monosubstitutions of the MCC epitope (27).
G35 recognition is more degenerate than the recognition by the 5C.C7 TCR, although less so if partial agonistic or antagonistic reactivities are also taken into consideration (Fig. 8). These observations suggest that although G35 cannot recognize most I-Ek–binding peptides, it may be able to efficiently recognize nearly every peptide–I-Ek complex to which 5C.C7 can respond. G35 may recognize other complexes that the 5C.C7 TCR can bind to only weakly as well as those with which 5C.C7 fails to interact. Although the G35 antibody is tolerant to alterations in the NH2 terminus of MHC-bound peptide, its specificity is very similar to the 5C.C7 TCR at the COOH terminus, particularly at residues 101 and 102. It is difficult to estimate the fraction of bound peptides that can be recognized by G35, but consideration of the restriction at peptide positions 98, 99, 101, and 102 that are not involved in I-Ek binding (14, 11, 3, and 5 residues of 20 tolerated, respectively) suggests a figure in the range of 1–2%. In addition, the variants that can stimulate the 5C.C7 T cell clone to produce IL-3 (including residues that antagonize stimulation by MCC complexes) are quite similar to those seen by G35, especially at the residues 101 and 102. These results suggest that the endogenous peptide antigens recognized by G35 must be very similar to MCC, particularly at the COOH-terminal residues.
Figure 8.
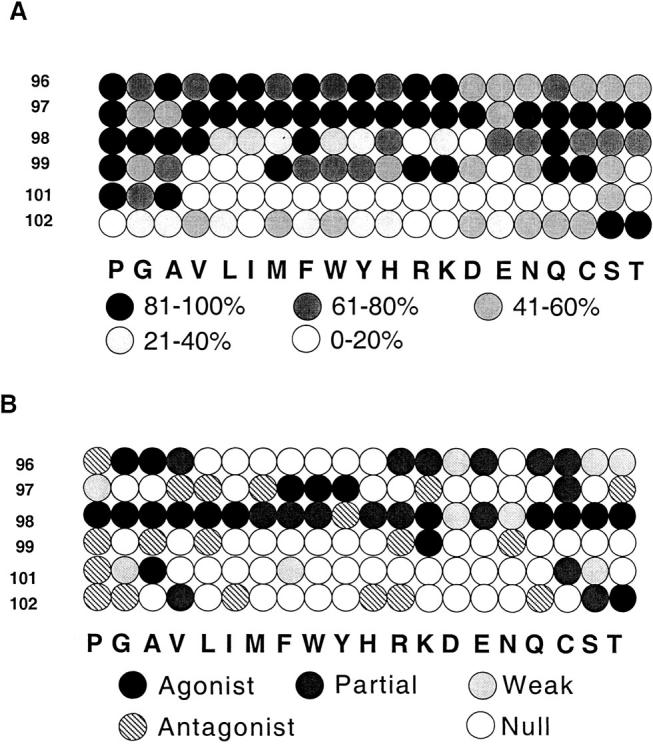
G35 sensitivity to I-Ek bound to single substitution peptide variants. Each circle represents a different peptide; as keyed, the number on the left indicates the amino acid that was replaced and the letters indicate which amino acid was substituted in each position. Replacement amino acids are ordered by rough chemical similarity. Only residues that do not affect MHC binding were analyzed. (A) Recognition by mAb G35. Black circles represent from 81 to 100% of G35 reactivity to MCC–I-Ek complex activity and each shade of gray represents a 20% decrease in activity as calculated by estimating the concentration of G35 necessary to achieve the half maximal signal from the WT MCC–peptide complexes. (B) Interaction with 5C.C7 TCR. Gray circles represent peptides that produce detectable levels of IL-3 when used to stimulate a 5C.C7-bearing cell line as indicated. Hatched circles, peptides that antagonize the stimulation of the cell line by WT MCC peptide–I-Ek complexes; white circles, peptides that did not antagonize or stimulate the 5C.C7 cell line.
Discussion
In summary, the G35 antibody blocks the positive selection of thymocytes expressing the 5C.C7 TCR but does not affect the differentiation of other I-Ek–restricted thymocytes. The distribution of G35 staining in the thymus and in the peripheral tissues shows that the antibody differentially recognizes certain thymic I-Ek–peptide complexes. Biochemical isolation of these complexes shows that they comprise a finite and unique subset of total thymic I-Ek– peptide complexes. We conclude that this antibody blocks thymocyte development by binding to the endogenous complexes that are required for positive selection of this particular MCC-reactive T cell clone.
Two additional conclusions may be inferred from these data. First, the fact that another I-Ek–reactive T cell clone (A18) and most nontransgenic CD4+ thymocytes develop normally in the presence of G35 suggests that different TCR specificities are selected on different subsets of thymic peptide–MHC complexes. Second, the recognition properties of G35 indicate that endogenous, positively selecting peptides are likely to be structurally related to the peptides that can stimulate mature T cells in the periphery.
What is the relationship between the endogenous peptide–MHC complexes that normally mediate the positive selection of a class II MHC–restricted receptor and those that can activate it in the periphery? The G35 data suggest that sequence similarity may play a role, at least in class II MHC–mediated positive selection. In the class I system, however, peptide sequence may be less instructive. In the experiments of Hogquist et al. (16) and Hu et al. (17), peptides were purified from class I MHC molecules derived from thymic epithelial or other cell lines and screened for their ability to positively select TCR transgenic thymocytes in vitro. Surprisingly, these peptides did not resemble the nominal peptide antigens for the receptors they studied. However, these endogenous peptides are likely to be different from the peptides that allow positive selection to occur in a normal animal for two reasons. First, because the class I MHC molecules expressed by the thymic APCs are improperly folded due to the mutations that facilitate peptide loading, they are expressed at artificially low levels. Second, to rescue selection, the peptides must be added to the cultures at extremely high concentrations that are unlikely to be approximated by the endogenous peptide expression in these cells. In fact, studies of APCs have shown that thousands of different peptides are typically expressed by a given cell type and that even the most abundant peptides typically do not comprise more than a few percent of the total (34–36). Thus, the findings using G35 may be more representative of the normal case in which peptide sequence and/or structure do play an important role in determining the consequences of TCR-peptide–MHC interactions in the thymus.
Although we do not yet know the exact sequences of the peptides that G35 recognizes, we do have an estimate of their abundance and diversity. The HPLC analyses of G35- and 14.4.4 reactive peptide–MHC complexes reveal that although G35 peptides are likely to be less diverse than 14.4.4 they still contain a number of different species (Fig. 7). The 10-fold difference in yield between the two columns suggests that G35-reactive peptides do not comprise more than 10% of the total peptides displayed on the cell surface. In fact, of the several predominantly displayed peptides identified in previous studies (34), none interact with G35 when bound to I-Ek (Baldwin, K.K., unpublished data). Thus, G35 probably interacts with peptides that are less abundant in the thymus than those previously identified.
These data not only underscore the potential importance of peptide sequence in thymic selection, they also provide some insight into the possible role of different cell types in these events. Several lines of evidence have suggested that radiation-resistant cortical thymic epithelial cells are required for positive selection (37–44) and that thymic medullary epithelium and bone marrow–derived cells induce negative selection far more efficiently than do cortical epithelial cells (45–50). However, in some experimental systems, cultured thymic epithelial cells can induce deletion (51–57), whereas some fibroblast-derived cells may mediate inefficient positive selection (58–60). One proposed difficulty in interpreting these results stems from the possibility that the pool of endogenous peptides displayed on the surface of these thymic APCs may have been different in the various experiments. Other sequence-level investigations of peptide expression in different tissues have not found significant differences between tissues or between cell types in the thymus (34). However, these analyses are technically very difficult and can quantify only the most abundant peptides bound to a particular MHC molecule in a given cell-type preparation. Based on these data, it is formally possible that thymic cortical epithelial cells express a small amount of a special peptide that permits them to induce positive selection. Alternatively, these cells may have another feature that predisposes them to induce positive rather than negative selection.
Our data suggest that special thymic peptides are not responsible for the unique ability of cortical epithelial cells to induce positive selection. If there were such a special peptide, G35 should block positive selection of all T cells, which it does not. In addition, G35 staining should be most intense in the thymic cortex. Instead, G35 staining is stronger in the thymic medulla than in the cortex, even when normalized for the overall MHC expression level. Despite this increased staining, we have not detected any negative selection of 5C.C7 thymocytes in the thymus. Thus, either the peptides that G35 recognizes in the medulla differ from those in the cortex, or cortical cells have peptide-independent unique qualities that permit efficient positive selection. However, without medullary and cortical cell–specific peptide sequence, we cannot distinguish between these alternatives.
Where else are these potentially weakly stimulatory complexes expressed? Staining of the peripheral lymphoid tissues with G35 indicates that these endogenous peptide– MHC complexes are found in both the spleen and lymph nodes. In addition, the peripheral staining varies in different regions of the same lymph node or spleen section even when relative levels of class II MHC are taken into account (Fig. 5). Thus, different APCs can express different peptide–MHC complexes even when they are closely associated in a single tissue. Although tissue-specific expression patterns have been noted previously (29, 61, 62), our data are among the first to suggest that the peptides that are responsible for positive selection in the thymus may also be expressed by cells in the periphery at similar (or higher) levels. In addition, recent reports from animals that lack peripheral MHC molecules have suggested that signaling induced by self-peptide–MHC complexes is required for maintenance of peripheral T cell pools (64–67). Therefore, it is possible that the complexes recognized by G35 in the periphery serve this function for mature 5C.C7 T cells. If so, additional studies using G35 might also provide clues about the identity, affinity for TCR, and localization of such complexes.
Acknowledgments
We gratefully acknowledge David King for help with the microbore HPLC analyses. We also thank Nelida Prado for animal husbandry; Dr. William Ho (University of Washington, Seattle) for help with FACS® analyses; Drs. Brigitte Stockinger and Tomas Zal (both from the National Institute for Medical Research, London, UK) for providing us with the A18 TCR transgenic mice; Drs. Barbara Fazekas de St. Groth (Centenary Institute of Cancer Medicine and Cell Biology, New South Wales, Australia) and William Ho for generating and characterizing the 5C.C7 TCR transgenic mice; and Drs. Irving Weissman and Anis Sen-Majumdar for help in establishing the thymic stromal cell cultures.
Abbreviation used in this paper
- MCC
moth cytochrome c.
Footnotes
K.K. Baldwin was a Howard Hughes Predoctoral Fellow. L.C. Wu is presently supported by a fellowship from the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation and previously by an Immunology Training Grant. The Howard Hughes Medical Institute provided postdoctoral support for P.A. Reay and support for this project in the laboratory of M.M. Davis. Additional support for A. Farr was provided by NIH grants AI24137 and AG04360.
K. Baldwin's present address is H.H.M.I./Center for Neurobiology and Behavior, College of Physicians and Surgeons, Columbia University, 701 West 168th St., New York, NY 10032.
K.K. Baldwin and P.A. Reay contributed equally to the work described in this paper.
References
- 1.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 2.Kisielow P, Teh HS, Bluthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–723. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 3.Berg LJ, Pullen AM, Fazekas de St B, Groth, Mathis D, Benoist C, Davis MM. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–1046. doi: 10.1016/0092-8674(89)90502-3. [DOI] [PubMed] [Google Scholar]
- 4.Bevan MJ. In thymic selection, peptide diversity gives and takes away. Immunity. 1997;7:175–178. doi: 10.1016/s1074-7613(00)80520-8. [DOI] [PubMed] [Google Scholar]
- 5.Hogquist KA, Gavin MA, Bevan MJ. Positive selection of CD8+T cells induced by major histocompatibility complex binding peptides in fetal thymic organ culture. J Exp Med. 1993;177:1469–1473. doi: 10.1084/jem.177.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashton-Rickardt PG. The role of peptide in the positive selection of CD8+ T cells in the thymus. Thymus. 1993;22:111–115. [PubMed] [Google Scholar]
- 7.Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Mice lacking the MHC class II-associated invariant chain. Cell. 1993;72:635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- 8.Miyazaki T, Wolf P, Tourne S, Waltzinger C, Dierich A, Barois N, Ploegh H, Benoist C, Mathis D. Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell. 1996;84:531–541. doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 9.Martin WD, Hicks GG, Mendiratta SK, Leva HI, Ruley HE, Van Kaer L. H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell. 1996;84:543–550. doi: 10.1016/s0092-8674(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 10.Fung-Leung WP, Surh CD, Liljedahl M, Pang J, Leturcq D, Peterson PA, Webb SR, Karlsson L. Antigen presentation and T cell development in H2-M-deficient mice. Science. 1996;271:1278–1281. doi: 10.1126/science.271.5253.1278. [DOI] [PubMed] [Google Scholar]
- 11.Hogquist KA, Jameson SC, Bevan MJ. Strong agonist ligands for the T cell receptor do not mediate positive selection of functional CD8+ T cells. Immunity. 1995;3:79–86. doi: 10.1016/1074-7613(95)90160-4. [DOI] [PubMed] [Google Scholar]
- 12.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 13.Ashton-Rickardt PG, Bandeira A, Delaney JR, Van Kaer L, Pircher HP, Zinkernagel RM, Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 14.Sebzda E, Wallace VA, Mayer J, Yeung RS, Mak TW, Ohashi PS. Positive and negative thymocyte selection induced by different concentrations of a single peptide. Science. 1994;263:1615–1618. doi: 10.1126/science.8128249. [DOI] [PubMed] [Google Scholar]
- 15.Sebzda E, Kundig TM, Thomson CT, Aoki K, Mak SY, Mayer JP, Zamborelli T, Nathenson SG, Ohashi PS. Mature T cell reactivity altered by peptide agonist that induces positive selection. J Exp Med. 1996;183:1093–1104. doi: 10.1084/jem.183.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogquist KA, Tomlinson AJ, Kieper WC, McGargill MA, Hart MC, Naylor S, Jameson SC. Identification of a naturally occurring ligand for thymic positive selection. Immunity. 1997;6:389–399. doi: 10.1016/s1074-7613(00)80282-4. [DOI] [PubMed] [Google Scholar]
- 17.Hu Q, Bazemore CR, Walker, Girao C, Opferman JT, Sun J, Shabanowitz J, Hunt DF, Ashton-Rickardt PG. Specific recognition of thymic self-peptides induces the positive selection of cytotoxic T lymphocytes. Immunity. 1997;7:221–231. doi: 10.1016/s1074-7613(00)80525-7. [DOI] [PubMed] [Google Scholar]
- 18.Ignatowicz L, Kappler J, Marrack P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84:521–529. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- 19.Fukui Y, Ishimoto T, Utsuyama M, Gyotoku T, Koga T, Nakao K, Hirokawa K, Katsuki M, Sasazuki T. Positive and negative CD4+ thymocyte selection by a single MHC class II/peptide ligand affected by its expression level in the thymus. Immunity. 1997;6:401–410. doi: 10.1016/s1074-7613(00)80283-6. [DOI] [PubMed] [Google Scholar]
- 20.Surh CD, Lee DS, Fung-Leung WP, Karlsson L, Sprent J. Thymic selection by a single MHC/peptide ligand produces a semidiverse repertoire of CD4+ T cells. Immunity. 1997;7:209–219. doi: 10.1016/s1074-7613(00)80524-5. [DOI] [PubMed] [Google Scholar]
- 21.Grubin CE, Kovats S, deRoos P, Rudensky AY. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- 22.Tourne S, Miyazaki T, Oxenius A, Klein L, Fehr T, Kyewski B, Benoist C, Mathis D. Selection of a broad repertoire of CD4+ T cells in H-2Ma0/0 mice. Immunity. 1997;7:187–195. doi: 10.1016/s1074-7613(00)80522-1. [DOI] [PubMed] [Google Scholar]
- 23.Seder RA, Paul WE, Davis MM, Fazekas de St B, Groth The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II–restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sen-Majumdar A, Lieberman M, Alpert S, Wiessman IL, Small M. Differentiation of CD3−4−8−thymocytes in short-term thymic stromal cell culture. J Exp Med. 1992;176:543–551. doi: 10.1084/jem.176.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wettstein DA, Boniface JJ, Reay PA, Schild H, Davis MM. Expression of a class II major histocompatibility complex (MHC) heterodimer in a lipid-linked form with enhanced peptide/soluble MHC complex formation at low pH. J Exp Med. 1991;174:219–228. doi: 10.1084/jem.174.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reay PA, Kantor RM, Davis MM. Use of global amino acid replacements to define the requirements for MHC binding and T cell recognition of moth cytochrome c (93–103) J Immunol. 1994;152:3946–3957. [PubMed] [Google Scholar]
- 28.Nelson AJ, Dunn RJ, Peach R, Aruffo A, Farr AG. The murine homolog of human Ep-CAM, a homotypic adhesion molecule, is expressed by thymocytes and thymic epithelial cells. Eur J Immunol. 1996;26:401–408. doi: 10.1002/eji.1830260220. [DOI] [PubMed] [Google Scholar]
- 29.Farr A, DeRoos PC, Eastman S, Rudensky AY. Differential expression of CLIP:MHC class II and conventional endogenous peptide:MHC class II complexes by thymic epithelial cells and peripheral antigen-presenting cells. Eur J Immunol. 1996;26:3185–3193. doi: 10.1002/eji.1830261252. [DOI] [PubMed] [Google Scholar]
- 30.Kruisbeek A, Mond J, Fowlkes B, Carmen J, Bridges S, Longo D. Absence of the Lyt-2−, L3T4+lineage of T cells in mice treated neonatally with anti-I-A correlates with absence of intrathymic I-A bearing antigen presenting cell function. J Exp Med. 1985;161:1029–1047. doi: 10.1084/jem.161.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruisbeek AM, Bridges S, Carmen J, Longo DL, Mond JJ. In vivo treatment of neonatal mice with anti-I-A antibodies interferes with the development of the class I, class II, and Mls-reactive proliferating T cell subset. J Immunol. 1985;134:3597–3604. [PubMed] [Google Scholar]
- 32.Small M, Van Ewijk W, Gown AM, Rouse RV. Identification of subpopulations of mouse thymic epithelial cells in culture. Immunology. 1989;68:371–377. [PMC free article] [PubMed] [Google Scholar]
- 33.Ho, W.Y.-W. 1995. Transgenic mouse models of T cell tolerance and in vitro T cell-B cell collaboration. Ph.D. thesis. Stanford University. 183 pp.
- 34.Marrack P, Ignatowicz L, Kappler JW, Boymel J, Freed JH. Comparison of peptides bound to spleen and thymus class II. J Exp Med. 1993;178:2173–2183. doi: 10.1084/jem.178.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt DF, Henderson RA, Shabanowitz J, Sakaguchi K, Michel H, Sevilir N, Cox AL, Appella E, Engelhard VH. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992;255:1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 36.Chicz R, Urban R, Lane W, Gorga J, Stern L, Vignali D, Strominger J. Predominant naturally processed peptides bound to HLA-DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature. 1992;358:764–768. doi: 10.1038/358764a0. [DOI] [PubMed] [Google Scholar]
- 37.Bill J, Palmer E. Positive selection of CD4+ T cells mediated by MHC class II-bearing stromal cell in the thymic cortex. Nature. 1989;341:649–651. doi: 10.1038/341649a0. [DOI] [PubMed] [Google Scholar]
- 38.Anderson G, Jenkinson EJ, Moore NC, Owen JJ. MHC class II-positive epithelium and mesenchyme cells are both required for T-cell development in the thymus. Nature. 1993;362:70–73. doi: 10.1038/362070a0. [DOI] [PubMed] [Google Scholar]
- 39.Anderson G, Owen JJ, Moore NC, Jenkinson EJ. Thymic epithelial cells provide unique signals for positive selection of CD4+CD8+thymocytes in vitro. J Exp Med. 1994;179:2027–2031. doi: 10.1084/jem.179.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka Y, Williams O, Tarazona R, Wack A, Norton T, Kioussis D. In vitro positive selection of alpha beta TCR transgenic thymocytes by a conditionally immortalized cortical epithelial clone. Int Immunol. 1997;9:381–393. doi: 10.1093/intimm/9.3.381. [DOI] [PubMed] [Google Scholar]
- 41.DeKoning J, DiMolfetto L, Reilly C, Wei Q, Havran WL, Lo D. Thymic cortical epithelium is sufficient for the development of mature T cells in relB-deficient mice. J Immunol. 1997;158:2558–2566. [PubMed] [Google Scholar]
- 42.Fort MM, Pardoll DM. Can bone marrow- derived thymic stromal cells mediate the positive selection of class I-restricted T cells? . Cell Immunol. 1996;171:74–79. doi: 10.1006/cimm.1996.0175. [DOI] [PubMed] [Google Scholar]
- 43.Schonrich G, Strauss G, Muller KP, Dustin L, Loh DY, Auphan N, Schmitt-Verhulst AM, Arnold B, Hammerling GJ. Distinct requirements of positive and negative selection for selecting cell type and CD8 interaction. J Immunol. 1993;151:4098–4105. [PubMed] [Google Scholar]
- 44.Markowitz JS, Auchincloss H, Jr, Grusby MJ, Glimcher LH. Class II-positive hematopoietic cells cannot mediate positive selection of CD4+ T lymphocytes in class II-deficient mice. Proc Natl Acad Sci USA. 1993;90:2779–2783. doi: 10.1073/pnas.90.7.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature. 1996;383:81–85. doi: 10.1038/383081a0. [DOI] [PubMed] [Google Scholar]
- 46.Le PT, Maecker HT, Cook JE. In situ detection and characterization of apoptotic thymocytes in human thymus. Expression of bcl-2 in vivo does not prevent apoptosis. J Immunol. 1995;154:4371–4378. [PubMed] [Google Scholar]
- 47.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 48.Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Meerwijk JP, Marguerat S, Lees RK, Germain RN, Fowlkes BJ, MacDonald HR. Quantitative impact of thymic clonal deletion on the T cell repertoire. J Exp Med. 1997;185:377–383. doi: 10.1084/jem.185.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burkly LC, Degermann S, Longley J, Hagman J, Brinster RL, Lo D, Flavell RA. Clonal deletion of V beta 5+ T cells by transgenic I-E restricted to thymic medullary epithelium. J Immunol. 1993;151:3954–3960. [PubMed] [Google Scholar]
- 51.Iwabuchi K, Nakayama K, McCoy RL, Wang F, Nishimura T, Habu S, Murphy KM, Loh DY. Cellular and peptide requirements for in vitro clonal deletion of immature thymocytes. Proc Natl Acad Sci USA. 1992;89:9000–9004. doi: 10.1073/pnas.89.19.9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spain LM, Berg LJ. Quantitative analysis of the efficiency of clonal deletion in the thymus. Dev Immunol. 1994;4:43–53. doi: 10.1155/1994/92025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vukmanovic S, Jameson SC, Bevan MJ. A thymic epithelial cell line induces both positive and negative selection in the thymus. Int Immunol. 1994;6:239–246. doi: 10.1093/intimm/6.2.239. [DOI] [PubMed] [Google Scholar]
- 54.Volkmann A, Zal T, Stockinger B. Antigen-presenting cells in the thymus that can negatively select MHC class II-restricted T cells recognizing a circulating self antigen. J Immunol. 1997;158:693–706. [PubMed] [Google Scholar]
- 55.Hugo P, Kappler JW, Godfrey DI, Marrack PC. Thymic epithelial cell lines that mediate positive selection can also induce thymocyte clonal deletion. J Immunol. 1994;152:1022–1031. [PubMed] [Google Scholar]
- 56.Tanaka Y, Mamalaki C, Stockinger B, Kioussis D. In vitro negative selection of alpha beta T cell receptor transgenic thymocytes by conditionally immortalized thymic cortical epithelial cell lines and dendritic cells. Eur J Immunol. 1993;23:2614–2621. doi: 10.1002/eji.1830231035. [DOI] [PubMed] [Google Scholar]
- 57.Hengartner H, Odermatt B, Schneider R, Schreyer M, Walle G, MacDonald HR, Zinkernagel RM. Deletion of self-reactive T cells before entry into the thymus medulla. Nature. 1988;336:388–390. doi: 10.1038/336388a0. [DOI] [PubMed] [Google Scholar]
- 58.Hugo P, Kappler JW, McCormack JE, Marrack P. Fibroblasts can induce thymocyte positive selection in vivo. Proc Natl Acad Sci USA. 1993;90:10335–10339. doi: 10.1073/pnas.90.21.10335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pawlowski T, Elliott JD, Loh DY, Staerz UD. Positive selection of T lymphocytes on fibroblasts. Nature. 1993;364:642–645. doi: 10.1038/364642a0. [DOI] [PubMed] [Google Scholar]
- 60.Bix M, Raulet D. Inefficient positive selection of T cells directed by haematopoietic cells. Nature. 1992;359:330–333. doi: 10.1038/359330a0. [DOI] [PubMed] [Google Scholar]
- 61.Murphy DB, Lo D, Rath S, Brinster RL, Flavell RA, Slanetz A, Janeway CA., Jr A novel MHC class II epitope expressed in thymic medulla but not cortex. Nature. 1989;338:765–768. doi: 10.1038/338765a0. [DOI] [PubMed] [Google Scholar]
- 62.Inaba K, Pack M, Inaba M, Sakuta H, Isdell F, Steinman RM. High levels of a major histocompatibility complex II–self peptide complex on dendritic cells from the T cell areas of lymph nodes. J Exp Med. 1997;186:665–672. doi: 10.1084/jem.186.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wülfing C, Rabinowitz JD, Beeson C, Sjaastad MD, McConnell HM, Davis MM. Kinetics and extent of T cell activation as measured with the calcium signal. J Exp Med. 1997;185:1815–1825. doi: 10.1084/jem.185.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tanchot C, Lemmonnier FA, Crarnau BP, Frietas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 65.Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- 66.Rooke R, Waltzinger C, Benoist C, Mathis D. Targeted complementation of MHC class II deficiency by intrathymic delivery of recombinant adenoviruses. Immunity. 1997;7:123–134. doi: 10.1016/s1074-7613(00)80515-4. [DOI] [PubMed] [Google Scholar]
- 67.Kirberg J, Berns A, von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex–encoded molecules. J Exp Med. 1996;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]