Abstract
Interleukin (IL)-12 is expressed mainly in antigen-presenting cells after challenge with microbial material or after CD40 activation. Although IL-12 was cloned from human Epstein-Barr virus (EBV)-transformed B cell lines, surprisingly, CD40 ligation on murine B cells did not lead to IL-12 production, suggesting that murine B cells do not produce IL-12. Here we demonstrate that a subset of human tonsillar B cells can be induced to express and secrete bioactive IL-12. The major stimulus to produce IL-12 in human B cells was CD40 ligation. In contrast, B cell receptor cross-linking did not induce IL-12. Expression of IL-12 after CD40 activation was restricted to CD38−IgD± non-germinal center (non-GC) B cells. CD40 ligation and interferon (IFN)-γ exhibited synergistic effects on IL-12 production, whereas IL-10 abrogated and IL-4 significantly inhibited IL-12 production by these B cells. In contrast to IL-12, production of IL-6 is conversely regulated, leading to significant increase after CD40 ligation in the presence of the T helper type 2 (Th2) cytokine IL-4. Cord blood T cells skewed towards either a Th1 or a Th2 phenotype maintained their cytokine expression pattern when restimulated with allogeneic resting B cells. Blockade of CD40 and/or IL-12 during T–B interaction significantly reduced IFN-γ production by the T cells. This suggests a model whereby B cells produce either IL-12 or IL-6 after contact with T cells previously differentiated towards Th1 or Th2. Furthermore, IL-12 and IL-6 might provide a positive feedback during cognate T–B interactions, thereby maintaining T cells' differentiation pattern during amplification of the immune response.
Keywords: interleukin 12, B lymphocytes, CD40, interferon γ, interleukin 10
The current model of T cell–mediated immune response suggests that priming and activation of T cells occur in secondary lymphoid organs (1). First, professional APCs, especially dendritic cells (DCs),1 present antigen to naive T cells in the T cell zone (2, 3). This initial priming step involves cell-to-cell contact between APCs and T cells that engages MHC molecules on the APC and TCR molecules on the T cell. Sufficient expression of adhesion and costimulatory molecules on the APCs assures efficient T cell activation (4–7), and the expression of cytokines by APCs, such as IL-12 and IL-6, can lead to T cell polarization (for reviews, see references 2–6). It is now widely accepted that the major sources of IL-12 are DCs (7–10) and activated macrophages (11, 12). IL-12 production is induced either directly by microbacterial compounds in a T cell–independent mechanism (13, 14) or by a T cell– dependent route that uses CD40L–CD40 interactions between T cells and APCs (8–10). Although CD40 ligation on B cells is a major signal for activation, differentiation, isotype class switching (15), and upregulation of cytokines and cell surface molecules (16), it remains unclear whether normal human B cells express IL-12 after CD40 activation (17). It has been reported that IL-6 expressed by APCs can induce T cells to produce IL-4 (18), and this cytokine might therefore be a factor responsible for inducing Th2 responses. IL-6 is expressed by a variety of cells including B cells, but monocytic cells are the major source (19).
Although there is no doubt regarding the key role of DCs in processing and presentation of antigen and initiation of a T cell immune response, questions remain about their role later during the immune response. Since in vivo data have demonstrated that antigen-bearing DCs disappear from secondary lymphoid organs after 24–48 h of initial antigen inoculation (13, 20), mechanisms of maintaining, amplifying, and diversifying this initial T cell activation have to exist to explain the subsequent expansion of the immune response after initiation (21). One explanation could be that this short period of antigen presentation by DCs is sufficient to induce not only T cell activation, but also expansion. In vivo data in murine model systems using tumor-specific peptide-pulsed DCs support this notion (22–28). However, when antigen-specific T cells in vivo are followed over time during an immune response, it has been clearly demonstrated that they migrate to the outer T cell zone (21) and then to the B cell area (29) where cognate T–B interactions between antigen-specific T and B cells occur, leading to further expansion of both T and B cells. This intermediate step is followed by the development of a germinal center (GC) reaction, leading to further expansion of antigen-specific B cells but also T cells inside the GC (21).
T cell polarization of naive T cells towards Th1 or Th2 subsets can occur in 48 h after activation (30, 31) and presumably occurs during interaction with DCs in the T cell zone. However, if polyclonal T cell populations are polarized towards Th1 or Th2 subtypes, they can be converted to the opposite phenotype when exposed thereafter to IL-4 or IL-12, respectively (32, 33). It remains unclear if and how T cell polarization is determined during the latter phases of the immune response, when T cells are migrating to the outer T cell zone and subsequently to the GC to meet antigen-specific B cells. It could be possible that T cells are resistant to any further skewing by the microenvironment once they have completed their polarization program. Alternatively, T cell polarization could be further stabilized by the microenvironment during migration, most likely during cognate T–B cell interactions, supporting not only B cell (34) but also T cell differentiation. Our working hypothesis is that a positive feedback loop between the antigen-presenting B cell and the T cell exists during cognate T–B interactions in the outer T cell zone, and that this mechanism is mediated by cytokines, including IL-6 and IL-12, analogous to that expressed by APCs. We would further hypothesize that the expression of these cytokines by B cells is precisely regulated by signals delivered by the activated T cells and not by signals mediated via the B cell receptor or through MHC signals. To test this hypothesis, we analyzed human tonsillar B cells for their expression of IL-12 and IL-6 and the signals that induce these cytokines. Although IL-12 was cloned from human EBV-transformed B cell lines (35–38), protein expression in normal human tonsillar B cells has not yet been described (17). We show here that IL-12 is induced in a small fraction of IgD+ and IgD− non-GC B cells after CD40 activation, increased by the Th1 cytokine IFN-γ and negatively regulated by IL-4 and, more significant, by IL-10. We detected IL-6 production by non-GC B cells (39) that was mainly induced after CD40 activation and IL-4 (40). In keeping with the model of Mamula and Janeway (1), we suggest that B cells are second line APCs able to support an ongoing T cell response initiated by DCs.
Materials and Methods
Donors.
Human PBMCs from healthy donors were obtained by phlebotomy or leukopheresis. Cord blood was obtained after delivery. Tonsils were obtained as discarded tissue after routine tonsillectomy. All specimens were obtained after approval by the Institutional Review Board. Informed consent for blood donations was obtained from all volunteers.
B Cells.
Tonsillar B cells were obtained from human tonsils. Cells were mechanically homogenized in medium containing magnesium and calcium and mashed through a 40-μm sieve to clear the cell solution from tissue fragments and cell clusters known to contain the vast majority of DCs (41). The single cell suspension obtained was subsequently ficoll density centrifuged and rosetted over sheep erythrocytes (42) followed by magnetic bead depletion (MBD) of CD4+, CD8+, CD14+, and CD56+ cells (16, 43–45). As determined by FACS® analysis, B cell purity always exceeded 97%, with few CD3+ cells. Of particular importance, no CD19−CD3− cells were found that stained with CD11a, CD14, CD33, CD83, or MHC class II. GC B cells were isolated by MBD using an anti-CD44 mAb (a gift of Dr. S. Cannistra, Dana-Farber Cancer Institute). This resulted in a cell population uniformly expressing CD19, CD20, and CD38, resembling the immunophenotype of GC B cells. For purification of resting non-GC B cells containing both naive IgD+ and memory IgD− CD38− B cells, a CD38 mAb (Coulter Corp.) was added during MBD, resulting in a population of cells >98% of which were CD38−CD19+CD20+ B cells.
T Cells.
Adult CD3+CD4+ T cells were obtained from PBMCs. Removal of adherent cells by plastic adherence was followed by MBD of non-T cells (16, 43, 44) using mAbs against CD11a (Mo1), CD14 (Mo2), CD19 (B4), CD8 (T8), and CD56 (3G8). Preparations were always >97% CD3+CD4+ as assessed by immunophenotypic analysis. Where indicated, T cells were further enriched for CD45RA+ T cells using a CD45RA+ mAb (UCHL1; gift of Dr. C. Morrimoto, Dana-Farber Cancer Institute) during MBD. Cells were always >75% CD45RA+ as determined by FACS® analysis. Cord blood T cells were purified by rosetting over sheep red blood cells followed by MBD as described above, and were always >97% CD3+CD45RA+CD4+.
CD40L and Mock Transfectants.
As described previously (16, 43, 44), murine NIH3T3 fibroblasts transfected with the human CD40L (t-CD40L cells) or vector alone (t-mock) were used for stimulation of human B cells. Phenotypic analyses were performed regularly on these cells and in all analyses >95% of t-CD40L cells were positive for human CD40L with a mean intensity of fluorescence (MIF) between 80- and 300-fold over background (MIF = 10), whereas t-mock cells were always found to be negative. Transfectants were grown in medium containing 45% DMEM (GIBCO BRL), 45% F12 (GIBCO BRL), 10% FCS, 2 mM glutamine (GIBCO BRL), and 15 μg/ml gentamicin (GIBCO BRL). For B cell cultures, t-CD40L and t-mock cells were lethally irradiated (96 Gy) and subsequently plated on 6- or 24-well plates (Costar Corp.) at a concentration of 0.4 × 105 cells/well. After an overnight culture at 37°C in 5% CO2, t-CD40L cells were adherent and could be used for coculture.
B Cell Cultures.
B cells were stimulated with various stimuli for up to 96 h in medium based on Iscove's MDM (GIBCO BRL) supplemented with 2% FCS, 50 μg/ml human transferrin (Boehringer Mannheim), 5 μg/ml human insulin (Sigma Chemical Co.), and 15 μg/ml gentamicin at 37°C in 5% CO2. B cells were stimulated at a concentration of 1 or 2 × 106 cells/ml with mAbs against the cell surface Ig (goat anti–human IgG and IgM Fc F(ab′)2 fragments, 10 μg/ml; Jackson ImmunoResearch Laboratories [46]), anti–MHC class II mAbs (9-49, 10 μg/ml [43, 44]), or the cytokines IL-2 (50 IU/ml; gift of Dr. J. Ritz, Dana-Farber Cancer Institute), IL-4 (10 ng/ml; Immunex), IL-6 (5 ng/ ml; Genzyme), IL-10 (10 ng/ml; Genzyme), and IFN-γ (20 ng/ ml; Genzyme), or combinations of these cytokines. For stimulation via CD40, cells were cocultured with adherent irradiated t-CD40L cells and with t-mock cells for control experiments.
DCs and Monocytes.
For control experiments, monocytes were isolated from peripheral blood by ficoll density centrifugation and rosetting over sheep erythrocytes. Nonrosetting cells were mostly CD14+ with few CD83+ DCs or CD19+ B cells. These cells were stimulated with IFN-γ (20 ng/ml) for 2 h followed by LPS (10 ng/ml) and IFN-γ (20 ng/ml) for 22 h before analysis for intracellular IL-12. For these experiments, Brefeldin A (Sigma Chemical Co.) was added at 10 μg/ml for the last 22 h. DCs were generated from monocyte-enriched fractions by culture with GM-CSF (50 ng/ml; Immunex) and IL-4 (10 ng/ml; Genzyme) for 5–8 d, followed by stimulation via CD40 using t-CD40L cells for 24–72 h (16). Supernatants of these cultures were harvested and stored at –80°C until use.
Immunofluorescence Studies.
Purity of cell populations under study was determined by dual-color FACS® analysis using directly conjugated mAbs for CD3 (CD3; Coulter Corp.), CD4 (T4; Coulter Corp.), CD8 (T8; Coulter Corp.), CD14 (Tük4, anti–human IgG and IgM Fc fragment F; DAKO), CD83 (HB15; Coulter Corp.), CD19 (B4; Coulter Corp.), CD20 (B1; Coulter Corp.), CD38 (HBV; Becton Dickinson), CD44 (DF1485; DAKO), CD56 (N901; Coulter Corp.), and surface (s)IgD (DAKO).
Detection of p70 and p40 IL-12 in B Cell Culture Supernatants.
Supernatants from B cell cultures were harvested after 18–96 h of culture and stored at –80°C until analysis. As positive controls, supernatants from blood-derived DCs were also analyzed. Both p70 IL-12 and p40 IL-12 were analyzed by sandwich ELISA using matched antibody pairs (R&D Systems). In brief, anti-p40 IL-12 (5 μg/ml) or p70 IL-12 (5 μg/ml) were plated overnight at room temperature in PBS onto MaxiSorb® plates (Nunc). Plates were washed using 0.05% Tween 20 in PBS, pH 7.4, and subsequently blocked using PBS containing 5% Tween 20, 5% sucrose, and 0.05% NaN3. Plates were again washed four times before standards (recombinant human [rh]IL-12 or rh p40 IL-12 in B-2) or supernatants from B cell cultures were incubated for 2 h at room temperature. Plates were again thoroughly washed followed by a 2-h incubation using biotinylated anti–IL-12 mAbs (350 ng/ml; R&D Systems). Plates were incubated subsequently with streptavidin–horseradish peroxidase (1:5,000; Zymed Laboratories, Inc.) for 1 h. TMB (DAKO) was used as substrate and 0.18 M H2SO4 to stop the enzymatic reaction after 30–60 min. Plates were read using an automated ELISA reader and analyzed with Soft MAX Pro® software (Molecular Devices Corp.).
IFN-γ Production by CD3+CD4+ T Cells as Bioassay for IL-12 Detection.
Human purified CD3+CD4+ T cells were stimulated with PMA (1 ng/ml; Sigma Chemical Co.) and CD28 mAbs (clone 3D10, 2 μg/ml) at 105 cells/ml in RPMI 1640 (Cellgro Mediatech) supplemented with 10% human AB serum (Sigma Chemical Co.), 2 mM glutamine (GIBCO BRL), 15 μg/ml gentamicin (GIBCO BRL), hereafter referred to as RPMI-10, in 200 μl final vol/well in 96-well plates (Falcon; Becton Dickinson) in triplicate for 48 h at 37°C in a 5% CO2 atmosphere before supernatants were harvested from the cultures. Exogenous rhIL-12 (R&D Systems) was added to these cultures at concentrations ranging from 1 to 1,000 pg/ml. To measure bioactive IL-12 from culture supernatants of the B cell cultures, a 50% vol of B cell culture supernatant was added to the T cell culture. Culture supernatants from the T cell cultures were then analyzed for IFN-γ by ELISA using matched pairs of anti–IFN-γ antibodies following the provider's instructions (Endogen). To confirm that IL-12 was indeed responsible for IFN-γ production by the T cells, a neutralizing mAb for IL-12 (10 μg/ml; R&D Systems) was added when indicated throughout the whole culture period. To control for equivalent activation and proliferation in these cultures, thymidine incorporation ([3H]Tdr, 1 mCi/well; DuPont-NEN) was measured for the last 16 h of a 72-h culture after supernatants had been removed.
Detection of p40/p70 IL-12 by Intracellular Staining.
Intracellular IL-12 in monocytes and B cells was detected by FACS® analysis using a directly labeled anti–IL-12 mAb (C11.5; PharMingen). B cells were stimulated with t-CD40L cells with or without IFN-γ (20 ng/ml). After 14–16 h, Brefeldin A (10 μg/ml) was added to the cultures and the cells were cultured for another 6–8 h. Addition of Brefeldin A during earlier time points or for shorter times at the end of the B cell cultures resulted in a decreased detection of intracellular cytokines. Cells were harvested, washed with PBS, and incubated with mouse Ig (60 μg/ml; Jackson ImmunoResearch Laboratories) for 10 min to block unspecific binding, followed by the addition of directly labeled mAbs to CD19, CD20, or sIgD for 30 min to detect extracellular expression of these molecules. After washing with PBS, cells were first paraformaldehyde fixed for 20 min using the Perm and FIX kit from Caltag, followed by permeabilization and intracellular staining for 30 min using either anti–IL-12 (C11.5; PharMingen) or anti–IL-6 mAbs (M02-1305; PharMingen). Cells were finally washed in PBS again and resuspended in PBS supplemented with 0.1% formaldehyde. All incubations were carried out at room temperature. Cells were immediately analyzed using a Coulter EPICS XL Elite flow cytometer. Data are displayed as dot plots of PE (x-axis) and FITC (y-axis) fluorescence (four decade log scales).
Cytokine Production Analysis in T–B Cultures.
Naive cord blood T cells were stimulated primarily with CD3 plus CD28 mAbs bound to tossyl beads in the presence of IL-2 and either IL-12 plus anti–IL-4 mAbs or IL-4 and anti–IFN-γ mAbs for 3–6 d. Cells were washed extensively, rested for 6–8 h on ice, and restimulated with allogeneic resting CD19+CD38−sIgD± B cells for 24–72 h before supernatants were analyzed for IFN-γ and IL-4 by ELISA. Where indicated, neutralizing anti–IL-12 mAb, blocking anti-CD40L mAb (anti-CD40L mAb clone M90, 10 μg/ml; Immunex), or both were added to the cultures. To test whether CD40-activated B cells produce IL-12 and can induce IFN-γ by T cells, adult CD3+CD4+ T cells (2 × 106 T cells/ml) from healthy individuals enriched for CD45RA+ (>75%) were stimulated in an allogeneic MLR with irradiated (32 Gy) CD40-activated CD19+CD38−sIgD± tonsillar B cells (106 cells/ml). T cells were stimulated for 5 d, then ficoll density centrifuged to remove nonviable cells before resting in medium for 24 h. Where indicated, neutralizing anti–IL-12 mAb (10 μg/ml; R&D Systems) was added. T cells were then restimulated with the same allogeneic CD40-activated B cells for up to 6 d depending on the assay performed thereafter. Supernatants from these cultures were harvested at different times during culture and were analyzed for IL-4, IL-6, IL-10, or IFN-γ by ELISA (Endogen). T cell proliferation during secondary stimulation was determined by [3H]thymidine incorporation in triplicate on days 2, 4, and 6. Following the same procedure described above, intracellular IFN-γ and IL-4 were analyzed in T–B cell cultures using anti–IFN-γ (4S.B3; PharMingen) and anti–IL-4 mAbs (MP4-25D2; PharMingen).
Results
CD40 Activation Is the Major Stimulus for Human Tonsillar B Cells to Produce IL-12.
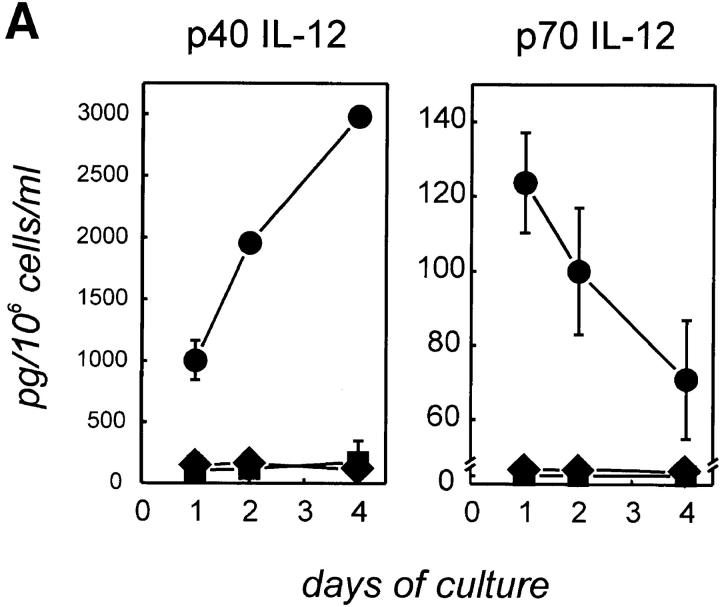
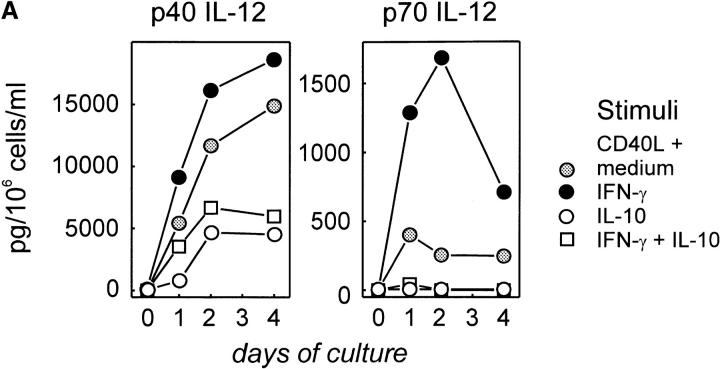
Since human DCs have been shown to produce IL-12 after CD40 activation (7–10), we first attempted to determine whether CD40 activation might also induce normal human B cells to produce IL-12. Tonsillar B cells (>97% CD19+) were isolated and cultured (a) in medium, (b) with control transfectants (t-mock), or (c) with CD40L transfectants (t-CD40L) for up to 96 h. Supernatants were examined for p40 and p70 IL-12 by ELISA. As shown in Fig. 1 A, unfractionated tonsillar B cells were induced to produce both p40 and the biologically active heterodimer p70 IL-12 after CD40 activation, whereas no IL-12 was detected in control cultures. Although p40 IL-12 significantly increased over time, the highest level of p70 IL-12 was detected after 24 h and declined thereafter. The decrease of the p70 heterodimer during later time points of the culture is most likely a result of dissociation of the two chains due to decreasing pH in these cultures. Control experiments demonstrated that similar amounts of rhIL-12 dissolved in PBS lead to significantly reduced detection of IL-12 with decreasing pH (to 7.2–6) as assessed by ELISA analysis (data not shown). As described for macrophages (47), the concentration of p40 IL-12 was always 10–50-fold higher than that of p70 IL-12.
Figure 1.
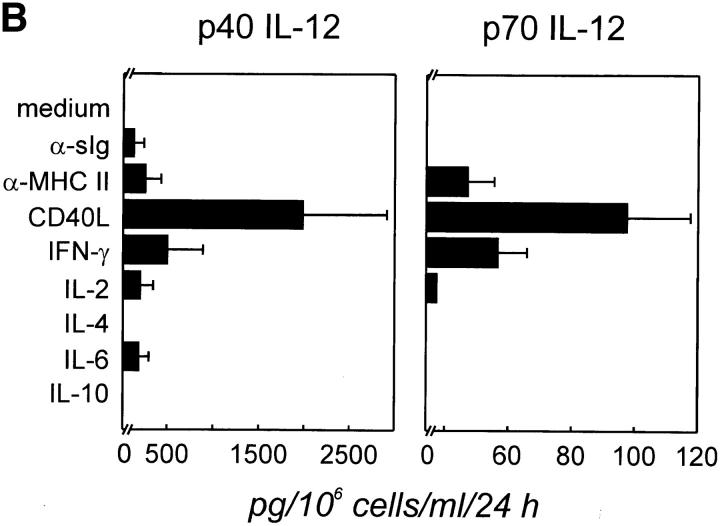
Expression of IL-12 p40 and p70 by human tonsillar B cells. (A) t-CD40L cells induce IL-12 p40 and p70 in B cells. Purified tonsillar B cells (>97% CD19+, 2 × 106 cells/well in 1 ml of medium) were cultured in medium (▪), on t-mock cells (♦), or on t–CD40L cells (•) for 24, 48, and 96 h when supernatants were harvested and analyzed for the production of IL-12 by ELISA. Results are representative for four individual experiments. Error bars represent 1 SD from the mean. (B) CD40L is the major signal inducing B cells to produce IL-12. Purified tonsillar B cells (2 × 106 cells/well in 1 ml of medium) were stimulated with various stimuli, including anti-sIgM and anti-sIgG F(ab′)2 (α-sIg), anti-MHC class II mAbs (9–49), t-CD40L cells, IFN-γ (20 ng/ml), IL-2 (25 IU/ml), IL-4 (10 ng/ml), IL-6 (5 ng/ml), and IL-10 (10 ng/ml), for 24 h when supernatant was harvested for IL-12 analysis by ELISA. Results represent the mean ± SD of a total of six individual experiments.
We next examined whether human tonsillar B cells could be induced to produce IL-12 by various other stimuli, including B cell receptor cross-linking using mAbs, antibodies against MHC class II molecules, Th1 cytokines IL-2 and IFN-γ, or Th2 cytokines IL-4, IL-6, and IL-10. Again, p40 IL-12 and the p70 IL-12 heterodimer were measured in B cell culture supernatants by ELISA after 24 h. In six individual experiments, CD40L was identified to be the most effective stimulus for IL-12 p40 production (mean ± SD in Fig. 1 B). Of all other stimuli tested, only IFN-γ, IL-2, and MHC class II cross-linking mAbs induced p40 IL-12 threefold above detection level. Significant amounts of p70 IL-12 were only detected in B cell cultures stimulated with CD40L, IFN-γ, or anti–MHC class II mAb. In control experiments, we analyzed human monocyte-derived DCs (generated by culture with GM-CSF and IL-4 for 5–8 d and further differentiated by CD40 activation for 24–72 h [10]) for their production of IL-12 using the same ELISA method. These in vitro–generated DCs produced higher amounts (two- to fivefold) of both IL-12 p40 and p70 than unseparated tonsillar B cells, with a different kinetics for p70 IL-12, with levels peaking at 48– 72 h after CD40 activation (data not shown). A potential reason for lower IL-12 production by B cells could be that the production of IL-12 is restricted to certain differentiation periods and therefore only a small fraction of tonsillar B cells might be responsible for IL-12 production.
Naive and Memory B Cells Produce IL-12.
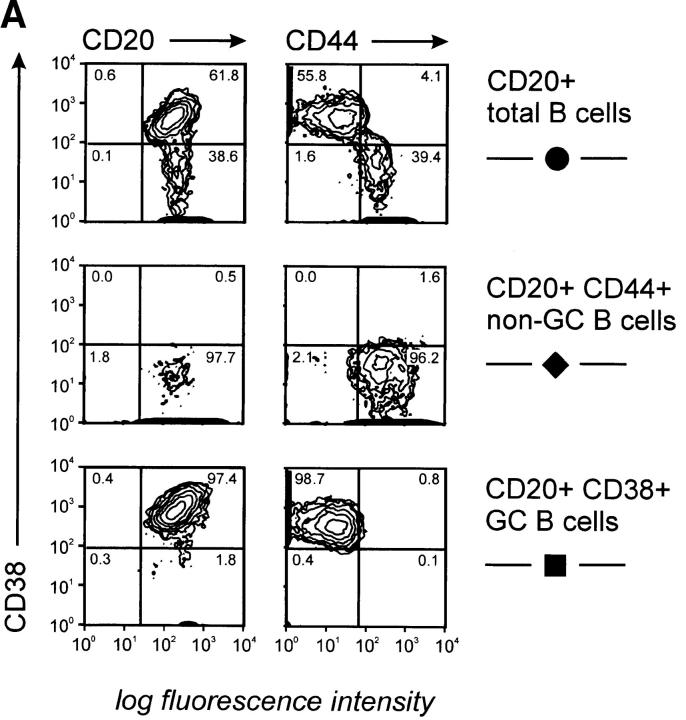
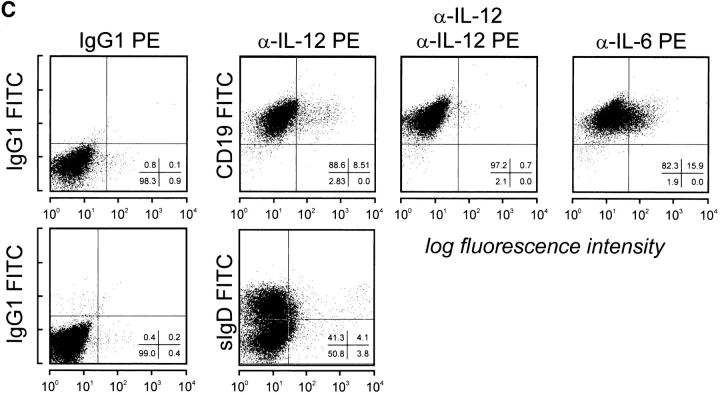
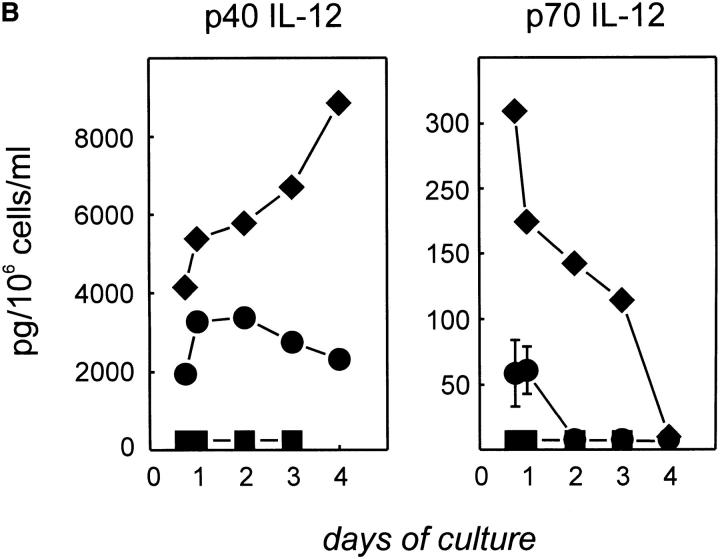
To determine further whether production of IL-12 p40 and p70 was restricted to B cell subsets, we purified GC B cells (CD19+ CD38+CD44−) and non-GC B cells (CD19+CD38− CD44+) from human tonsillar B cells (Fig. 2 A) and stimulated them with CD40L for 24–96 h. These experiments clearly identified non-GC B cells as the source of IL-12 p40 and p70 (Fig. 2 B). To further confirm that non-GC B cells produce IL-12, we determined intracellular IL-12 by FACS® analysis. Purified non-GC B cells were stimulated by CD40L for 14–16 h before Brefeldin A was added to the cultures for the remaining 6–8 h of culture. Cells were harvested and analyzed for intracellular IL-12 using directly conjugated anti–IL-12 mAb. As can be seen in Fig. 2 C, only a small percentage (8.5%) of these B cells stained intracellularly for IL-12. Preincubation with an unlabeled anti– IL-12 mAb of the same specificity abrogated (Fig. 2 C) and preincubation with rhIL-12 significantly decreased (data not shown) the staining for intracellular IL-12 in these cells, further demonstrating specific staining for IL-12. The intensity of staining of different tonsillar samples varied widely, whereas the percentage of positive cells was always between 2 and 9% of total cells (total of five experiments; Fig. 2 C, and data not shown). IL-12 production was detected in both sIgD+ and sIgD− non-GC B cells after CD40 activation, indicating that naive and memory B cells can be induced to produce IL-12 (Fig. 2 C). GC B cells did not induce IL-12 p70, and this was not due to increased cell death during culture as measured by trypan blue exclusion test. Although low amounts of p40 IL-12 could be detected after 96 h in some GC B cell cultures, this was not a consistent finding. This low level of production might be explained either by the outgrowth of few contaminating non-GC B cells or alternatively by the differentiation of GC B cells towards memory B cells, regaining the capability to produce IL-12. To address this, GC B cells were differentiated into memory B cells by CD40 activation in the presence of IL-2 and IL-10. Cells were passaged on day 4, and at day 7 of culture cells were restimulated via CD40 only. IL-12 was measured subsequently. Cells cultured in this manner acquired a memory-like phenotype and regained their capacity to produce IL-12, although at significantly lower levels than in the unseparated non-GC B cell population (data not shown).
Figure 2.
CD20+CD38−CD44+ sIgD± B cells produce IL-12 p40 and p70. (A) Two-color flow cytometry for CD20, CD38, and CD44 of isolated tonsillar B cells, purified CD20+CD38−CD44+ sIgD± non-GC B cells, and CD20+CD38+CD44− GC B cells. (B) IL-12 p40 and p70 are mainly induced by non-GC B cells. B cells (•, total; ♦, non-GC; ▪, GC) were cultured on t-CD40L cells for 18, 24, 48, 72, or 96 h when supernatants were harvested and analyzed for the production for IL-12 by ELISA. Results are representative of three individual experiments. Error bars represent 1 SD from the mean of the ELISA analysis. (C) Detection of IL-12 in non-GC B cells by intracellular staining. Purified tonsillar resting CD19+ CD44+CD38− sIgD± non-GC B cells were cocultured with t-CD40L cells. Brefeldin A (10 μg/ml) was added for the last 6–8 h of a 24-h culture. Cells were harvested and stained for extracellular CD19 and sIgD, and intracellular p40/p70 IL-12 and IL-6, and analyzed by FACS® analysis. Specificity controls were carried out by preincubation of the cells with saturating concentrations of an unlabeled IL-12 mAb of similar specificity or by adding saturating concentrations of rhIL-12 (data not shown). Shown here is one of five experiments. As positive control, intracellular IL-6 concentrations were analyzed, known to be induced after CD40 activation (reference 40).
B Cell IL-12 Production after CD40 Ligation Is Further Enhanced by IFN-γ and Blocked by IL-10.
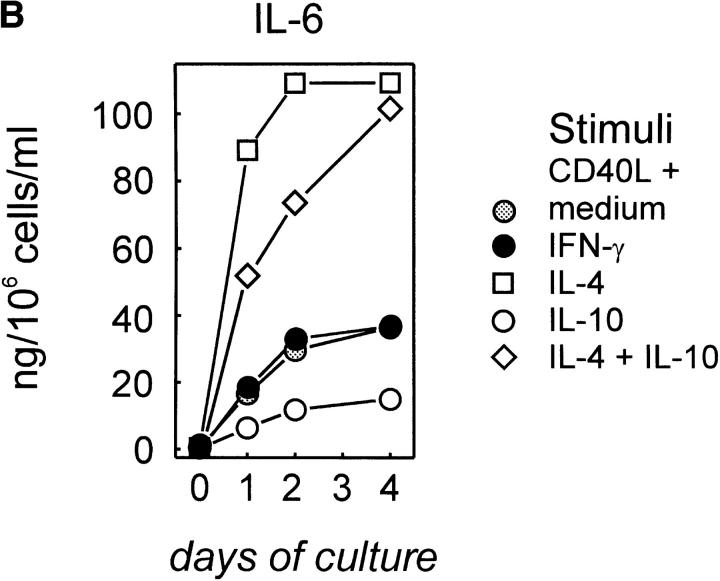
CD40 activation of naive B cells occurs in the interfollicular areas where T cells previously activated by DCs express the ligand for CD40 (15). These preactivated T cells also produce cytokines, including IL-2, IL-4, IL-6, IFN-γ, and IL-10. IL-6 production by unseparated tonsillar B cells (48) as well as IL-12 produced by DCs (9) and macrophages (49, 50) are negatively regulated by IL-4 and IL-10. Therefore, we tested the effect of these T cell–derived cytokines on the expression of CD40-induced p40 IL-12 and p70 IL-12 in human non-GC B cells. IFN-γ in combination with CD40 activation slightly increased p40 IL-12 accumulation (Fig. 3 A) but significantly increased p70 IL-12 accumulation in these cultures. In contrast, IL-10 dramatically decreased both IL-12 p40 and p70 (Fig. 3 A). The combination of CD40L and IFN-γ did not restore IL-12 production in the presence of IL-10, suggesting a dominant inhibitory effect of IL-10. Other Th2 cytokines were less inhibitory. IL-4 inhibited CD40L moderately, and IL-6 had only a minor impact on the amount of IL-12 in the supernatant of these B cell cultures (data not shown). In contrast to IL-10, the inhibitory effect of IL-4 could be partially reversed by IFN-γ. IL-2 showed no significant effects and could restore neither IL-4– nor IL-10–mediated inhibition (data not shown). In control experiments and confirming the findings of others (39), we detected very high levels of IL-6 in cultures of non-GC B cells (Fig. 3 B) but not GC B cells (data not shown) stimulated with CD40L. The addition of IL-4 dramatically increased IL-6 production, addition of IL-2 and IFN-γ had no influence, but IL-10 significantly decreased IL-6 production by the CD40-activated B cells (Fig. 3 B). However, in the presence of IL-4, IL-10 did not reduce IL-6 production, suggesting again a hierarchy of signals with IL-4 dominant over IL-10, IL-2, and IFN-γ. Taken together, these data suggest that CD40 activation of B cells is the major stimulus of IL-12 and IL-6. However, the two cytokines are differentially regulated by T cell cytokines: IL-2 and IFN-γ increase IL-12 production and IL-4 increases IL-6 production. In contrast, IL-10 can suppress IL-12 and IL-6 production in the absence of IL-4.
Figure 3.
Regulation of IL-12 and IL-6 production by T cell cytokines. Non-GC B cells were cultured on t-CD40L cells in the presence of IFN-γ (20 ng/ml), IL-4 (10 ng/ml), IL-10 (5 ng/ml), or their combinations. Supernatants were harvested after 24, 48, or 96 h and analyzed for IL-12 by ELISA. (A) IL-12 production. (B) IL-6 production. One representative experiment of three experiments performed is shown here.
B Cell–derived IL-12 Is Bioactive.
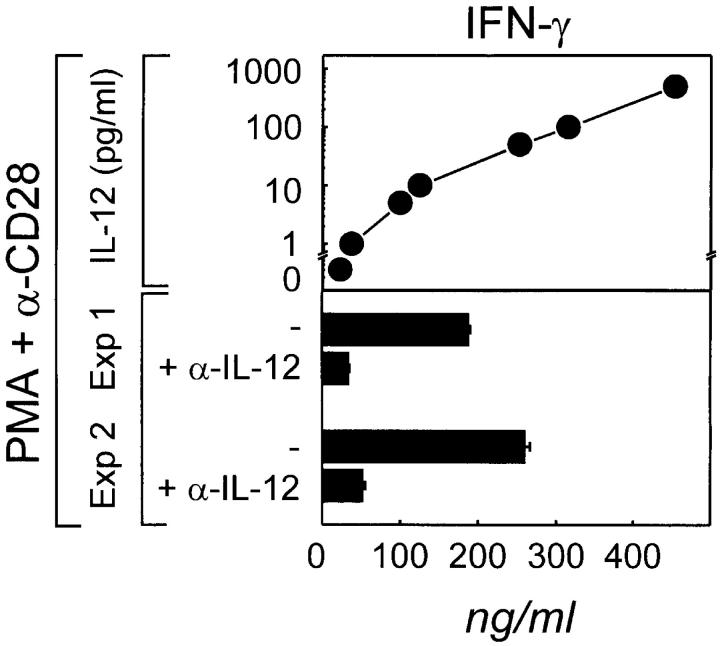
To determine whether the p70 IL-12 heterodimer detected in supernatants of CD40-activated tonsillar B cells is bioactive, we measured the influence of B cell culture–derived supernatants on the IFN-γ production of activated CD3+CD4+ T cells. In control cultures, T cells were stimulated with PMA and anti-CD28 mAbs in the presence of increasing concentrations of exogenous rhIL-12. IFN-γ could be detected at low levels in cultures without exogenous IL-12, but increased significantly from 22 to 480 ng/ml IFN-γ when between 1 and 1,000 pg/ml of exogenous IL-12 was added. The addition of supernatants from B cell cultures (24-h cultures) at 50% vol induced IFN–γ production by the T cells equivalent to the effect of 48–114 pg/ml rIL-12 (Fig. 4). This suggests that ∼50–250 pg/ml of bioactive IL-12 is present in B cell culture supernatants after 24 h, confirming our ELISA results. The addition of neutralizing anti–IL-12 mAb (10 μg/ml) to T cells stimulated in the presence of B cell culture supernatants reduced IFN-γ production by >80%, further supporting that these supernatants contained bioactive IL-12. To further confirm that IL-12 was derived from CD40-activated B cells, we cocultured allogeneic CD4+ T cells with previously CD40-activated non-GC B cells and rechallenged the T cells with the same target cells to analyze the cytokine pattern in these cultures (Table I). Similar to experiments described previously using DCs (9, 10), T cell–derived IFN-γ production is mainly dependent on the production of IL-12 by CD40-activated non-GC B cells. Blockade of IL-12 during challenge reduced IFN-γ production during rechallenge by ∼85%, whereas T cell proliferation in the presence of anti– IL-12 mAbs decreased only ≤25%. IL-4 and IL-10 were not detected in any culture supernatant (Table I).
Figure 4.
B cell–derived IL-12 induces T cells to produce IFN-γ. CD3+CD4+CD45RA+ enriched T cells (98% CD3+ CD4+ > 75% CD45RA) were stimulated with PMA (1 ng/ml) plus CD28 mAbs (2 μg/ml) with or without rhIL-12 p70 at concentrations ranging from 1 to 1,000 pg or with supernatants (a 50% vol of 200 μl final vol) from tonsillar B cells cultured for 24 h on t-CD40L cells. Where indicated, neutralizing anti–IL-12 mAb (α–IL-12) was added. After 3 d, supernatants were harvested and analyzed for IFN-γ production by ELISA.
Table I.
Cytokine Production and Proliferation during Rechallenge with CD40-activated Irradiated Non-GC B Cells
| Primary activation | Rechallenge | IFN-γ | IL-4 | IL-6 | IL-10 | Proliferation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ng/ml | pg/ml | pg/ml | pg/ml | cpm | ||||||||
| Experiment 1 | ||||||||||||
| CD40-B | CD40-B | 14,791 ± 2,350 | <40 | 71.5 ± 16.3 | <40 | 297,101 ± 62,519 | ||||||
| CD40-B + α–IL-12 | CD40-B | 2,272 ± 575 | <40 | 139.0 ± 38.2 | <40 | 221,185 ± 17,542 | ||||||
| Experiment 2 | ||||||||||||
| CD40-B | CD40-B | 10,006 ± 1,077 | <40 | 41.0 ± 19.8 | <40 | 225,844 ± 26,765 | ||||||
| CD40-B + α–IL-12 | CD40-B | 1,321 ± 39 | <40 | 124.0 ± 33.9 | <40 | 219,761 ± 7,865 |
Preferential production of IFN-γ by T cells stimulated with CD40-activated non-GC B cells is mainly due to B cells' IL-12 production. CD3+CD4+ T cells enriched for the CD4+CD45RA+ subpopulation (>75% CD45RA) were primarily activated with CD40-activated irradiated non-GC B cells (72-h preactivation of the B cells) with or without anti–IL-12 mAbs (10 μg/ml) for 5 d, rested for 48 h in medium alone, and rechallenged with the same irradiated B cells. Supernatants of these cultures were harvested after 24, 48 (shown here), 72, or 96 h and analyzed by ELISA for IFN-γ, IL-4, IL-6, or IL-10 (detection level of all ELISA was 40 pg/ml). T cell proliferation was measured by [3H]Tdr incorporation for the last 18 h of a 72-h culture period. Shown here are mean ± SD of triplicates. CD40-B, CD40-activated irradiated non-GC B cells.
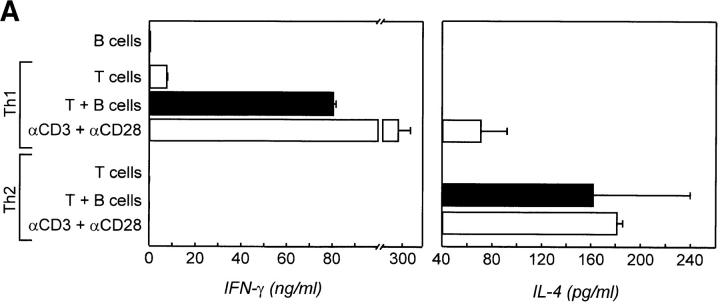
Resting B Cells Maintain the Cytokine Profile of Previously Activated T Cells.
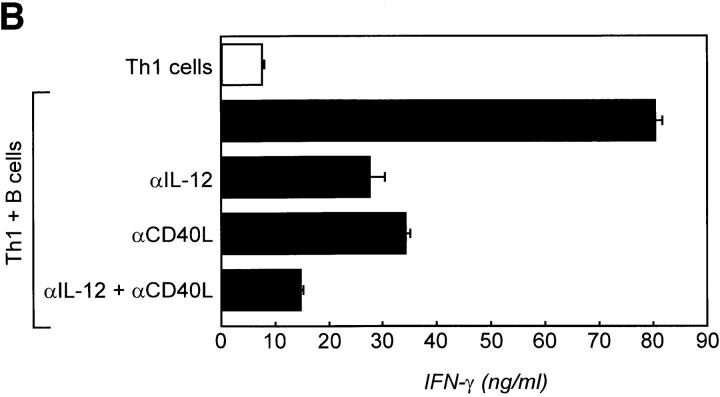
Recent in vivo analysis demonstrated that T cells primarily activated by DCs migrate towards the B cell zone to interact with antigen-specific naive and/or memory B cells and that CD40L–CD40 interactions occur during this phase of the immune response (29). To study this interaction with regard to our observation that CD40 and IFN-γ stimulation induces these B cells to produce IL-12, the following experiment was performed. Naive cord blood T cells were primarily stimulated with anti-CD3 and anti-CD28 mAbs bound to tossyl beads representing artificial APCs in the presence of IL-12 and anti–IL-4 mAbs to skew towards a Th1 phenotype, or with IL-4 and anti– IFN-γ mAbs to skew towards a Th2 phenotype. Cells were restimulated after 3–6 d with freshly isolated irradiated allogeneic resting CD19+CD38+ sIgD± tonsillar B cells, and cytokines were measured 48 h later. As shown in Fig. 5 A, T cells skewed towards a Th1 phenotype produced significant amounts of IFN-γ in response to incubation with resting allogeneic B cells as well as when restimulated with CD3 and CD28 mAb beads. In contrast, Th2 cells could not be induced to produce IFN-γ. However, Th2 cells were induced to produce small amounts of IL-4 in the presence of the B cells. IL-4 was also barely detectable in cultures primarily skewed towards Th1 cells during restimulation with CD3 and CD28 mAb beads, but not when restimulated with resting allogeneic B cells. Staining for intracellular IFN-γ and IL-4 showed that the CD3+ T cells were the source of these cytokines in these cultures (data not shown). To further support the role of CD40L– CD40 interaction and IL-12 production by B cells for the sustained Th1 phenotype, the same experiments were performed in the presence of neutralizing anti–IL-12 mAb and/or blocking anti-CD40L mAb during T–B cultures (Fig. 5 B). Blockade of IL-12 or CD40L significantly reduced IFN-γ production, whereas blocking both almost totally abrogated IFN-γ in the supernatant of these cultures.
Figure 5.
Resting B cells sustain the cytokine profile of previously activated T cells. (A) To mimic the interaction of DCs and naive T cells, cord blood CD3+CD4+CD45RA+ T cells were primed with CD3 and CD28 mAbs bound to magnetic beads in the presence of IL-2 and either IL-12 or IL-4 to skew towards a Th1 or Th2 phenotype. Cells were rested and restimulated with allogeneic tonsillar resting B cells (1:1 ratio) or CD3 plus CD28 mAb beads as a positive control. Supernatants of these cultures were harvested 48 h later and analyzed for IFN-γ or IL-4 by ELISA. (B) To determine the role of CD40L–CD40 interaction and IL-12 for the induction of IFN-γ in Th1 cells, CD40L and/or IL-12 were blocked during T–B cell cultures. IFN-γ production in these cultures was assessed as described.
Discussion
IL-12 was originally identified in and cloned from human EBV-transformed B cell lines (35–38), and indirect evidence exists that this cytokine is expressed in normal peripheral blood–derived human B cells (49, 51). However, the major stimuli for the induction of IL-12 by normal human B cells in secondary lymphoid organs and its regulation have been unclear. Moreover, recent data from murine models suggest that IL-12 is not expressed in murine B cells (52, 53). CD40 ligation, a major signal for the induction of IL-12 in APCs, does not lead to IL-12 by murine B cells (52, 53). In contrast to the findings in murine B cells, we demonstrate that human tonsillar non-GC B cells are induced to produce IL-12 after CD40 activation, but not by B cell receptor cross-linking. The Th1 cytokine IFN-γ is synergistic with CD40, whereas Th2 cytokines IL-4 and IL-10, but not IL-6, are inhibitory for IL-12 production by B cells. IL-12 produced by these human B cells is bioactive and induces IFN-γ production by allogeneic CD3+CD4+ T cells. IL-6 has been recently suggested as the cytokine expressed by APCs leading to Th2 skewing (18). Tonsillar non-GC B cells expressed IL-6 after CD40 activation, and expression was markedly increased in the presence of IL-4. In contrast to IL-12, IL-6 was not suppressed by IL-10 in the presence of IL-4. These data suggest an additional level of feedback stimulation and inhibition that occur during cognate T–B interactions (1, 34).
Although human peripheral blood B cells have been suggested to contribute to the IL-12 production of human PBMCs (51) induced by Staphylococcus aureus Cowan I (SAC), it has been recently reported that murine B cells from BALB/c mice fail to produce IL-12 in response to SAC and other stimuli (52). In this context, it will be of interest whether a general difference between human and murine B cells exists, or whether this is a specific phenomenon of BALB/c mice. This is of particular interest, since this syngeneic strain has been shown neither to develop nor to maintain Th1 responses to Leishmania major (54). This is despite the fact that DCs of BALB/c mice do not differ in their capacity to produce IL-12 from C57BL/6 mice (13), known to develop Th1 responses to L. major (55). Although the defect in mounting a Th1 response in BALB/c mice is believed to result from a defect in the T cell compartment, it is not well characterized whether additional defects in the B cell compartment exist (56). C57BL/6 and C3H mice usually demonstrate Th1 responses to L. major that can be redirected towards Th2 in the presence of anti– IL-12 mAbs (57). However, after termination of treatment with anti–IL-12 mAbs, the immune response switches back to a Th1 response, which is maintained thereafter (57). It is tempting to speculate whether B cells from these mice might differ from BALB/c mice in their capacity to produce IL-12. Studies using CD40−/− mice have also unraveled that the induction of IL-12 after CD40 activation is necessary for the generation and maintenance of Th1 responses (7–10). However, it has not been studied directly whether IL-12 production is restricted to APCs, or if adoptively transferred CD40+ B cells could overcome the lack of Th1 responses in CD40−/− mice.
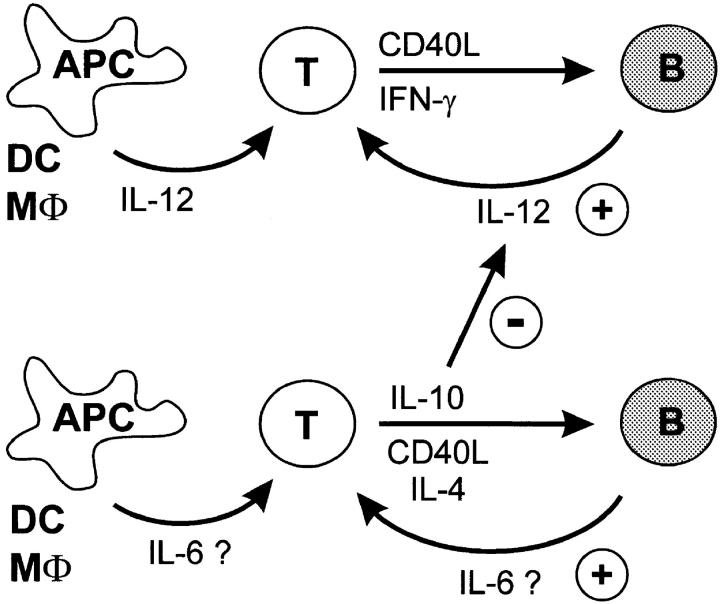
The amount of IL-12 produced by human DCs after CD40 activation is severalfold higher than we could demonstrate for human B cells. Cella and colleagues reported p40 IL-12 production by DCs in excess of 100 ng/ml and p70 IL-12 production higher than 10 ng/ml (10). In this context, it is clear that human B cells produce lower amounts of IL-12 than DCs and are not the major source of IL-12. Our own experiments (data not shown) using monocyte-derived DCs support these earlier findings, although we could not detect equally high levels as reported (10). When and why do B cells produce IL-12? Since CD40L and IFN-γ were the major stimuli for human non-GC B cells to produce IL-12, it is most likely that previously activated T cells expressing CD40L and IFN-γ induce naive or memory B cells to express IL-12 during their first contact (21, 29). The production of IL-12 by B cells after cognate interaction with T cells could serve as a positive feedback mechanism during the expansion of Th1 cells, ensuring the polarization of these T cells and other T cells recruited to the site (Fig. 6). Our data suggest that the simultaneous production of CD40-induced IL-6, a cytokine indicated as an APC-derived factor inducing Th2 differentiation (18), does not seem to influence this feedback pathway. In contrast, the Th2 cytokines IL-4 and IL-10 are potent inhibitors of B cell–derived IL-12. From these data we conclude that the inhibition of IL-12 production after CD40 activation is similarly regulated by human macrophages, DCs (58), and B cells. Similar to IL-12, IL-6 production induced by CD40 ligation in human B cells was further regulated by T cell cytokines. The Th2 cytokine IL-4 dramatically increased IL-6 production by these B cells and IL-10 did not inhibit this effect, suggesting a positive feedback mechanism by Th2-polarized cells. Of note, when IL-4 was absent, IL-10 reduced CD40-mediated IL-6 production, suggesting that the effect of IL-10 on production of IL-6 by B cells depends on the cytokine milieu present.
Figure 6.
B cells maintain a Th1 phenotype by a positive feedback mechanism via CD40 and IL-12. Resting naive and memory B cells produce IL-12 when encountering activated Th1 cells expressing CD40L and IFN-γ, sustaining the Th1 phenotype. In contrast, when resting B cells encounter Th2 cells producing IL-4, they do not induce IFN-γ production but rather maintain the T cells' IL-4 production.
Indeed, CD40L is expressed in vivo in the outer T cell zone (59) and the B cell area (29), areas where CD40L+ T cells are juxtaposed to antigen-specific B cells. Our data strongly indicate that the production of IL-12 and IL-6 is a consequence of the interaction of resting B cells with already activated T cells. Indeed, during T–B contact, CD40L–CD40 interaction appears to be the most important signal for IL-12 and IL-6 production, and this is further regulated by T cell–derived cytokines. Since B cells did not produce IL-12 or significant amounts of IL-6 (data not shown) to sIg cross-linking, it seems unlikely that B cells primarily activated by antigen are responsible for skewing T cell differentiation. Therefore, we add to the model initially presented by Janeway and colleagues (1) and introduce the production of these cytokines by B cells in a positive feedback mechanism. B cells may play an important role for amplification and maintenance of an immune response primarily initiated by DCs or macrophages (Fig. 6). When T cells are primarily activated by DCs in the interfollicular area, they migrate 24–48 h later to the outer T cell zone (21) and subsequently to the B cell area (29) where they meet antigen-specific B cells. It is this interaction that leads to massive expansion of both T and B cells (60, 61). When B cells meet IFN-γ–producing T cells expressing surface CD40L, they produce IL-12, further reinforcing IFN-γ production by the adjacent T cells (Fig. 6). In contrast, T cells that express CD40L in the context of IL-4 and IL-10 suppress IL-12 production but further support IL-4 production by these T cells. Naive T cells could be primed in the presence of exogenous IL-4 and then restimulated with resting B cells to produce low levels of IL-4. Blocking of CD40L completely abrogated IL-4 in these cultures, whereas addition of anti–IL-6 mAb slightly decreased IL-4 production (data not shown), suggesting that although IL-6 plays a role in regulating T cell responses, other factors are also likely to be important.
Finally, our results are relevant for immunotherapeutic strategies. We have previously described a simple method to generate large numbers of B cells from human peripheral blood by stimulation with CD40L and IL-4. These cells are highly efficient APCs and can present antigen to both CD4+ and CD8+ T cells. Preliminary results suggest that these B cells produce IL-12 (Schultze, J.L., unpublished results), and this is the most likely explanation for the induction of the very high amounts of IFN-γ by the T cells stimulated with CD40-activated B cells (16). Therefore, this makes these B cells a very attractive alternative to DCs for ex vivo or in vivo T cell–mediated immunotherapy.
Acknowledgments
We thank Glenn Dranoff, David Sherr, Robert Vonderheide, and David Frank for critically reading the manuscript.
Abbreviations used in this paper
- DC
dendritic cell
- GC
germinal center
- MBD
magnetic bead depletion
- MIF
mean intensity of fluorescence
- rh
recombinant human
- sIg
surface immunoglobulin
- t-CD40L
CD40 ligand transfectants
- t-mock
control transfectants
Footnotes
J.L. Schultze was a Fellow of the Lymphoma Research Foundation of America when most of the work was performed and is currently a Special Fellow of the Leukemia Society of America. This work was supported by National Institutes of Health grant CA66996 to L.M. Nadler.
References
- 1.Mamula MJ, Janeway CA., Jr Do B cells drive the diversification of immune responses? . Immunol Today. 1993;14:151–152. doi: 10.1016/0167-5699(93)90274-O. [DOI] [PubMed] [Google Scholar]
- 2.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 3.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 4.Constant SL, Bottomly K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu Rev Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- 5.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 6.Carter LL, Dutton RW. Type 1 and type 2: a fundamental dichotomy for all T-cell subsets. Curr Opin Immunol. 1996;8:336–342. doi: 10.1016/s0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 7.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O'Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–5079. [PubMed] [Google Scholar]
- 8.Heufler C, Koch F, Stanzl U, Topar G, Wysocka M, Trinchieri G, Enk A, Steinman RM, Romani N, Schuler G. Interleukin-12 is produced by dendritic cells and mediates T helper 1 development as well as interferon-gamma production by T helper 1 cells. Eur J Immunol. 1996;26:659–668. doi: 10.1002/eji.1830260323. [DOI] [PubMed] [Google Scholar]
- 9.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skeen MJ, Miller MA, Shinnick TM, Ziegler HK. Regulation of murine macrophage IL-12 production. Activation of macrophages in vivo, restimulation in vitro, and modulation by other cytokines. J Immunol. 1996;156:1196–1206. [PubMed] [Google Scholar]
- 12.Reiner SL, Zheng S, Wang ZE, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+T cells during initiation of infection. J Exp Med. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sousa CR, Hieny S, Scharton-Kersten T, Jankovic D, Charest H, Germain RN, Sher A. In vivo microbial stimulation induces rapid CD40 ligand–independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson LL, Sayles PC. Interleukin-12, dendritic cells, and the initiation of host-protective mechanisms against Toxoplasma gondii. . J Exp Med. 1997;186:1799–1802. doi: 10.1084/jem.186.11.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, Vankooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 16.Schultze JL, Michalak S, Seamon MJ, Dranoff G, Jung K, Daley J, Delgado JC, Gribben JG, Nadler LM. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–2765. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pistoia V. Production of cytokines by human B cells in health and disease. Immunol Today. 1997;18:343–350. doi: 10.1016/s0167-5699(97)01080-3. [DOI] [PubMed] [Google Scholar]
- 18.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4–producing CD4+T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 20.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4+T cells. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLennan IC, Gulbranson-Judge A, Toellner KM, Casamayor-Palleja M, Chan E, Sze DM, Luther SA, Orbea HA. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. 1997;156:53–66. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 22.Flamand V, Sornasse T, Thielemans K, Demanet C, Leo O, Urbain JM, Moser M. Vaccination with tumor-antigen-pulsed dendritic cells induces in vivo resistance to a B cell lymphoma. Adv Exp Med Biol. 1993;329:611–616. doi: 10.1007/978-1-4615-2930-9_102. [DOI] [PubMed] [Google Scholar]
- 23.Flamand V, Sornasse T, Thielemans K, Demanet C, Bakkus M, Bazin H, Tielemans F, Leo O, Urbain J, Moser M. Murine dendritic cells pulsed in vitro with tumor antigen induce tumor resistance in vivo. Eur J Immunol. 1994;24:605–610. doi: 10.1002/eji.1830240317. [DOI] [PubMed] [Google Scholar]
- 24.Grabbe S, Beissert S, Schwarz T, Granstein RD. Dendritic cells as initiators of tumor immune responses: a possible strategy for tumor immunotherapy? . Immunol Today. 1995;16:117–121. doi: 10.1016/0167-5699(95)80125-1. [DOI] [PubMed] [Google Scholar]
- 25.Gabrilovich DI, Cunningham HT, Carbone DP. IL-12 and mutant p53 peptide-pulsed dendritic cells for the specific immunotherapy of cancer. J Immunother. 1996;19:414–418. doi: 10.1097/00002371-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Paglia P, Chiodoni C, Rodolfo M, Colombo MP. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Celluzzi CM, Mayordomo JI, Storkus WJ, Lotze MT, Falo LD., Jr Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zitvogel L, Mayordomo JI, Tjandrawan T, DeLeo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide–pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1–associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 30.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Kamogawa Y, Bottomly K, Flavell RA. Polarization of IL-4- and IFN-γ-producing CD4+ T cells following activation of naive CD4+ T cells. J Immunol. 1997;158:1085–1094. [PubMed] [Google Scholar]
- 32.Sornasse T, Larenas PV, Davis KA, de Vries JE, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+T cells, analyzed at the single-cell level. J Exp Med. 1996;184:473–483. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy E, Shibuya K, Hosken N, Openshaw P, Maino V, Davis K, Murphy K, O'Garra A. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996;183:901–913. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature. 1994;367:425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi M, Fitz L, Ryan M, Hewick RM, Clark SC, Chan S, Loudon R, Sherman F, Perussia B, Trinchieri G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J Exp Med. 1989;170:827–845. doi: 10.1084/jem.170.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stern AS, Podlaski FJ, Hulmes JD, Pan YC, Quinn PM, Wolitzky AG, Familletti PC, Stremlo DL, Truitt T, Chizzonite R, et al. Purification to homogeneity and partial characterization of cytotoxic lymphocyte maturation factor from human B-lymphoblastoid cells. Proc Natl Acad Sci USA. 1990;87:6808–6812. doi: 10.1073/pnas.87.17.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gubler U, Chua AO, Schoenhaut DS, Dwyer CM, McComas W, Motyka R, Nabavi N, Wolitzky AG, Quinn PM, Familletti PC, et al. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–4147. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf SF, Temple PA, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick RM, et al. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 39.Burdin N, Galibert L, Garrone P, Durand I, Banchereau J, Rousset F. Inability to produce IL-6 is a functional feature of human germinal center B lymphocytes. J Immunol. 1996;156:4107–4113. [PubMed] [Google Scholar]
- 40.Clark EA, Shu GL. Association between IL-6 and CD40 signaling. IL-6 induces phosphorylation of CD40 receptors. J Immunol. 1990;145:1400–1406. [PubMed] [Google Scholar]
- 41.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JL, Barker CR. T and B cell separation by sheep-red-cell rosetting. Lancet. 1973;1:558–559. doi: 10.1016/s0140-6736(73)90382-6. [DOI] [PubMed] [Google Scholar]
- 43.Schultze JL, Cardoso AA, Freeman GJ, Seamon MJ, Daley J, Pinkus GS, Gribben JG, Nadler LM. Follicular lymphomas can be induced to present alloantigen efficiently: a conceptual model to improve their tumor immunogenicity. Proc Natl Acad Sci USA. 1995;92:8200–8204. doi: 10.1073/pnas.92.18.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schultze JL, Seamon MJ, Michalak S, Gribben JG, Nadler LM. Autologous tumor infiltrating T cells cytotoxic for follicular lymphoma cells can be expanded in vitro. Blood. 1997;89:3806–3816. [PubMed] [Google Scholar]
- 45.Bleul CC, Schultze JL, Springer TA. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J Exp Med. 1998;187:753–762. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Astier A, Avraham H, Manie SN, Groopman J, Canty T, Avraham S, Freedman AS. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. J Biol Chem. 1997;272:228–232. doi: 10.1074/jbc.272.1.228. [DOI] [PubMed] [Google Scholar]
- 47.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 48.Burdin N, van Kooten C, Galibert L, Abrams JS, Wijdenes J, Banchereau J, Rousset F. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40- activated human B lymphocytes. J Immunol. 1995;154:2533–2544. [PubMed] [Google Scholar]
- 49.D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon γ production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D'Andrea A, Ma X, Aste-Amezaga M, Paganin C, Trinchieri G. Stimulatory and inhibitory effects of interleukin (IL)-4 and IL-13 on the production of cytokines by human peripheral blood mononuclear cells: priming for IL-12 and tumor necrosis factor α production. J Exp Med. 1995;181:537–546. doi: 10.1084/jem.181.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D'Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chan SH, Kobayashi M, Young D, Nickbarg E, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guery JC, Ria F, Galbiati F, Adorini L. Normal B cells fail to secrete interleukin-12. Eur J Immunol. 1997;27:1632–1639. doi: 10.1002/eji.1830270707. [DOI] [PubMed] [Google Scholar]
- 53.Maruo S, Oh-hora M, Ahn HJ, Ono S, Wysocka M, Kaneko Y, Yagita H, Okumura K, Kikutani H, Kishimoto T, et al. B cells regulate CD40 ligand-induced IL-12 production in antigen-presenting cells (APC) during T cell/ APC interactions. J Immunol. 1997;158:120–126. [PubMed] [Google Scholar]
- 54.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. . Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 55.Guler ML, Gorham JD, Hsieh CS, Mackey AJ, Steen RG, Dietrich WF, Murphy KM. Genetic susceptibility to Leishmania: IL-12 responsiveness in TH1 cell development. Science. 1996;271:984–987. doi: 10.1126/science.271.5251.984. [DOI] [PubMed] [Google Scholar]
- 56.Gorham JD, Guler ML, Steen RG, Mackey AJ, Daly MJ, Frederick K, Dietrich WF, Murphy KM. Genetic mapping of a murine locus controlling development of T helper 1/T helper 2 type responses. Proc Natl Acad Sci USA. 1996;93:12467–12472. doi: 10.1073/pnas.93.22.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hondowicz BD, Scharton-Kersten TM, Jones DE, Scott P. Leishmania major-infected C3H mice treated with anti-IL-12 mAb develop but do not maintain a Th2 response. J Immunol. 1997;159:5024–5031. [PubMed] [Google Scholar]
- 58.Takenaka H, Maruo S, Yamamoto N, Wysocka M, Ono S, Kobayashi M, Yagita H, Okumura K, Hamaoka T, Trinchieri G, Fujiwara H. Regulation of T cell-dependent and -independent IL-12 production by the three Th2-type cytokines IL-10, IL-6, and IL-4. J Leukocyte Biol. 1997;61:80–87. doi: 10.1002/jlb.61.1.80. [DOI] [PubMed] [Google Scholar]
- 59.Van den Eertwegh AJ, Noelle RJ, Roy M, Shepherd DM, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E. In vivo CD40–gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T–B cell interactions. J Exp Med. 1993;178:1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gulbranson-Judge A, MacLennan I. Sequential antigen-specific growth of T cells in the T zones and follicles in response to pigeon cytochrome c. Eur J Immunol. 1996;26:1830–1837. doi: 10.1002/eji.1830260825. [DOI] [PubMed] [Google Scholar]
- 61.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]