Abstract
The mechanisms underlying initiation and maintenance of CD4 T cell responses after DNA vaccination were studied using a construct coding for nonsecreted fifth component of complement (C5) protein, thus restricting the availability of antigen. The only cell types to express C5 were keratinocytes at the site of DNA application and a small number of dendritic cells present in the draining lymph nodes. Antigen expression persisted for up to 12 wk in keratinocytes, but dendritic cells did not express C5 beyond 2 wk after vaccination. Cross-priming of dendritic cells by C5 expressed in keratinocytes did not occur unless keratinocyte death was induced by irradiation in vitro. CD4 T cells were activated in the draining lymph nodes only and subsequently migrated to the spleen, where memory T cells persisted for longer than 40 wk despite the absence of a source of persistent antigen. While DNA vaccination resulted in transfection of a small proportion of dendritic cells only, it led to general activation of all dendritic cells, thus providing optimal conditions for effective T cell activation and maintenance of memory.
Keywords: DNA, vaccination, dendritic cells, cross-presentation, T cell memory
Genetic immunization with naked DNA has been shown to induce humoral as well as cellular immune responses with high efficiency, emphasizing the enormous potential of this strategy for vaccination purposes (1). DNA applied either intramuscularly or intradermally is mostly taken up by muscle cells or keratinocytes, respectively (2, 3). However, these cells are unable to initiate primary T cell responses. Several recent studies have provided evidence for the involvement of dendritic cells (DC)1 in priming naive T cells after DNA vaccination (4–6). In many cases, the constructs used for vaccination led to secretion of the synthesized protein, so that it remained unclear to what extent DC participated in stimulating an immune response after direct transfection or through taking up antigen released by other transfected cells.
Successful priming of T cell responses generally requires the presence of adjuvant, whose role appears to lie in activating APCs. This is especially the case for CD8 T cells which are strictly dependent on T cell help unless they interact with activated DC (7–9). Resting DC can be activated by inflammatory cytokines, bacterial products, and certain viruses, resulting in upregulation of MHC class II molecules and costimulatory molecules (10). Unmethylated CpG motifs in nonvertebrate DNA have been described to have a potent adjuvant effect (11–15). Here we show that vaccination with a DNA construct encoding a protein that is not secreted induces strong, long-lived CD4 T cell responses that are initiated in the draining lymph nodes by a small number of DC that express the antigen. Although DNA vaccination results in direct transfection of only a very small proportion of DC, it leads to general activation of all DC found in the draining lymph nodes, thus providing optimal conditions for effective T cell activation.
Materials and Methods
Mice
A/J (C5−) mice were maintained under specific pathogen-free conditions at the National Institute for Medical Research, London. A18 TCR transgenic Rag-1−/− C5− mice recognize epitope 106-121 of the serum protein C5 in the context of H2-Ek using a receptor composed of Vβ8.3 and Vα11.1a as described previously (16).
BrdU Labeling and Detection
For continuous BrdU labeling, mice received one i.p. injection of 1 mg BrdU (Sigma Chemical Co.) in PBS and then were given 0.8 mg/ml BrdU in the drinking water, which was changed every 3 d. Single cell suspensions of lymph node or spleen were stained with anti-CD4 (PharMingen) and anti-Vβ8.3 mAb (17), and resuspended in 200 μl of PBS/1% paraformaldehyde for 20 min on ice. After washing with PBS, the samples were fixed with cold 70% ethanol for 4 min, followed by another fixation step with 100 μl of PBS/1% paraformaldehyde/0.01% Tween 20 for 30 min at room temperature followed by 30 min on ice, and washed with PBS. Subsequently, the samples were treated with DNase I (Boehringer Mannheim), 200 μl/sample for 30 min at 37°C. After washing with PBS, the cells were resuspended in 45 μl of PBS/5% FCS/0.5% Tween 20 and stained with anti-BrdU mAb (Becton Dickinson).
Confocal Microscopy
Positively selected DC from draining lymph nodes of mice vaccinated with green fluorescent protein (GFP)-C5 vaccine were stained with biotinylated N418, followed by Streptavidin Texas red (PharMingen). After washing with PBS, cells were dropped onto glass coverslips and mounted on glass slides with colorless nail lacquer. The confocal images were acquired at the Confocal and Image Analysis Lab at the National Institute For Medical Research, London. The confocal system used was an upright Leica TCS-NT with an Argon/Krypton laser source.
Construction of DNA Vaccine
The full-length C5 cDNA (5.4 kb) was cloned into pTracer vector (Invitrogen BV, UK) under the human cytomegalovirus (CMV) promoter. To optimize and positively regulate the expression, 0.9 kb of 5′ untranslated intronic sequence of the immediate early gene of cytomegalovirus (intron A) was cloned in between the CMV promoter and C5 cDNA (18). In brief, C5 cDNA in pBluescript (Stratagene Inc.) was digested with Bsp120I/NotI restriction enzymes, followed by separation of C5 from the agarose gel electrophoresis and the 5.4-kb fragment released from the construct was excised from the gel and purified using the Geneclean protocol (Anachem Ltd., UK). The pTracer vector was linearized using a unique NotI site in the multiple cloning region of the plasmid and the terminal phosphates were removed using Shrimp Alkaline Phosphatase, (Boehringer Mannheim) treatment for 30 min at 37°C, followed by purification of the linearized vector by the Geneclean method. The 5.4-kb NotI/Bsp120I–C5 DNA fragment and the NotI linearized/phosphatased vector were ligated together in a 3:1 ratio, respectively, using T4 DNA ligase as per standard protocol. The ligated plasmid was then transformed into Escherichia coli bacterial DH5α and plasmid was purified as described in QIAGEN plasmid purification hand book (QIAGEN, Ltd., UK).
Vaccination
DNA vaccine (∼150 μg/mouse in 100 μl volume) was applied by scarification of the ear skin with a 25G needle. At various time points after vaccination, the cervical lymph nodes were removed for further analysis. Keratinocytes were prepared by digesting the dorsal halves of ears with 0.25% trypsin for 25 min at 37°C. Trypsin was washed out with 20% FCS Iscove's modified Dulbecco's medium and the epidermal sheets were peeled off with forceps, resuspended in culture medium, or lysed for RNA preparation.
Immunization
Mice were injected subcutaneously above the footpads with 10 μg C5 in complete Freund's adjuvant. For determination of antibodies, microtiter ELISA plates were coated with C5 at 10 μg/ ml (50 μl) in carbonate buffer, pH 9.5, and serial dilutions of serum were tested for the presence of anti-C5 antibodies using secondary alkaline phosphatase–coupled anti-IgG1 and -IgG2a antibodies.
FACS® Analysis
Analytical flow cytometry was carried out using a FACScan® (Becton Dickinson) and the data were processed using the Cellquest software (Becton Dickinson). Three color staining performed with FITC-, PE-, and biotin-conjugated mAbs followed by streptavidin red 670 (GIBCO BRL, Paisley, UK). Anti-CD4 (H129.19, PharMingen) was used as PE-conjugate, anti-TCR Vβ8.3 (7G8.2) was conjugated with FITC using standard procedures. Biotinylated anti-CD44, -CD69, -CD62-L (PharMingen) were used followed by streptavidin red 670.
Magnetic Cell Sorting
Positive selection of lymph node cells was carried out by magnetic cell sorting with the Vario-MACS (Miltenyi Biotec Inc., Germany) according to the manufacturer's instructions. Lymph nodes were digested with a cocktail of 0.1% DNase I (fraction IX; Sigma Chemical Co.) and 1.6 mg/ml collagenase (CLS4; Worthington Biochemical Corp.) at 37°C for 1 h. For positive selection of DC, cells were stained with biotinylated N418 (19), followed by labeling with streptavidin-magnetic beads. Positively selected cells and the nonselected fractions were collected for further analysis.
cDNA Generation, Amplification, and Analysis
For generation of cDNA, cells were lysed and total RNA was extracted from the lysate in the presence of RNase inhibitors according to the RNeasy RNA isolation kit manual (QIAGEN, Ltd.). cDNA was generated from the mRNA template using a 15-mer poly dT oligonucleotide (Genosys Biotechnologies, Ltd.) and the Superscript reverse transcriptase (RT) enzyme according to Superscript Preamplification System protocol (GIBCO BRL). Expression of the C5 construct was detected by PCR using C5-specific primers under general PCR conditions.
Functional T Cell Activation Tests
IL-2 Production by T Cells from Injected Mice.
Draining lymph nodes from vaccinated or protein injected mice were digested with collagenase/DNase cocktail (see above), washed, and 2 × 105 lymphocytes were cultured in round-bottom 96-well plates with 2 × 104/well bone marrow–derived DC and C5 antigen. Bone marrow–derived DC as APC were generated as described previously (20). In some cases (see Fig. 2 E) DC were pulsed with antigen for 2 h, followed by extensive washing to minimize carry over of antigen into the culture. After 48 h of culture, 50 μl of supernatants were transferred to IL-2–dependent CTLL ATCC TIB 214 cells, and CTLL proliferation was assessed by [3H]thymidine incorporation over 18 h.
Figure 2.
(A) RT-PCR was performed for C5 and β-actin for each sample, as indicated on top. The panel on the left shows RT-PCR from cells of 5.4- vaccinated mice. NDC-DC depleted lymph node cells. On the right RT-PCR from DC of control vector–vaccinated mice and from keratinocytes of 5.4-vaccinated mice are shown. (B and C) Quantitative PCR for the presence of C5 cDNA in (B) lymph nodes and (C) spleen of 5.4-vaccinated mice. The number of PCR cycles is indicated below each lane. (D) Confocal image of two DC isolated from draining lymph nodes of 5.4-vaccinated mice. N418 expression is in red, green fluorescence (GFP) shows the presence of the vaccination construct. Bright field image is overlaid to illustrate the dendritic shape of the cells. (E) IL-2 production obtained with 2 × 104/well DC isolated from draining lymph nodes of vaccinated mice determined by comparison with a standard curve. The standard curve was set up by culturing mixtures of untreated and C5-pulsed (and extensively washed) bone marrow–derived DC with A18 hybridoma cells. The ratio of unpulsed/pulsed DC is shown on the abscissa, and the counts obtained by coculture of A18 hybridoma cells and DC from vaccinated mice intersects the standard curve at a ratio of 98 unpulsed to 2 pulsed DC. (F) IL-2 production by A18 hybridoma cells cultured with serially titrated numbers of DC which were either pulsed with 5 μg/ml C5 (---○---) or cocultured with untreated keratinocytes (□) or with 1,000 Rad irradiated keratinocytes (⋄).
IL-2 Production by A18 T Cell Hybridoma Cells.
5 × 104/well A18 hybridoma cells were cultured with APC as indicated for 24 h. Supernatants were then tested for the presence of IL-2 as described above.
Limiting Dilution Assay.
For assessment of C5-specific T cell precursors, serial dilutions of spleen cell suspensions (24 replicates for each cell concentration) were cultured in the presence of 2 × 104 bone marrow–derived DC and 10 μg C5 protein in Iscove's modified Dulbecco's medium (Sigma Chemical Co.) containing 5% FCS, 2 × 10−3 M l-glutamine, 100 U/ml penicillin, 100 μg/ ml streptomycin, 5 × 10−5 M ME (all from Sigma Chemical Co.). Control wells received T cells and DC, but not C5 protein. After 48 h of culture, 50 μl of supernatants was transferred to IL-2–dependent CTLL cells, and CTLL proliferation was assessed by [3H]thymidine incorporation over 18 h. Reactions were considered positive in wells that exhibited proliferation greater than the mean plus three times the standard deviation value of control wells. The frequency of antigen-specific cells was calculated by regression analysis of the number of negative wells at each dilution of responder cells.
Keratinocyte/DC Cocultures.
Ear cell suspensions were added to 6-well Costar plates (40 mm diameter) and allowed to adhere for 2 h. Nonadherent cells were washed off and some of the keratinocyte monolayers were subjected to 1,000 Rad irradiation before addition of 106/well bone marrow–derived DC. Keratinocyte/DC mixtures were incubated overnight. The DC were then washed off and added in serially titrated numbers to 5 × 104/well A18 T hybridoma cells. IL-2 production by hybridoma cells was determined 24 h later.
Results
Induction of Long-lived CD4 T Cell, But Not B Cell Responses with DNA Vaccine Encoding Intracellularly Expressed C5 Protein.
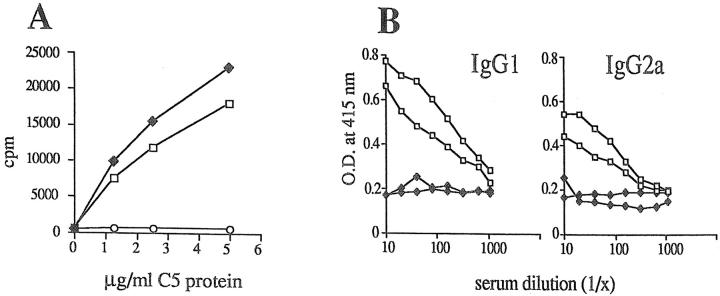
The DNA construct 5.4 containing full-length C5 cDNA under control of the human CMV promoter was applied to the ears of mice by scarification. Control mice were vaccinated with the corresponding empty vector DNA or with C5 protein in CFA. 10 d later the draining lymph nodes were taken and tested in vitro for generation of C5-specific T cell responses. 5.4 vaccination and immunization with C5 protein generated C5-specific T cell responses upon restimulation with C5 protein in vitro, whereas mice vaccinated with empty vector did not mount a response (Fig. 1 A). However, only mice that had received C5 protein had generated C5-specific antibodies 3 wk later, although splenocytes from both groups generated strong C5-specific T cell responses upon restimulation in vitro (data not shown). To increase the chance for an antibody response, two mice from each group were reimmunized 3 wk after protein immunization or 5.4 vaccination with either C5 protein or vaccine and then bled for assessment of the antibody responses to C5 9 d later. As shown in Fig. 1 B, C5 protein–immunized mice produced anti-C5 antibodies of the IgG1 subclass as previously reported (21), whereas 5.4-vaccinated mice did not produce C5-specific antibodies of any subclass even after revaccination. We followed precursor frequencies for C5-specific IL-2 producing T cells in a cohort of 5.4-vaccinated mice for 40 wk and found that maximal frequencies are detectable around 4 wk after vaccination and decline slowly thereafter, but still remain above nontreated background controls at 40 wk after vaccination (Table I).
Figure 1.
(A) IL-2 production of cells from draining lymph nodes of 5.4-vaccinated (150 μg/mouse, ♦), control vector– vaccinated (○), or protein- immunized (10 μg in CFA, □) mice. Lymph nodes from two mice per group were pooled. The figure shows proliferation of IL-2–dependent CTLL cells. (B) Two 5.4-vaccinated (♦) and two C5 protein–immunized (□) mice were revaccinated or reinjected with 10 μg C5 protein/ CFA, respectively, 3 wk after the first injection. 9 d later their sera were tested for the presence of IgG1 and IgG2a anti-C5 antibodies by ELISA.
Table I.
Frequency of C5-specific T Cells per 106 Spleen Cells
| Weeks after vaccination | Untreated | DNA vaccinated | A18TCRtg untreated | |||
|---|---|---|---|---|---|---|
| 0 | 5 | 5 | 10,000 | |||
| 4 | 5.7 | 110 | 13,333 | |||
| 8 | 5 | 66.6 | 10,000 | |||
| 12 | 5 | 50 | 8,000 | |||
| 16 | 5.7 | 30 | 13,333 | |||
| 20 | 5 | 25 | 10,000 | |||
| 24 | 5.7 | 25 | 10,000 | |||
| 28 | 5 | 25 | 8,000 | |||
| 32 | 5 | 25 | 10,000 | |||
| 36 | 5 | 25 | 13,333 | |||
| 40 | 5 | 25 | 10,000 |
The frequency of C5-specific T cells per 106 spleen cells was calculated by regression analysis of the number of negative wells (24 replicates per cell concentration) at each dilution of responder cells.
DC Isolated from Draining Lymph Nodes of Vaccinated Mice Express C5 Protein.
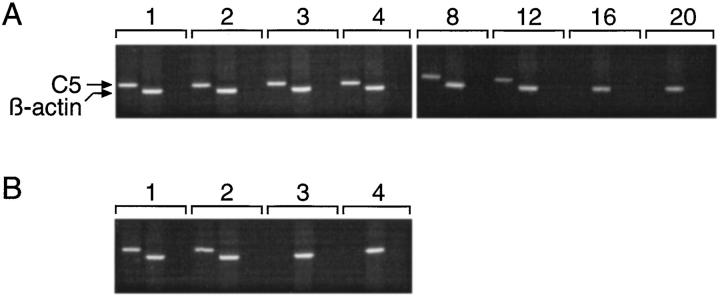
DC from 5.4- and vector control– vaccinated mice were isolated by magnetic cell sorting from draining lymph node cells and spleen and then assessed for the expression of C5 by RT-PCR. In addition, a suspension of ear skin cells (containing mainly keratinocytes) was analyzed by RT-PCR. As shown in Fig. 2 A DC from draining lymph nodes, but not spleen of 5.4-vaccinated mice, express C5. Keratinocytes from the ears of 5.4-vaccinated mice also showed a positive RT-PCR. There was no C5 message detectable in DC from vector vaccinated–control mice. Furthermore, the cell population depleted of DC from draining lymph nodes of 5.4-vaccinated mice, containing B and T cells, did not show a positive RT-PCR signal. This indicates that, in 5.4-vaccinated mice, a proportion of DC, which presumably migrated from the site of DNA application to the draining lymph nodes, had taken up the DNA construct and synthesized C5 protein. With the exception of keratinocytes at the site of DNA application no other cell population was found to contain C5 message. In contrast to this, C5 DNA was demonstrable in lymph node and spleen, albeit at much smaller amounts in the latter (Fig. 2, B and C). This suggests that DNA applied to ear skin gains access to the circulation.
A Small Proportion of DC Is Transfected and Cross-presentation of Protein from Transfected Keratinocytes Occurs Only in a Situation of Excessive Cell Death.
In an attempt to quantify the number of DC in draining lymph nodes which express the C5 construct, we set up functional studies and confocal analysis. The 5.4 construct used contains GFP expressed under a second promoter to allow direct visualization of transfected cells by fluorescence microscopy. FACS® analysis of DC isolated by magnetic cell sorting 10 d after vaccination did not give a clear signal, presumably because of the very low incidence of transfection. However, GFP containing DC were clearly identifiable by confocal microscopy (Fig. 2 D). DC from draining lymph nodes of 5.4-vaccinated mice were then tested for their capacity to activate the C5-specific T cell hybrid A18. In parallel a standard curve was set up by combining various numbers of DC that had been pulsed with C5 protein, followed by washing, with nonpulsed DC and A18 T cells. Comparing the T cell responses obtained, we estimate that ∼2% of the DC in the draining lymph nodes of vaccinated mice processed and presented C5 in the context of MHC class II molecules for recognition by A18 T cells (Fig. 2 E). A recent study (4) also illustrated that DC from vaccinated mice could stimulate T cells in the absence of added antigen. However, in this case the protein encoded by the DNA construct was secreted so that it was not possible to define to what extent the stimulatory capacity of DC was due to direct transfection, compared with uptake of protein synthesized and released by other transfected cells. Although the 5.4 C5 construct does not give rise to secreted C5 protein, there is now substantial evidence that DC can take up protein from other cells by a mechanism referred to as cross-priming (22–24). We tested this by coculturing keratinocytes from 5.4-vaccinated mice with untreated DC which were subsequently assayed for their ability to activate the A18 hybrid. This coculture assay is very sensitive, detecting as little as 6 ng/ml C5 (25), but in the presence of intact keratinocytes, which expressed C5 as shown by RT-PCR, there was no evidence of any protein transfer to DC (Fig. 2 F). In contrast, exposure of keratinocytes to 1,000 Rad irradiation before coculture with DC resulted in strong C5 presentation by the DC. This indicates that cell death induced by this treatment allows cross-priming of dendritic cells for presentation in the context of MHC class II.
DNA Vaccination Results in Activation of DC.
DC isolated by magnetic cell sorting from draining lymph nodes of 5.4- or control vector–vaccinated mice were tested for expression of cell surface molecules that are important in T cell stimulation, such as CD40, B7.1, B7.2, and ICAM-1. Both 5.4 vaccine and vector resulted in strong upregulation of these molecules on all DC found in the draining lymph nodes compared with DC from untreated controls (Fig. 3), illustrating that DNA vaccination, in a nonspecific manner, provides the necessary stimuli for activation of DC; this is in accordance with recent observations that bacterial DNA and synthetic CpG oligodeoxynucleotides promote DC activation and maturation in vitro (26).
Figure 3.
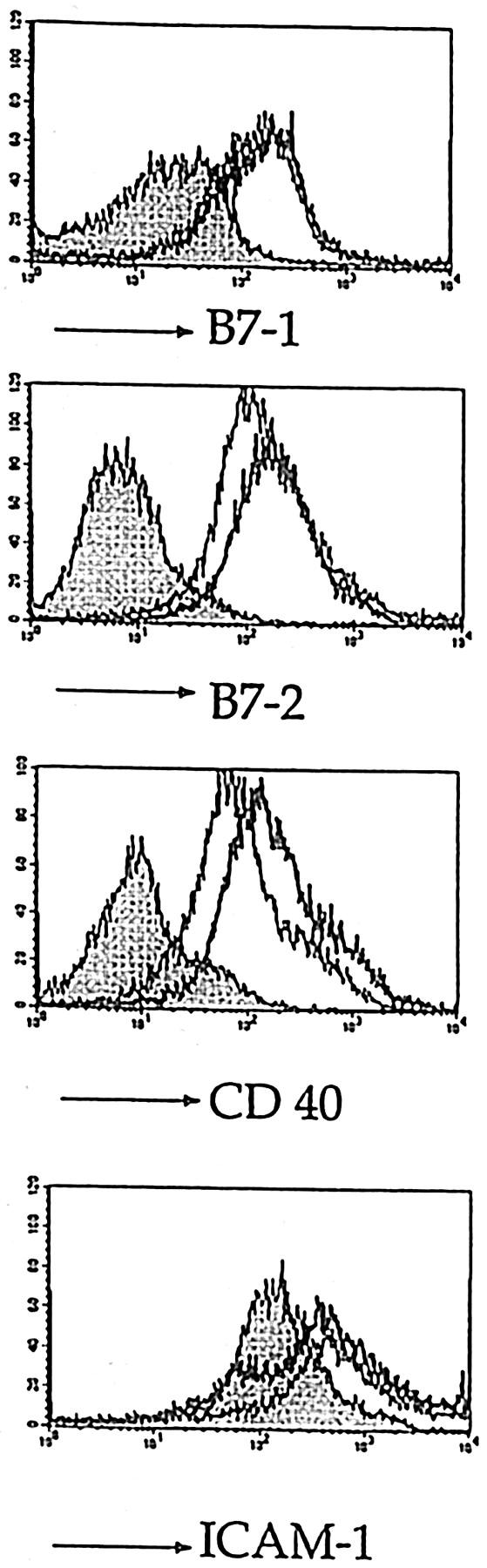
FACS® analysis of DC isolated 3 d after vaccination by magnetic cell sorting from draining lymph nodes of 5.4-vaccinated (thick lines), vector-vaccinated (thin lines), and untreated mice (shaded histograms). The figure shows expression of activation markers B7.1, B7.2, CD40, and ICAM-1 on gated N418 positive DC.
Kinetics and Site of T Cell Activation after DNA Vaccination of Transgenic Mice.
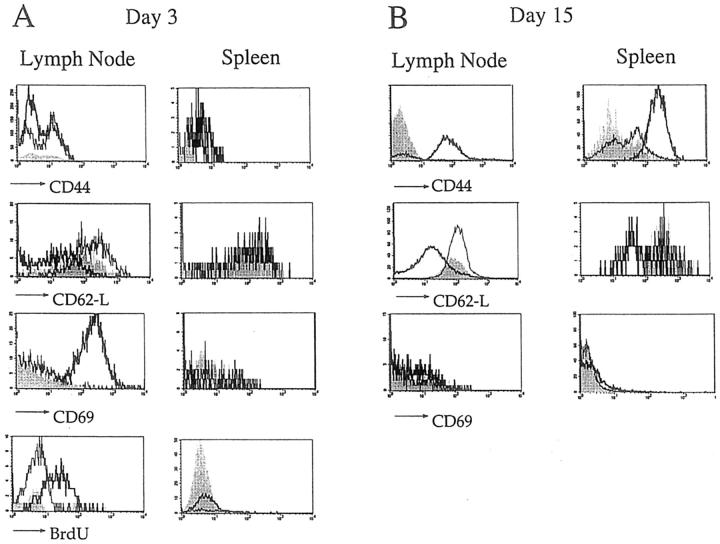
To investigate the sites, kinetics, and extent of T cell activation after DNA vaccination, we made use of T cell receptor transgenic (A18 TCRtg) mice, as normal mice have very low precursor frequencies for C5-specific T cells (Table I). All CD4 T cells found in the A18 TCRtg strain on a Rag−/− background are specific for epitope 106-121 of C5 (16) and have a naive phenotype as evidenced by their low expression of CD44 and CD69 and high expression of CD62-L. A cohort of A18 TCRtg mice was vaccinated with the 5.4 or vector control construct. At the same time two vaccinated mice per group were injected with BrdU and received BrdU in the drinking water for the next 3 d. 3 d later, the draining lymph nodes and spleens from BrdU-treated mice were analyzed for expression of activation markers on CD4 T cells and incorporation of BrdU. In 5.4-vaccinated mice, all CD4 T cells from lymph nodes draining the site of vaccination had incorporated BrdU, indicating activation and division (Fig. 4 A). CD69, an early activation marker, was upregulated and CD62-L showed some degree of downregulation, whereas no significant changes were observed in CD44 levels yet. CD4 T cells from mice vaccinated with vector control construct or from untreated mice retained their naive phenotype. Whereas CD4 T cells from draining lymph nodes of 5.4-vaccinated mice all appear to be activated 3 d after vaccination, splenic CD4 T cells had neither incorporated BrdU nor showed any changes in activation markers. This indicates that T cell activation is initiated in the draining lymph nodes and that, in contrast to DC, antigen-specific T cells are only activated by DNA encoding the corresponding antigen; there was no nonspecific activation by vector DNA alone. By day 15 after vaccination, all CD4 T cells in lymph node and spleen of 5.4-vaccinated mice expressed an activated phenotype, characterized by downregulation of CD62-L and upregulation of CD44 (Fig. 4 B), whereas T cells in control mice showed no signs of activation.
Figure 4.
Lymph node and spleen cells from A18 TCRtg mice vaccinated with the 5.4 construct, (thick lines), the control vector (thin lines), or untreated (shaded histograms) were analyzed (A) 3 d and (B) 15 d later by three-color FACS® analysis. The figure shows expression of CD44, CD62-L and CD69 (and BrdU labeling for day 3 after vaccination) on gated CD4 and Vβ8.3-positive T cells.
The Source of Stimulatory Antigen Is Short-lived in Vaccinated Mice.
To test for the continuous presence of antigen in 5.4-vaccinated mice, two approaches were used. First, DC of the draining lymph nodes and keratinocytes at the site of injection were analyzed for expression of C5 by RT-PCR from 1 to 20 wk after vaccination. Fig. 5 shows that, for DC, C5 expression was not demonstrable later than 2 wk after vaccination, whereas keratinocytes continued to express C5 longer, until week 12. In addition, a sensitive in vivo test using naive transgenic A18 T cells was used to detect the presence of a persistent source of antigen for T cell priming. Naive A18 T cells carrying a different Thy 1 allotype (Thy1.1) and labeled with the fluorescent dye carboxyfluorescein diacetate-succinimidylester (CFSE) to allow monitoring of cell division (27) were injected into host mice that had been vaccinated 20 d before. 7 d after injection, the mice were killed and the phenotype of the injected T cell population was analyzed in FACS® (Fig. 6). Thy1.1 A18 T cells found in the draining lymph nodes of mice vaccinated 20 d before had retained their CFSE label and naive phenotype. Likewise, injected Thy1.1 A18 T cells present in the spleen had a naive phenotype (data not shown). Thus, it appears that 20 d after vaccination there is no longer any source of stimulatory antigen present.
Figure 5.
RT-PCR analysis was performed for keratinocytes from (A) 5.4-vaccinated mice and (B) DC isolated from draining lymph nodes of 5.4-vaccinated mice by magnetic cell sorting. RT-PCR was performed for C5 and β-actin at different time points after vaccination, as indicated on top (week 1 to week 20). The third lane for each time point is a control showing the absence of DNA contamination in the RNA samples (omission of RT before the PCR reaction).
Figure 6.
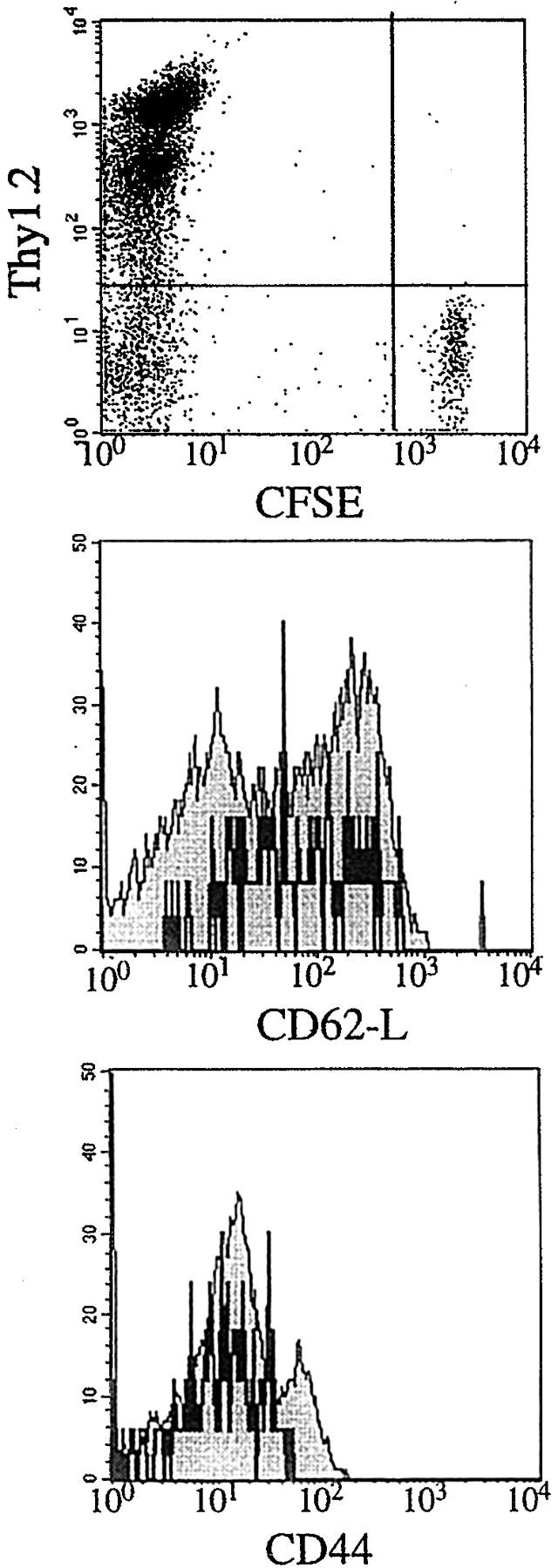
Three-color FACS® analysis of cells from draining lymph nodes (pooled from two mice vaccinated with 5.4 20 d before i.v. injection with 106 CFSE-labeled naive Thy1.1 A18 TCRtg T cells). 7 d after injection of naive T cells, lymph node cells were analyzed by FACS® for expression of CFSE, Thy1.2, Thy1.1, and activation markers. The dot plot shows CFSE levels on Thy1.2 negative (Thy1.1 positive) injected T cells. Histograms for the activation markers CD44 and CD62-l show cells gated for expression of Thy1.1 (black lines) or Thy1.2 (endogenous T cells; shaded histograms).
Discussion
The success of DNA vaccination is linked to initiation of T cell responses by DC. There are various routes by which DC can obtain the protein they present. They can either take up protein secreted by other cell types which were transfected after DNA vaccination, or pick up antigen from transfected cells, an as yet ill-defined mechanism referred to as cross-presentation (24, 28, 29). Additionally, they can be transfected themselves (2, 5, 6). In this paper we used a DNA construct encoding a form of C5 protein which is not secreted by transfected cells with the aim to limit its accessibility to APCs and investigate the consequences for priming and maintenance of CD4 T cell responses. It is not known what causes the block in secretion of C5, but we determined recently that addition of an optimal leader sequence to the construct results in C5 secretion. The exclusive intracellular expression of the 5.4 construct after transfection into many different cell types, such as fibroblasts, B cells, macrophages, and hepatocytes, has been tested extensively (25). In addition, its restricted localization is supported by the absence of antibody responses after vaccination. Thus, C5 message was only detectable in keratinocytes at the site of vaccination and in a small proportion of DC within the draining lymph nodes. We assume that these migrated there from the site of DNA application in the ear skin, although we cannot exclude the possibility that DC also were transfected in the draining lymph nodes themselves because of relatively high levels of DNA at this site (Fig. 2 B). Functional analysis of their stimulatory capacity for C5-specific T cells suggested that ∼2% of the lymph node DC presented antigen. A recent study (6) likewise concluded that activation of CD8 T cells is achieved through direct transfection of a small proportion of DC that migrate from the skin to the draining lymph nodes. Although it is possible that this reflects the number of directly transfected DC, we cannot formally exclude the possibility that some of these DC acquired C5 protein expressed in keratinocytes by cross-presentation. Notably, no antigen was transferred to DC from intact keratinocytes, whereas irradiation of keratinocytes provided a strong stimulus for cross-presentation.
It is still a matter of debate whether normal cell turnover by apoptosis in vivo results in release of antigenic material which could be obtained for presentation by DC or whether this event only occurs in inflammatory situations encountered during necrosis of cells or tissue (30), or in infection induced apoptosis as described by Albert et al. (22). While there is no obvious sign of inflammation, such as swelling after scarification of the ears, it is nevertheless possible that some keratinocytes die in a manner that results in cross-presentation by skin DC, which then migrate to the draining lymph nodes to activate T cells. However, this event is unlikely to take place at later time points after vaccination when the site of scarification has healed.
Although keratinocytes were shown to express C5 up to 12 wk after vaccination, the presence of antigen in these cells is unlikely to be responsible for the persistence of T cell memory beyond 40 wk. DC have been described to have a half-life of <1 wk in mouse spleen, and those DC present in lymphoid organs no longer proliferate (31, 32). We found no evidence of C5 expressing DC in the spleen, indicating that the transfected DC found in draining lymph nodes do not migrate to other lymphoid organs. The absence of C5 expression in DC beyond 2 wk after vaccination, together with the fact that a fresh cohort of naive transgenic T cells failed to be activated when injected into mice that had been vaccinated 20 d before, support the conclusion that there is no long-lived source of antigenic material present. Considering the absence of an antibody response, it is also unlikely that antigen was stored in the form of antigen–antibody complexes on follicular DC, which are known to be highly efficient depots of long-lived antigen (33). Therefore, it seems that DNA vaccination can induce memory CD4 T cells that can be long-lived without repeated antigenic stimulation.
The potent immunostimulatory activity of unmethylated CpG motifs present in bacterial plasmid DNA (for review see reference 34) may have important implications for priming and maintenance of T cell responses. The data in this paper show that DNA provides activation signals to DC. While only a very low fraction of DC expressed C5, all DC in the draining lymph nodes showed an activated phenotype. It is highly likely that scarification itself provided an activation signal to Langerhans cells in the skin, provoking them to migrate to the draining lymph nodes. However, it is unlikely that all DC found in the draining lymph nodes were derived from Langerhans cells, suggesting instead that DNA present in the lymph nodes was responsible for activation of resident DC and DC that may have been recruited from the circulation. Influx of DC or expansion from precursors may take place after DNA vaccination since total DC numbers in draining lymph nodes increase (6). It has been reported that DNA vaccines administered by the gene gun method gain access to the circulation, because immediate removal of the ear after vaccination does not prevent an immune response (35, 36). This seems to happen after scarification as well, since PCR analysis showed strong positive signals for C5 DNA in lymph node and weaker expression in spleen. Therefore, small amounts of DNA reaching the circulation apparently exert stimulatory activity on many cell types, but fail to be transcribed and translated into protein.
In view of recent data showing the crucial importance of DC activation for stimulation of CD8 T cell responses, the finding that DNA vaccination nonspecifically activates a large number of dendritic cells suggests that this type of genetic immunization provides ideal conditions not only for CD4 T cell responses, but especially for the initiation of CD8 T cell responses. Therefore, the success in achieving long-lived memory responses by DNA vaccination may lie in optimal activation and expansion of most antigen-specific T cell precursors, rather than in long-term storage of antigen leading to periodic restimulation.
Footnotes
Abbreviations used in this paper: CFSE, carboxyfluorescein diacetate-succinimidylester; CMV, human cytomegalovirus; DC, dendritic cells; GFP, green fluorescent protein; RT, reverse transcriptase.
References
- 1.Donnelly JJ, Ulmer B, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 2.Raz E, Carson DE, Parker SE, Parr TB, Abai AM, Aichinger G, Gromkowski SH, Singh M, Lew D, Yankauckas MA, et al. Intradermal gene immunization: the possible role of DNA uptake in the induction of cellular immunity to viruses. Proc Natl Acad Sci USA. 1994;91:9519–9523. doi: 10.1073/pnas.91.20.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 4.Casares S, Inaba K, Brumeanu T-D, Steinman RM, Bona CA. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II–restricted viral epitope. J Exp Med. 1997;186:1481–1486. doi: 10.1084/jem.186.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 6.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+T cells after gene gun immunization. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 8.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 9.Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin-4 and downregulated by tumor necrosis factor-α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun S, Kishimoto H, Sprent J. DNA as an adjuvant: capacity of insect DNA and synthetic oligodeoxynucleotides to augment T cell responses to specific antigen. J Exp Med. 1998;187:1145–1150. doi: 10.1084/jem.187.7.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 14.Roman M, Martin-Orozco E, Goodman JS, Nguyen MD, Sato Y, Ronaghy A, Kornbluth RS, Richman DD, Carson DA, Raz E. Immunostimulatory DNA sequences function as T helper-1–promoting adjuvants. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen MD, Silverman GJ, Lotz M, Carson DA, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 16.Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II–restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Förster I, Hirose R, Arbeit JM, Clausen BE, Hanahan D. Limited capacity for tolerization of CD4+T cells specific for a pancreatic beta cell neo-antigen. Immunity. 1995;2:573–585. doi: 10.1016/1074-7613(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 18.Chapman BS, Thayer RM, Vincent KR, Haigwood NL. Effect of intron A from human cytomegalovirus immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 1991;14:3979–3986. doi: 10.1093/nar/19.14.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volkmann A, Zal T, Stockinger B. Antigen-presenting cells in the thymus that can negatively select MHC class II–restricted T cells recognizing a circulating self antigen. J Immunol. 1997;158:693–706. [PubMed] [Google Scholar]
- 21.Stockinger B, Zal T, Zal A, Gray D. B cells solicit their own help from T cells. J Exp Med. 1996;183:891–899. doi: 10.1084/jem.183.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I–restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 23.Kurts C, Kosaka H, Carbone FR, Karamalis F, Miller JFAP, Heath WR. Class I–restricted cross- presentation of exogenous self-antigens leads to deletion of autoreactive CD8+T cells. J Exp Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone marrow– derived cells in presenting MHC class I–restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 25.Brazil MI, Weiss S, Stockinger B. Excessive degradation of intracellular protein in macrophages prevents presentation in the context of major histocompatibility complex class II molecules. Eur J Immunol. 1997;27:1506–1514. doi: 10.1002/eji.1830270629. [DOI] [PubMed] [Google Scholar]
- 26.Sparwasser T, Koch ES, Vabulas RM, Heeg K, Lipford GB, Ellwart JW, Wagner H. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045–2054. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 28.Corr M, Lee DJ, Carson DA, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doe B, Selby M, Barnett S, Baenziger J, Walker CM. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow derived cells. Proc Natl Acad Sci USA. 1996;93:8578–8583. doi: 10.1073/pnas.93.16.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 31.Metlay JP, Puré E, Steinman RM. Control of the immune response at the level of antigen presenting cells: a comparison of the function of dendritic cells and B lymphocytes. Adv Immunol. 1989;47:45–116. doi: 10.1016/s0065-2776(08)60662-8. [DOI] [PubMed] [Google Scholar]
- 32.Steinman RM, Lustig DS, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. III. Functional properties in vivo. J Exp Med. 1974;139:1431–1445. doi: 10.1084/jem.139.6.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tew JG, Phipps RP, Mandel TE. The follicular dendritic cell: long-term antigen retention during immunity. Immunol Rev. 1980;53:29–59. doi: 10.1111/j.1600-065x.1980.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 34.Pisetsky DS. Immune activation by bacterial DNA: a new genetic code. Immunity. 1996;5:303–310. doi: 10.1016/s1074-7613(00)80256-3. [DOI] [PubMed] [Google Scholar]
- 35.Klinman DM, Sechler JMG, Conover J, Gu M, Rosenberg AS. Contribution of cells at the site of DNA vaccination to the generation of antigen-specific immunity and memory. J Immunol. 1998;160:2388–2392. [PubMed] [Google Scholar]
- 36.Barry MA, Johnston SA. Biological features of genetic immunization. Vaccine. 1997;8:788–791. doi: 10.1016/s0264-410x(96)00265-4. [DOI] [PubMed] [Google Scholar]