Abstract
The bacterial toxin protein A from Staphylococcus aureus (SpA) interacts with B cell antigen receptors encoded by variable region heavy chain (VH) clan III genes via a V region framework surface that has been highly conserved during the evolution of the adaptive immune system. We have investigated the consequences of exposure to this prototypic B cell superantigen, and found that treatment of neonates or adults induces a T cell–independent deletion of a large supraclonal set of susceptible B cells that includes clan III/VH S107 family–expressing lymphocytes. In studies of different SpA forms, the magnitude of the induced deletion directly correlated with the VH-specific binding affinity/avidity. Upon cessation of SpA exposure, the representation of conventional splenic (B-2 subset) lymphocytes normalized; however, we found that the VH family–restricted deficit of peritoneal B-1 cells persisted. SpA treatment also induced a persistent loss of splenic S107-μ transcripts, with a loss of certain natural antibodies and specific tolerance to phosphorylcholine immunogens that normally recruit protective antimicrobial responses dominated by the S107-expressing B-1 clone, T15. These studies illustrate how a B cell superantigen can exploit a primordial Achilles heel in the immune system, for which B-1 cells, an important source of natural antibodies and host immune responses, have special susceptibility.
Keywords: tolerance, repertoire, clonal selection, immunoglobulin genes, host immunity
Introduction
Superantigens (SAgs) are products of microbial pathogens that have the capacity to adversely affect immune responsiveness by interactions with highly represented conserved sites in the V regions of the antigen receptors of host lymphocytes. These proteins have been studied to elucidate their possible roles in pathogenesis of inflammatory and autoimmune diseases, and these nonimmune binding interactions have also provided an invaluable window into the signaling, cytokine, and cognate parameters of T lymphocyte antigen receptor (TCR)-mediated stimulation. Analogous in vitro interactions of several naturally occurring proteins with B cell antigen receptors (BCRs) have also been described (for a review, see reference 1); however the implications for in vivo exposure to a B cell SAg are largely unexplored.
Of all putative B cell SAgs, protein A of Staphylococcus aureus (SpA) has been the best characterized. Despite the fact that this 42-kD secreted membrane protein does not appear to play an essential role in the metabolism or survival of the bacterium, SpA is produced by most (or all) clinical isolates 2. Consequently, it has been postulated that the highly refined Ig binding properties of SpA evolved to play a role in the host–pathogen relationship. Staphylococcal virulence has been shown to be enhanced by SpA in experimental models 3 but the responsible pathophysiologic mechanism(s) have not been determined.
The immunomodulatory activities of SpA are likely aided by its oligovalent organization. It is composed of five 56–61-amino acid homologous extramembrane domains in tandem 4, and each domain possesses both the well-known Fcγ binding specificity and a separate binding site that is specific for Fab-containing VH regions from the structurally related clan III families 5 6. Furthermore, in vitro stimulation with S. aureus has been reported to preferentially select for human B cells expressing genes from the VH3 family 7.
The special molecular features of the Fab-binding specificity of SpA, which were first identified in correlation with antibody sequence usage 1 8 9, have recently been elucidated in crystallographic analyses of a human IgM Fab–SpA domain cocomplex. This interaction was shown to be mediated by a clan III–restricted surface, distant from the CDR loops responsible for the recognition of conventional antigens 10, which involves 13 contact residues in the VH framework (FR)1 and FR3 subdomains that have been conserved during the evolution of the adaptive immune system 11 12. As a direct consequence, this unconventional type of VH-restricted BCR-mediated binding activity is highly represented in immune systems of diverse mammalian species, including the human system in which the VH3 family of clan III composes nearly half of all inherited VH genes. It is also prevalent in amphibian and avian species that have been studied 12. In the mouse, the homologous clan III families S107, J606, 7183, and DNA4 commonly convey this binding activity although the affinities of these interactions vary 12 13 14.
In a recent report, we showed that >5% of mature B cells in naive BALB/c mice possess this nonimmune binding activity, and it is also displayed by ∼12% of constitutively IgM-secreting splenic cells and a comparable proportion of circulating natural IgM 15. Most importantly, we found that neonatal exposure to a chemically modified form of SpA that is devoid of Fc-binding activity induced an acute loss of >80% of SpA-reactive splenic B cells. Although this cellular representation in the spleen later normalized, there was still a long-lasting loss of SpA-reactive IgM-secreting cells (ISCs) and an equivalent loss of circulating SpA-reactive IgM, which persisted, despite the presence of SpA-specific T cells, when evaluated >1 yr later 15. However, these studies did not identify which B cells are susceptible to SAg-mediated deletion, and the implications for host immune responsiveness were not further considered.
We have now investigated the molecular and cellular mechanisms responsible for the immunomodulatory activities of this model B cell SAg. To determine the functional features responsible for its immunological properties, we have compared the host's response to native SpA to treatment with several forms of SpA that vary in their Fc- and Fab-binding activities. Within these studies, we have also identified the VH-defined supraclonal B cell set most affected by treatment. Moreover, we found that SpA exposure adversely affected the levels of certain natural antibodies and caused a selective tolerance to immunogens important for host defense against many bacterial as well as protozoan, fungal, and nematode pathogens 16. Based on these findings, we present a model explaining how this microbial toxin can cause an immunosuppression in the host B cell compartment based on VH usage, and discuss why B-1 cells are especially susceptible to the induction of long-lasting effects.
Materials and Methods
Mutagenesis and Cloning of Domain D′ Derivatives.
To create novel recombinant forms of SpA, the L17D and I31A mutations 17 were introduced into the gene for domain D (DD′) of SpA in the pDomD′ plasmid 5, and termed mDomD′. By overlap PCR methods, a two domain product, dimeric mDD′ (dimDD′), and four domain product, tetra-mutant domainD′ (tetmDD′), were created in the pRSET system (Invitrogen) (Silverman, G.J., manuscript in preparation).
Mice and Immunogens.
Mice were obtained from The Jackson Laboratory and bred under specific pathogen-free conditions under the supervision of the University of California San Diego Animal Subjects Program. Applying a previously reported protocol 15, beginning within 24 h of birth mice were treated with either hen egg lysozyme (HEL; Sigma-Aldrich), OVA (Sigma-Aldrich), tetmDomD′, domain D′, rSpA (Repligen), or SpA that was iodinated (MSpA) to selectively ablate Fc-binding activity 18 19, which had been purified to remove endotoxin. These neonatal mice received PBS or 100 μg of protein in PBS, intraperitoneally every other day for the first 2 wk of life (eight doses of 100 μg protein). Adult mice, at least 6 wk of age, received 1 mg of protein every other day for five doses. In all experiments, mice were age and sex matched.
To assess immune responsiveness, certain groups of BALB/c × C57BL/6 F1 mice were challenged with the phosphorylcholine (PC)-KLH conjugate (50 μg/dose; Biosearch Technologies), whereas others received the thymus-independent type 2 (TI-2) immunogens of B1355 dextran (50 μg/dose; gift of Norman Klinman, The Scripps Research Institute, La Jolla, CA) or pronase-treated extract of 108 CFU of heat-killed R36A Streptococcus pneumoniae (gift of David Briles, University of Alabama at Birmingham, Birmingham, AL) in CFA (Difco). Alternatively, mice were challenged intravenously with 2 μg of pneumococcal cell wall polysaccharide (C-PS; Serumstaatinstitut) in saline. Blood was collected 10 d later for serologic assays, as described 15.
Immunoassays of Antibody Responses.
The antibody response to MSpA and control antigens was quantified as described previously 15. In brief, microtiter wells were coated overnight with protein, dextran, or C-PS at 5 μg/ml in PBS. After blocking with 2% BSA/PBS, serum samples diluted in block were incubated for 4 h at room temperature. The amount of bound antibody was determined by incubation with horseradish peroxidase (HRP)-labeled affinity-purified goat F(ab′)2 anti–mouse Ig-, IgM-, IgG-, or IgG subclass–specific reagents (Jackson ImmunoResearch Laboratories), with values obtained after incubation of substrate for 15 min. The anti-PC response from the S107/clan III–encoded T15 set was measured by development with a saturating concentration of the T15 clone–specific rat IgG2a, T139.2 (20; gift of Matthew Scharff, Albert Einstein College of Medicine, New Hyde Park, NY), or with the T15-specific biotinylated mouse IgG1, AB1-2 (21; gift of John Kearney, University of Alabama at Birmingham, Birmingham, AL), with the recombinant avian mAb, LJ-26, which has restricted recognition of clan III products (including 7183, J606, S107, and DNA4) but not clan I or II products 12. For quantitation, we used a calibration curve of a T15 IgM mAb. The J558/clan I–encoded antibody response to α1–3 dextran was ascertained using an HRP–anti-λ (Jackson ImmunoResearch Laboratories), and the IgMλ, M104E, was used in a standard curve.
To compare relative Fab-binding activity of the SpA forms, 100 μl aliquots of an mAb at fixed concentration were diluted in 1% BSA/PBS with 10 μg/ml of Fc (Jackson ImmunoResearch Laboratories) and incubated for 4 h at room temperature with one of the SpA forms at a range of concentrations; these were later incubated for 1 h in wells coated with tetmDomD′. Plates were developed with HRP-conjugated anti–mouse IgG or IgM (Jackson ImmunoResearch Laboratories), as appropriate. To evaluate relative Fc-binding activity, wells were coated with SpA, and proteins were incubated with a fixed concentration of biotinylated IgG Fc and later developed using streptavidin-alkaline phosphatase (Kirkegaard & Perry Laboratories). Relative inhibition values were determined by comparison to a standard curve of the protein without inhibitor.
Enzyme-linked Immunospot Assays.
The frequencies of Ig- and specific antibody–secreting splenocytes and bone marrow were quantitated as described previously 15. In parallel studies, wells were coated with either goat affinity-purified anti–mouse IgM or IgG (Jackson ImmunoResearch Laboratories), MSpA, control protein antigens of OVA, HEL, or FCS, LJ-26 12, AB1-2, T139.2, Tc54.8 (Fig. 1), or isotype controls.
Figure 1.
A scheme of sets of murine VH genes and SAg reactivity. In many strains, genes from clan I and clan II are the source of most of the repertoire, whereas clan III represents a nonoverlapping set. Most clan III products interact with the Fab-binding site of SpA and also with LJ-26, a recombinant avian mAb. Each recognizes similar but nonidentical sets. These also identify S107-encoded antibodies, including those expressing the T15-specific VS107.1 rearrangement that generally display among the highest levels of SpA-binding affinity.
Flow Cytometry Analysis.
Adapting previously reported methods 15 19 22 to quantify the representation of SAg-susceptible B cells, cells from a mononuclear cell scatter gate were evaluated. For studies of T15i mice, we then gated on all allophycocyanin-labeled anti-B220+ cells (clone RA3-6B2), and then evaluated the representation of surface peridinin chlorophyll protein (PerCP)- or PE-labeled anti-IgMa (clone CS-1) to identify the T15i transgene–expressing cells, and FITC-labeled anti–Mac-1 (clone M1/70) for peritoneal cells. For splenic studies, PerCP-labeled anti-IgMa and FITC-labeled anti-IgD (clone 11-26c.2a) were used. For other studies of peritoneal B-1 cells, we gated on IgDlowB220+ cells, as described previously 22. The binding of biotin-labeled anti–clan III (clone LJ-26; reference 12), detected with PerCP-streptavidin, was then evaluated in B-1a and B-1b subsets based on surface PE-labeled CD5 (clone 53-7.3). Staining was performed in the presence of Fc block, with gates based on staining with fluorochrome-labeled isotype controls. Replicate samples were stained with FITC-labeled anti–mouse IgM (clone 11/410), or anti-κ and anti-λ to confirm equivalent Ig expression. Data were acquired using a FACSCalibur™ (Becton Dickinson) and analyzed with CellQUEST™ (Becton Dickinson) or FlowJo software (Tree Star, Inc.). Dead cells were excluded based on light scatter and propidium iodide uptake. All commercial reagents were obtained from BD PharMingen.
Reverse Transcription PCR/Southern Blot Surveys of VH Family-μ Expression.
As described previously 15, total RNA was isolated from 4 × 107 fresh splenic cells. After DNase treatment and phenol chloroform extraction, 3 μg of total RNA was reverse transcribed. For amplification, 1/10 of the first strand reaction product was added to tubes containing 5 μl of 10× PCR buffer (Boehringer), 5 μl of 25 mM MgCl2, 1 μl of 10 mM dNTP (Amersham Pharmacia Biotech), and 1 μl of each 50 mM primer solution (Operon). Nuclease-free H2O was added to a final reaction volume of 49.5 μl. Samples in triplicate were amplified using a hot start of 95°C for 3 min then 2 U of Taq Polymerase (Boehringer) was added; then 30 cycles of 95°C for 1 min, 60°C for 1 min, and 72°C for 1 min, followed by a final 72°C for 5 min. β-Actin cDNA content was used to normalize total cDNA amounts added in the reactions. Each of the VH family–specific separate reactions used the same antisense Cμ1-derived primer (5′-ccc atg gcc acc aga ttc tta-3′) and one of a previously described series of sense VH FR1-derived oligo primers 15 23, and after electrophoretic separation on 2.5% agarose gels and membrane transfer, blots were probed with VH family–specific plasmid probes (gift of Roy Riblet, Medical Biology Institute, La Jolla, CA), with images analyzed as described 15.
Quantification of S107-μ Transcript Expression.
Applying the Taqman™ (PerkinElmer) PCR-based 5′ nuclease system 24, S107 mRNA transcripts were measured using the primers S107FR1-sh (5′-AGC TTG GTA CAG CCT GGG-3′) and mu3AS-sh (5′-ATG GCC ACC AGA TTC TTA-3′) and the internal S107-specific FR1 sequence probe (S107Frprob, 5′-TTC TCT GAG WCT CTC CTG TGC AAC T-3′), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcripts (PerkinElmer) were quantitated. Each 50-μl reaction tube contained 10 ng of cDNA, and a final concentration of 1× Taqman™ Universal PCR Mix (PerkinElmer), 900 nM forward primers, 900 nM reverse primers, and Taqman™ probe (450 nM for s107FR1-sh probe or 200 nM for murine GAPDH). For negative control template, we used a VH12 cell line (NC1-A7; gift of Alan Whitmore, University of North Carolina at Chapel Hill, Chapel Hill, NC). For a calibration curve, 10 ng of S107.1-μ–containing plasmid was added in six 1:10 serial dilutions to 10 ng of negative control cDNA. Probes were 5′ labeled with a reporter (6-carboxy-fluorescein) and at the 3′ end with a quencher (6-carboxytetramethyl-rhodamine). Amplification conditions were 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. All samples were run in triplicate with data collected from the ABI Prism 7700 (PerkinElmer). To compare the amount of reporter dye with the quenching dye, we generated amplification plots. The fluorescence threshold (C T) was set to bisect the exponential phase of fluorescence increase. Hence, with higher copy number in a sample, fewer reaction cycles were required to attain the threshold level of fluorescence, and the lower the designated sample C T. To normalize the amount of RNA in each tube, target C T values were normalized to the GAPDH C T.
Statistical Analysis.
Comparisons between different groups used the nonparametric rank sum Wilcoxon and Mann-Whitney one-tailed U test.
Results
Affinity/Avidity-dependent B Cell SAg Properties.
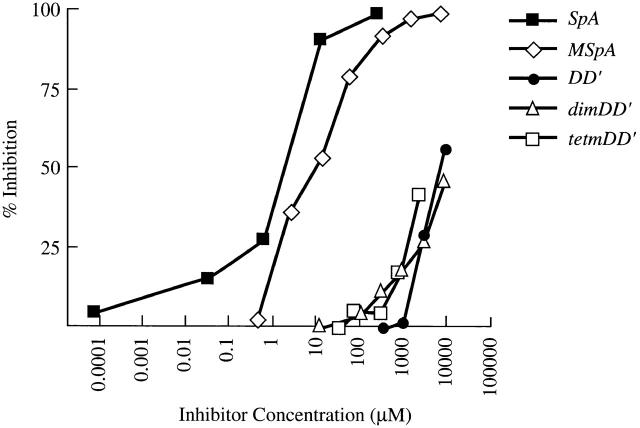
In our recent report, we demonstrated that neonatal treatment with a chemically modified form of SpA (MSpA) induced a persistent suppression of MSpA-reactive splenic ISCs 15. To better define the features responsible for B cell immunomodulatory properties of this natural bacterial toxin, we assembled a panel of different forms of SpA, and the Ig-binding properties of these proteins were compared in direct binding and inhibition studies with human and murine mAbs. In a representative study (Fig. 2), all of these proteins displayed the same clan III–specific Fab-binding specificity, although relative affinity/avidity varied greatly (Table ). Native SpA displayed the strongest Fab-binding activity, whereas MSpA exhibited about fivefold weaker activity. Compared with native SpA, the monomeric domain D′ of SpA, which is capable of simultaneously binding a VH3 Fab and an Fcγ molecule via distinct sites 5, exhibited ∼5,000-fold weaker Fab-binding activity. By introduction of the L17D and I31A mutations that are reported to ablate Fc-binding activity 17, a genetic mutant domain D′ (mDD′) was generated and oligomeric forms were also engineered. Although not anticipated, the mutant dimeric and tetrameric mutant SpA forms, dimDD′ and tetmDD′, exhibited ∼2,000–5,000-fold weaker Fab-binding activity than native SpA. In separate studies, the MSpA and mutant SpA forms were devoid of detectable Fcγ activity, whereas the greatest binding activity was displayed by SpA, and by comparison the domain D monomer had ∼500-fold weaker inhibitory activity (not shown).
Figure 2.
Recombinant forms of SpA display a broad range of Fab-binding avidities. The relative Fab-binding activities of five different forms of SpA were compared in inhibition immunoassays of the binding of an S107-encoded IgG in solution to wells coated with an SpA variant. In these studies, the activity of native SpA is compared with a form of SpA created by chemical modification (MSpA), an SpA monomer, domain D (DD′), and dimeric (dimtetDD′) and tetrameric (tetmDD′) forms of domain D′, which include two site-specific mutations that remove Fc-binding activity.
Table 1.
Relative Activities of SpA Forms
| SpA form | MW | No. of domains | Fab-binding | Fc-binding | |
|---|---|---|---|---|---|
| kD | M | M | |||
| SpA | Native | 42 | 5 | 2 × 10−6 | 4 × 10−7 |
| MSpA | Iodinated | 42 | 5 | 1 × 10−5 | ND |
| DD′ | Native | 11 | 1 | 1 × 10−2 | 2 × 10−4 |
| DimDD′ | L17D I31A | 24 | 2 | 1 × 10−2 | ND |
| TetmDD′ | L17D I31A | 37 | 4 | 5 × 10−3 | ND |
MW, molecular mass.
Fab-binding Avidity Determines Level of Immunosuppression.
To investigate the in vivo biologic activities of different proteins, mice were treated during the first 2 wk of life and then evaluated months later. In general, neonatal treatment with SpA or control protein antigen resulted in a lasting increase in the overall frequency of splenic constitutive ISCs (Fig. 3 A; reference 15). To evaluate whether treatment altered the representation of ISCs reactive with the clan III–specific Fab-binding site of SpA (i.e., SAg-reactive ISCs), we ascertained the frequencies of cells producing IgM that bind to MSpA-coated wells 15 and found that in naive adult mice, 12.5 ± 0.7% (mean ± SEM) of splenic ISCs were SAg reactive and this proportion was not altered by prior treatment with a control protein antigen (HEL, 12.3 ± 1.1%; Fig. 3 A) 15. Treatment with the tetrameric mutant SpA form, which has weak Fab-binding activity and no Fc-binding activity, induced a minor decrease (10.3 ± 0.7%) in the representation of SAg-reactive ISCs that was not significantly less than in control groups (P = 0.095). By contrast, treatment with the domain D′ monomer induced a significant level of inhibition (7.9 ± 0.02%, P = 0.03). A greater level of suppression was induced by MSpA, which has relatively strong Fab-binding activity and no Fc-binding activity (6.25 ± 0.4%, P < 0.001). However, the greatest suppression was detected in mice treated with native SpA, which has the strongest Fab-binding activity (2.8 ± 0.4%, P < 0.004). Essentially the same patterns of suppressive activity were also detected in bone marrow ISCs, where the effects in the MSpA- and SpA-treated groups were similar (Fig. 3 B). A similar effect was also demonstrated for cells in the peritoneal cavity, the treatment site (not shown). Even though total IgM levels were generally increased compared with naive mice (not shown), SpA treatment of neonatal mice also induced a decrease in the levels of MSpA-binding circulating IgM 15. Notably, a total dose of only 50 μg of MSpA often induced a similar level of suppression, but the responses of mice within this treatment group were more heterogeneous (not shown). Hence, the level of supraclonal suppression of SAg-reactive ISCs directly correlated with the relative Fab-binding affinity and not the Fc-binding activity.
Figure 3.
The level of loss of SAg-reactive ISCs in bone marrow and spleen after neonatal or adult treatment with SpA. Level of suppression correlates with avidity of Fab-binding activity and does not require T cells. BALB/c mice treated as (A and B) neonates or as (C and D) adults, or (E and F) neonatally treated TCR-β−/−δ−/− or control C57BL/6 mice, received intraperitoneal injections of saline (Naive), a control antigen (HEL), a genetic mutant tetrameric form of SpA (tetmDD′), an SpA monomer (DD′), a chemically modified form of SpA (MSpA), or native SpA. The frequencies of IgM-specific ISCs in the spleen (A, C, and E) and bone marrow (B, D, and F) were quantitated 2–5 mo later by enzyme-linked immunospot assay. Replicate wells coated with anti-IgM established the total frequency of IgM-ISCs (left); with MSpA determined the frequency of ISCs that interact with the Fab-binding site of SpA (middle); or with a control antigen established that the frequency of ISCs with nonspecific binding activity was <10/106 total cells (not shown). For each mouse, the relative proportion of ISCs with SAg reactivity was determined by dividing the frequency of MSpA-reactive IgM-ISCs by the frequency for total ISCs (right). The frequency of PC-specific ISCs was below the limits of detection (not shown; reference 59). +, mean values for each group. For comparisons to control protein-treated groups, significant values are indicated as follows for SpA-treated groups: *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001. Values represent the frequency of each type of IgM-secreting cell per million mononuclear cells, or the relative percentage of these IgM-secreting cells among all IgM-secreting cells (%), as indicated.
Adult Mice Are Also Susceptible to SAg-induced Immunosuppression.
To ascertain whether adult mice are also affected by SpA treatment, 7-wk-old BALB/c mice were given proportionately larger doses and evaluated 1 mo later. In these treated mice, the frequency of total splenic ISCs was not affected, whereas SAg-reactive splenic ISCs were significantly reduced by treatment with MSpA (4.81 ± 0.95%, P < 0.001) or SpA (3.41 ± 0.039%, P < 0.0005) compared with control groups (Fig. 3 C). Similar suppressive effects were also found on constitutive ISCs in the bone marrow (Fig. 3 D).
T Lymphocytes Are Not Required for SAg-induced B Cell Suppression.
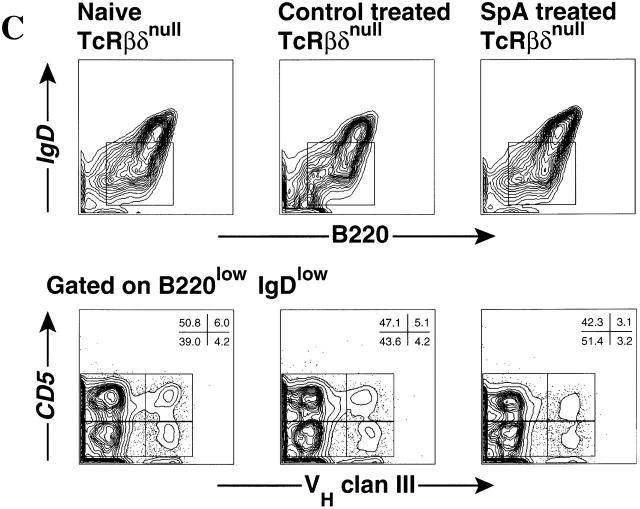
To investigate whether T lymphocytes play a critical role in SpA-induced B cell suppression, we evaluated the effect of neonatal treatment on mice with homozygous deficiencies in both TCR β and δ chains (TCR-β−/−δ−/−), which are devoid of mature T cells 25 26. Akin to the control C57BL/6 groups, neonatal treatment of T cell–deficient mice with a protein antigen also resulted in increased overall levels of splenic ISCs, while these mice were also susceptible to SpA-induced selective decreases in the frequency and relative representation of SAg-reactive ISCs in the spleen (Fig. 3 E) and bone marrow (Fig. 3 F). Compared with control groups, SpA treatment reduced by 35% the proportion of clan III–expressing splenic ISCs (P = 0.007; Fig. 3 E), identified by a broadly reactive clan III Ig–specific marker (LJ-26 antibody) 12. The T15 B cell clonal set represents only a small component of VH clan III/S107–expressing cells, and using a VHT15-specific marker (Tc68) we found that in these treated mice the representation among ISCs of these products was reduced by 76% (P = 0.0002) in the spleen (Fig. 3 E) and by 82% in bone marrow (P = 0.026; Fig. 3 F). Compared with control groups, levels of circulating natural Ig-bearing T15-specific idiotypic markers, which included IgM, IgG2b, and IgG3 responses, were each found to be significantly reduced (not shown). These results document that SpA-induced suppression is mediated via a B cell–targeted mechanism affecting certain S107-expressing B cells, which is not dependent on T cell influences.
SAg Induces a Selective VH Family–targeted Deficit in the Expressed Ig Repertoire.
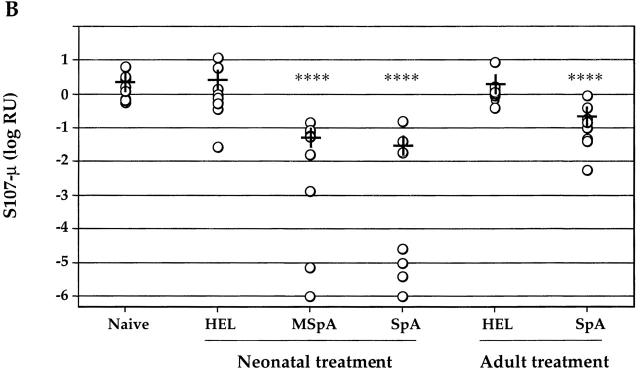
To ascertain whether these defects in SAg-reactive ISCs and circulating IgM reflect losses restricted to VH family–defined supraclonal B cell sets, reverse transcription (RT)-PCR/Southern blots were performed. Compared with naive age-matched mice, akin to the finding of increased IgM levels, we found that treatment with a control protein induced a nonspecific increase in transcript expression of μ rearrangements of each of seven different VH families studied (Fig. 4 A). Significantly, MSpA or SpA treatment induced a selective expression deficit of only the clan III/S107 family (Fig. 4 A). A similar selective suppression of VH S107 transcript expression was also documented after SpA treatment of adult mice (not shown).
Figure 4.
SAg-treated mice display a loss of S107 VH family–specific μ rearrangements in the spleen. (A) RT-PCR/Southern blot surveys of VH-μ transcript expression were performed using splenic RNA. Different VH FR1–specific primers were used to amply transcripts from families of clan I (J558), clan II (Q52), and clan III (S107, J606, and 7183), and results for several major VH families are shown. Results for BALB/c mice treated as neonates are shown, but similar results were also obtained for mice treated as adults then evaluated 1 mo later (not shown). Relative β-actin content is demonstrated by staining with Sybr Green Dye II (Molecular Probes). For each treatment group, signals for fivefold serial dilutions of splenic cDNA from two different mice are demonstrated. Nonspecific posttreatment increases were also documented for μ transcripts of the Vgam3 and X24 families (not shown). (B) Quantitative assays of splenic S107-μ transcripts for groups that received neonatal or adult treatment are displayed. Relative S107 C T values were calculated using the untreated group as the denominator. Replicate values displayed <10% coefficient of variation (not shown). +, mean values for each group. For comparisons with control protein-treated groups, significance is indicated as follows: ****P < 0.001.
To quantitate the deficit, we used a real-time PCR–based assay system, which demonstrated that neonatal treatment with MSpA or SpA significantly reduced splenic S107-μ transcript expression levels by a mean 97.7 and 98.6% (P < 0.001) compared with control age-matched mice, respectively (Fig. 4 B). Notably, in many of these SAg-treated mice, S107 transcript levels were actually orders of magnitude lower than the groups' mean values, indicating much greater levels of suppression. Furthermore, for mice treated as adults and evaluated 1 mo later, SpA exposure also induced a mean 90% reduction in S107-μ transcript expression (P < 0.001). In addition, after treatment with MSpA, five of seven mice displayed >90% reductions of S107-μ transcript expression (not shown). Hence, in vivo exposure to SpA targets a supraclonal B cell set, resulting in a nearly complete loss of S107 transcript expression that appears to be linked to the selective loss of certain clan III–constitutive antibody-forming cells that are a source of natural antibodies.
SAg Exposure Alters the Natural Antibody Repertoire and Induces Tolerance to PC.
To evaluate the consequences of SAg exposure on natural antibodies of defined clonal origin, we measured the levels of antibodies to PC, an immunodominant moiety present in many microbial pathogens 16. In many murine strains, antibodies to PC predominantly derive from T15 B cells that employ a specific pairing of canonical VH and Vκ rearrangements (23 27; Fig. 1). Notably, these T15 clones are represented at high frequency in the B-1 pool without prior immune exposure even in germ-free mice 27 28, and provide defense from systemic infection from virulent pneumococci 29 and Salmonella typhi 30. Only higher avidity Fab-binding forms (e.g., MSpA or SpA) inhibited levels of PC-specific natural antibodies, including those from the T15 clone (Fig. 5 A). In contrast, levels of natural antibodies to α1–3 dextran, which arise from a separate B-1 clonal set expressing a defined clan I/J558 rearrangement, were increased in each of the treatment groups (Fig. 5 B), reflecting the nonspecific increase of total circulating IgM (not shown). Hence, SpA exposure selectively suppressed a VH-targeted set of antigen-specific natural antibodies.
Figure 5.

SpA or MSpA induces persistent loss of natural IgM anti-PC antibodies, whereas natural IgM anti-α1–3 dextran antibodies are nonspecifically increased. Neonatal BALB/c mice were treated with control proteins or different forms of SpA for the first 2 wk of life, and natural IgM antibody levels were quantitated 3–5 mo later. For groups of four mice, serial dilutions of antibody reactivities in ELISA are indicated with mean ± SEM.
To assess the impact of SAg exposure on the immune competence of the host, mice in different neonatal treatment groups were challenged with dextran and PC immunogens that induce TI-2 responses. Compared with the vigorous responses in naive or control protein-treated groups, all of the mice neonatally treated with MSpA or SpA were tolerant to challenge with S. pneumoniae extract, whereas the mice treated with the domain D′ monomer of SpA displayed highly heterogeneous anti-PC responses (Fig. 6 A). In other challenged groups, mice treated as neonates with SpA were also nonresponsive to the PC-containing purified C-PS TI-2 immunogen (not shown), and were also tolerant to the T cell–dependent PC immunogen PC-KLH (Fig. 6 B) that also recruits a response dominated by T15 B cell clone(s) 31. Treatment of adult mice with SpA induced the same selective tolerance to the PC-containing pneumococcal immunogen (Fig. 6 C). Therefore, SAg treatment induces tolerance in an antigen-specific response requiring clan III/S107–expressing B cell clonal sets; in the absence of this response, other clonal sets are not recruited into this response, whereas a response requiring an clan I/J558–expressing B cells is unaffected (Fig. 6A and Fig. C).
Figure 6.

Neonatal or adult treatment with SpA causes a persistent tolerance to PC-containing immunogens. Postimmune responses were measured 10 d after challenge of 3-mo-old mice (A) with pronase-treated extract of R36A pneumococcal bacteria and B1355 dextran in CFA, and (B) with PC-KLH emulsified in CFA. (C) Adult BALB/c mice were treated with SpA, and then challenged 7 d later. Detection of anti-PC responses used the T15 clone–specific AB1-2 antibody, which recognizes an idiotope requiring coexpression of both the T15 VH and VL regions (reference 21), and also the clan III–restricted avian mAb, LJ-26 (reference 12). Groups are indicated by the protein with which they were conditioned and subsequently challenged, with comparison to control groups of naive mice challenged with R36A and dextran in CFA (Naive/R36A Dex), and to naive mice that received CFA alone (Naive/CFA). Values are relative antibody units per milliliter. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001.
SpA Induces a Loss of Detectable Clan III–expressing B-1 Cells in the Peritoneal Cavity.
To consider the cellular mechanisms responsible for SpA-induced defects, we have evaluated T15i transgenic “knockin” mice that have B cells expressing the canonical T15/S107 VH rearrangement. In heterozygotic mice, clonal representation of B cells can be readily tracked, as B cells expressing endogenous VH regions bear the IgMb allotype, whereas expression of the VHT15 transgene is linked to the IgMa allotype. Confirming their earlier characterization 32, in these mice most peritoneal VHT15-expressing B cells, which coexpress diverse endogenous L chains, bear the phenotype of B-1b cells (i.e., B220lowIgDlowMac-1+CD5−). Clarifying our previously reported findings in BALB/c mice, 1 d after completion of neonatal SpA treatment, heterozygous mice exhibited a >90% reduction of VHT15 transgene–expressing splenic B cells (not shown). Furthermore, treatment of adult 6-wk-old mice with a single 0.5-mg dose of SpA also induced an acute selective loss of >60% of VHT15-expressing splenic and peritoneal B cells when evaluated 1 d later (not shown).
To evaluate the clonal representation associated with long-lasting functional defects, we studied T15i heterozygous mice months after the last SpA exposure. At these late time points, there were no major differences in the representation of transgene-expressing splenic conventional B cells (IgDhiB220+) (Fig. 7 B), presumably because these B cells are continually replenished from the bone marrow. However, in the peritoneal cavity, a major anatomic residence of B-1 cells, neonatal SpA treatment resulted in a highly significant mean 78% reduction (P = 0.0006) in the frequency of VHT15 transgene–expressing peritoneal B-1 cells (Mac-1+B220+) (7.9 ± 1.1%, n = 7) compared with control treated mice (36.5 ± 3.2%, n = 6) (Fig. 7 A). Using the clan III (LJ-26) marker, comparable losses of transgene-expressing B-1 cell populations were also demonstrated in the peritoneum but not the spleen (not shown). We also demonstrated similar findings in studies of adult TCR-deficient mice with polyclonal endogenous B cell populations. In the T cell–deficient mice that had received neonatal SpA treatment, the representation of clan III–expressing peritoneal B-1a (LJ-26+IgDlowB220+CD5+) cells was significantly reduced (8.1 ± 0.49%, n = 4, P < 0.016), representing a mean 30% reduction, compared with naive (11.5 ± 0.36%) or control treated mice (10.3 ± 0.52%). Similar significant losses were also demonstrated in the clan III–expressing B-1b (LJ-26+IgDlowB220+CD5−) cells of SpA-treated mice (6.8 ± 0.48%, P < 0.015) compared with naive (9.7 ± 0.14%) and control treated mice (9.75 ± 0.65%) (Fig. 7 C). In contrast, the representation of conventional splenic B cells in these mice was not significantly affected (not shown). These findings demonstrate that a limited interval of exposure to SpA can induce a long-lasting clonal loss of clan III/S107–expressing peritoneal B-1 cells.
Figure 7.
Persistent clonal loss of VHT15 and clan III–expressing peritoneal B-1 cells. In each multigroup study, naive, SpA- or control protein-treated mice were included, with comparisons to naive BALB/c and C57BL/6 mice. Mice were treated as neonates, and then evaluated 7–21 wk later in microfluorimetric assays. Representative results from studies of T15i mice (A) peritoneal cavity and (B) splenic mononuclear cells are displayed. For these analyses, gates were set so that <0.8% of C57BL/6 (IgMb)B220+ cells were reactive with the IgMa-specific reagent. Representative results are shown from the (C) peritoneal cells of matched groups of TCR-β2/2δ2/2 mice, evaluated 16 wk after the last exposure. In these results, control mice were treated with HEL, with equivalent findings in control β-galactosidase–treated mice (not shown). For each B cell subset, the IgMa or LJ-26 reactivity was used to derive the percentages representative of clan VHIII–expressing B cells.
Discussion
We have demonstrated that in vivo exposure to staphylococcal protein A, a model B cell SAg, results in a selective targeting and immunosuppression of a supraclonal set of B lymphocytes that express VH clan III antibody genes, and both neonatal and mature mice are susceptible. In general, the magnitude of the induced B cell defect correlated with the relative avidity of Fab-binding interaction of the SAg. While the structural basis of this SAg–BCR interaction is no doubt distinct from those associated with binding of conventional antigens, these findings are consistent with earlier observations in which high affinity ligands are most effective at inducing B cell tolerance in classical models 33 and in transgenic Ig models 34 35.
In surveys of the murine antibodies, many genes from clan III–related VH families commonly encode for binding of this prototypic bacterial B cell SAg 13 14, but certain of these genes, especially those from the S107 family, generally encode for antibodies with greater SpA binding activity 14. These findings parallel data from earlier studies of phage-display antibody libraries, in which SpA preferentially selected for phagemid clones expressing certain human germline VH3 genes that conveyed greater SpA binding activity 5 8. In the current studies, we have now demonstrated that in vivo exposure to SpA can induce a nearly complete loss of constitutively expressed splenic S107-μ transcripts and natural IgM, including anti-PC antibodies. In view of the cumulative data, we can only interpret the loss of these S107-expressing B cell clones as due, at least in part, to their higher binding affinity for SpA.
The susceptibility of T cell–deficient mice to SAg-induced immune defects indicates that this process, which is associated with long-lasting clonal loss, does not have an obligatory T cell dependence. In fact, induced PC-specific tolerance persists in the presence of SpA-specific T cells 15. Hence, SAg-induced tolerance has features that appear distinct from experimental models of B cell tolerance in which the unavailability of antigen-specific T cells is a primary determinant in whether B cell interactions with nominal antigen result in clonal anergy and/or deletion 36 37.
The capacity to induce a persistent “hole” in the B cell repertoire is not unique to SpA, as similar outcomes have also been previously reported upon treatment with certain specific B cell ligands/antigens, or with antiidiotypic antibodies that act as their surrogates 38. However, we know that in naive mice, PC-specific antibody-forming cells normally represent only an estimated 1/50,000 splenocytes 39, indicating that PC-specific B cells represent only a limited component of the large supraclonal set affected by SAg exposure. Hence, the distinction of the SpA response derives in part from the much larger scale of the induced supraclonal defect. In addition, the immunological properties of SpA are also special, as this naturally occurring “tolerizing agent” affects host responses to other immunogens by virtue of its ability to interact with supraclonal sets via VH family–specific interactions (i.e., it has superantigenic properties).
Our earlier surveys identified two distinct temporal phases in BALB/c mice during the response to neonatal treatment with this B cell SAg. Throughout both early and late phases, suppression of levels of SAg-reactive IgM was a consistent finding 15, whereas the late phase response, beginning at ∼6 wk of life, was distinguished by the acquisition of SpA-specific T cell responsiveness and the induction of SAg-specific IgG1 antibody production. In the current studies, T cell–deficient mice were also found to be sensitive to the induced B cell supraclonal suppression, but the features characteristic of the late phase of the response were absent in these mice. This late phase T cell–dependent response likely involves conventional (B-2) lymphocytes in germinal center–type reactions in secondary lymphoid tissue that elaborate clonally focussed MSpA-reactive IgG1 antibody responses (Silverman, G.J., manuscript in preparation). Therefore, because these IgG1 responses from conventional B cells can reconstitute in BALB/c mice, our data indicate that it is the effects of this bacterial toxin on a separate B cell pool that is responsible for the persistent induced defects in anti-PC responses and natural antibody production.
Several compelling pieces of evidence implicate the targeting of clones within the B-1 pool as the origin of the long-lasting immunological defects. Most relevant, B-1 cells are believed to be the source of constitutively produced natural IgM antibodies that provide the primary defense against many common microbial pathogens 40 41. Moreover, the susceptible set includes T15 B cells, the source of S107-encoded anti-PC antibodies that dominate these natural IgM and postimmunization protective responses, which adoptive transfer studies have rigorously demonstrated reside predominantly, if not solely, within the B-1 pool 42 43. We found that SAg exposure resulted in a near complete loss of splenic S107-μ transcripts, but these findings, from RNA-based assays, are greatly influenced by the representation of antibody-forming cells that contain 100–1,000-fold greater amounts of antibody-specific transcripts than resting B cells. Hence, the dramatic reductions in S107-μ transcript levels undoubtedly reflect the loss of an SAg-reactive set of constitutive ISCs that derive from B-1 cells to produce natural antibodies.
The special immunobiology of B-1 cells explains their susceptibility to the persistent VH-targeted effects of SpA. For the developing murine B-1 pool, entry of newly generated lymphocytes is reportedly complete by the end of the neonatal period, and thereafter these B cells are self-sustaining and cannot be replenished by the lymphoneogenesis that perpetually supplements the conventional (B-2) compartment in the adult 44 45 46. Also relevant, compared with splenic follicular B cells, adult peritoneal B-1 cells are poor responders to in vitro stimulation with anti-IgM, an experimental surrogate for a BCR signal. In fact, BCR-mediated apoptosis is a common outcome of high level antigenic stimulation of these extrafollicular cells 47 48. Strong in vivo cross-linking of cell surface Igs has also been reported to induce apoptotic death of mature B cells in the peritoneal cavity 48. Presumably, these features of B-1 cells are also responsible for the persistent clonal loss that results from instillation of IgM allotype–specific antibody into allotype heterozygotic mice 49. Based on these observations, it is predictable that even a limited exposure to a potent natural BCR-targeted agent would induce a supraclonal hole in the B-1 repertoire that would persist, likely indefinitely. In earlier studies, we demonstrated a transient supraclonal deletion in the spleen and bone marrow immediately after SAg treatment 15; however, the clonal representation at these sites later normalized, presumably because of replenishment of conventional B cells from the central compartment. Consistent with this hypothesis, in surveys of splenic VH-δ rearrangements that are representative of mature naive conventional follicular B cells (i.e., sIgDhi), we did not detect a lasting SAg-induced supraclonal defect (Silverman, G.J., manuscript in preparation). These findings contrast with the persistent loss of VHS107-μ transcripts characterized in the current studies, which correlates with the supraclonal loss of splenic constitutive ISCs that have been linked to the B-1 pool. Hence, we believe that the lasting SpA-induced loss of SAg-reactive clan III–derived peritoneal B-1 cells reflects their special vulnerability for the induction of persistent clonal defects by this bacterial Fab-binding protein.
B-1 cells are associated with a distinct repertoire that includes well-defined clonal sets that produce natural antibodies responsible for housekeeping and antimicrobial functions. From lessons first appreciated for the T15 clone, it appears that the binding specificities of B-1 cells commonly derive from canonical antibody gene rearrangements formed in primary sequence-directed splicing events 23 50 51 and are subsequently subject to ligand-mediated clonal selection 52; hence, B-1 cells have been postulated to convey a repertoire naturally selected during the evolution of the adaptive immune system 53. From this viewpoint, targeting of host clan III B cells, especially those providing natural immune defenses 29 41, may provide a great advantage to a pathogen. Clan III genes are expressed in the immune systems of phylogenetically distant species as far back as elasmobranchs, and in certain species these antibody genes are the earliest expressed during development 11. In particular, clan III VH families and SpA-binding activity are highly represented in mammalian immune systems 12, and in humans >30% of B cells are targeted by SpA 19. Moreover, the current findings, which elucidate the immunological properties of SpA, should also help us to understand the effects of other postulated B cell SAgs 54 55 56. In particular, our findings may provide insight into how the SAg properties of HIV-1–associated retroviral products may contribute to a predisposition to certain infections 57, especially the otherwise inexplicably high frequency of pneumococcal sepsis 58.
In summary, our findings indicate that staphylococci produce a toxin that can impair host defenses through effects on B lymphocytes, for which B-1 cells have special vulnerability. These interactions are mediated by an unconventional binding site on clan III–associated VH region framework subdomains that have been highly conserved in evolution 10. From these findings, we speculate that this common pathogen has developed a toxin with B cell SAg properties to exploit a primordial Achilles heel in the immune system.
Acknowledgments
We acknowledge the kind gift from Dr. Klaus Rajewsky of T15i transgenic mice, which were kindly transferred to us by Drs. Betty Diamond and Christine Grimaldi. We also thank Drs. Alan Stall, David Nemazee, and Mark Shlomchik for helpful discussions.
This work was supported by grants AI40305, AR40770, and AI46637 from the National Institutes of Health, and a Biomedical Sciences Award from the Arthritis Foundation.
Note added in proof. Confirming the importance of the T15/clan III B cell clone, in a recent report Mi et al. provided direct evidence that genetic manipulation that knocks out the S107.1/clan III gene results in greatly impaired immune defense from pneumococcal infection. (Mi, Q.S., L. Zhou, D.H. Schulze, R.T. Fischer, A. Lustig, L.J. Rezanka, D.M. Donovan, D.L. Longo, and J.J. Kenny. 2000. Proc. Natl. Acad. Sci. USA. 97:6031–6036.)
Footnotes
Abbreviations used in this paper: BCR, B cell antigen receptor; C-PS, pneumococcal cell wall polysaccharide; FR, framework; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HEL, hen egg lysozyme; HRP, horseradish peroxidase; ISC, IgM-secreting cell; MSpA, chemically modified SpA; PC, phosphorylcholine; PerCP, peridinin chlorophyll protein; RT, reverse transcription; SAg, superantigen; SpA, Staphylococcus aureus protein A; TI-2, thymus-independent type 2.
References
- Silverman G.J. B cell superantigenspossible roles in immunodeficiency and autoimmunity. Semin. Immunol. 1998;10:43–55. doi: 10.1006/smim.1997.0104. [DOI] [PubMed] [Google Scholar]
- Langone J.J. Protein A of Staphylococcus aureus and related immunoglobulin receptors produced by streptococci and pneumonococci. Adv. Immunol. 1982;32:157–252. [PubMed] [Google Scholar]
- Patel A.H., Nowlan P., Weavers E.D., Foster T. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect. Immun. 1987;55:3103–3110. doi: 10.1128/iai.55.12.3103-3110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlen M., Guss B., Nilsson B., Gatenbeck S., Philipson L., Lindberg M. Complete sequence of the staphylococcal gene encoding protein A. A gene evolved through multiple duplications. J. Biol. Chem. 1984;259:1695–1702. [PubMed] [Google Scholar]
- Roben P., Salem A., Silverman G.J. VH3 antibodies bind domain D of staphylococcal protein A. J. Immunol. 1995;154:6437–6446. [PubMed] [Google Scholar]
- Jansson B., Uhlen M., Nygren P.A. All individual domains of staphylococcal protein A show Fab binding. FEMS Immunol. Med. Microbiol. 1998;20:69–78. doi: 10.1111/j.1574-695X.1998.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Kristiansen S.V., Pascual V., Lipsky P.E. Staphylococcal protein A induces biased production of Ig by VH3-expressing B lymphocytes. J. Immunol. 1994;153:2974–2984. [PubMed] [Google Scholar]
- Sasano M., Burton D.R., Silverman G.J. Molecular selection of human antibodies with an unconventional bacterial B cell superantigen. J. Immunol. 1993;151:5822–5839. [PubMed] [Google Scholar]
- Sasso E.H., Silverman G.J., Mannik M. Human IgM molecules that bind staphylococcal protein A contain VHIII H chains. J. Immunol. 1989;142:2778–2783. [PubMed] [Google Scholar]
- Graille M., Stura E.A., Corper A.L., Sutton B., Taussig M., Charbonnier J.-B., Silverman G.J. Crystal structure of a Staphylococcus aureus protein A domain complexed with the Fab fragment of a human IgM antibodystructural basis for recognition of B-cell receptors and superantigen activity. Proc. Natl. Acad. Sci. USA. 2000;97:5399–5404. doi: 10.1073/pnas.97.10.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham P.M., Schroeder H.W., Jr. Antibody structure and the evolution of immunoglobulin V gene segments. Semin. Immunol. 1994;6:347–360. doi: 10.1006/smim.1994.1045. [DOI] [PubMed] [Google Scholar]
- Cary S., Lee J., Wagenknecht R., Silverman G.J. Characterization of superantigen induced clonal deletion with a novel clan III-restricted avian monoclonal antibody. Exploiting evolutionary distance to create antibodies specific for a conserved VH region surface. J. Immunol. 2000;164:4730–4741. doi: 10.4049/jimmunol.164.9.4730. [DOI] [PubMed] [Google Scholar]
- Seppala I., Kaartinen M., Ibrahim S., Makela O. Mouse Ig coded by VH families S107 or J606 bind to protein A. J. Immunol. 1990;145:2989–2993. [PubMed] [Google Scholar]
- Cary S., Krishnan M.R., Marion T., Silverman G.J. The murine clan VH III related 7183, J606 and S107 and DNA4 families commonly encode for binding to a bacterial B cell superantigen. Mol. Immunol. 1999;36:769–776. doi: 10.1016/s0161-5890(99)00085-1. [DOI] [PubMed] [Google Scholar]
- Silverman G.J., Nayak J.V., Warnatz K., Cary S., Tighe H., Curtiss V.E. The dual phases of the response to neonatal exposure to a VH family-restricted staphylococcal B-cell superantigen. J. Immunol. 1998;161:5720–5732. [PubMed] [Google Scholar]
- Harnett W., Harnett M.M. Phosphorylcholinefriend or foe of the immune system. Immunol. Today. 1999;20:125–129. doi: 10.1016/s0167-5699(98)01419-4. [DOI] [PubMed] [Google Scholar]
- Cedergren L., Andersson R., Jansson B., Uhlen M., Nilsson B. Mutational analysis of the interaction between staphylococcal protein A and human IgG1. Protein Eng. 1993;6:441–448. doi: 10.1093/protein/6.4.441. [DOI] [PubMed] [Google Scholar]
- Romagnani S., Giudizi M.G., del Prete G., Maggi E., Biagiotti R., Almerigogna F., Ricci M. Demonstration on protein A of two distinct immunoglobulin-binding sites and their role in the mitogenic activity of Staphylococcus aureus Cowan I on human B cells. J. Immunol. 1982;129:596–602. [PubMed] [Google Scholar]
- Silverman G.J., Sasano M., Wormsley S.B. Age-associated changes in binding of human B lymphocytes to a VH3-restricted unconventional bacterial antigen. J. Immunol. 1993;151:5840–5855. [PubMed] [Google Scholar]
- Desaymard C., Giusti A.M., Scharff M.D. Rat anti-T15 monoclonal antibodies with specificity for VH- and VH-VL epitopes. Mol. Immunol. 1984;21:961–967. doi: 10.1016/0161-5890(84)90154-8. [DOI] [PubMed] [Google Scholar]
- Kearney J.F., Barletta R., Quan Z.S., Quintans J. Monoclonal vs. heterogeneous anti-H-8 antibodies in the analysis of the anti-phosphorylcholine response in BALB/c mice. Eur. J. Immunol. 1981;11:877–883. doi: 10.1002/eji.1830111106. [DOI] [PubMed] [Google Scholar]
- Stall, A.M., and S.M. Wells. 1996. FACS analysis of murine B-cell populations. Weir's Handbook of Experimental Immunology. Blackwell Science, Cambridge, MA. 63.1–63.17.
- Feeney A.J. Predominance of the prototypic T15 anti-phosphorylcholine junctional sequence in neonatal pre-B cells. J. Immunol. 1991;147:4343–4350. [PubMed] [Google Scholar]
- Gerard C.J., Olsson K., Ramanathan R., Reading C., Hanania E.G. Improved quantitation of minimal residual disease in multiple myeloma using real-time polymerase chain reaction and plasmid-DNA complementarity determining region III standards. Cancer Res. 1998;58:3957–3964. [PubMed] [Google Scholar]
- Itohara S., Mombaerts P., Lafaille J., Iacomini J., Nelson A., Clarke A.R., Hooper M.L., Farr A., Tonegawa S. T cell receptor delta gene mutant miceindependent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A.R., Rudnicki M.A., Iacomini J., Itohara S., Lafaille J.J., Wang L., Ichikawa Y., Jaenisch R., Hooper M.L. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Sigal N.H., Pickard A.R., Metcalf E.S., Gearhart P.J., Klinman N.R. Expression of phosphorylcholine-specific B cells during murine development. J. Exp. Med. 1977;146:933–948. doi: 10.1084/jem.146.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N.H., Gearhart P.J., Klinman N.R. The frequency of phosphorylcholine-specific B cells in conventional and germfree BALB/C mice. J. Immunol. 1975;114:1354–1358. [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae . J. Exp. Med. 1982;156:1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecquet S.S., Ehrat C., Ernst P.B. Enhancement of mucosal antibody responses to Salmonella typhimurium and the microbial hapten phosphorylcholine in mice with X-linked immunodeficiency by B-cell precursors from the peritoneal cavity. Infect. Immun. 1992;60:503–509. doi: 10.1128/iai.60.2.503-509.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taki S., Schmitt M., Tarlinton D., Forster I., Rajewsky K. T cell-dependent antibody production by Ly-1 B cells. Ann. NY Acad. Sci. 1992;651:328–335. doi: 10.1111/j.1749-6632.1992.tb24632.x. [DOI] [PubMed] [Google Scholar]
- Taki S., Meiering M., Rajewsky K. Targeted insertion of a variable region gene into the immunoglobulin heavy chain locus. Science. 1993;262:1268–1271. doi: 10.1126/science.8235657. [DOI] [PubMed] [Google Scholar]
- Weigle W.O. Immunological unresponsiveness. Adv. Immunol. 1973;16:61–122. doi: 10.1016/s0065-2776(08)60296-5. [DOI] [PubMed] [Google Scholar]
- Healy J.I., Goodnow C.C. Positive versus negative signaling by lymphocyte antigen receptors. Annu. Rev. Immunol. 1998;16:645–670. doi: 10.1146/annurev.immunol.16.1.645. [DOI] [PubMed] [Google Scholar]
- Nemazee D.A., Burki K. Clonal deletion of B lymphocytes bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Fulcher D.A., Lyons A.B., Korn S.L., Cook M.C., Koleda C., Parish C., Fazekas de St B., Groth, Basten A. The fate of self-reactive B cells depends primarily on the degree of antigen receptor engagement and availability of T cell help. J. Exp. Med. 1996;183:2313–2328. doi: 10.1084/jem.183.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnatz K., Kyburz D., Brinson D.C., Lee D.J., Von Damm A., Engelhart M., Corr M., Carson D.A., Tighe H. Rheumatoid factor B cell tolerance via autonomous Fas/FasL-independent apoptosis. Cell. Immunol. 1999;191:69–73. doi: 10.1006/cimm.1998.1415. [DOI] [PubMed] [Google Scholar]
- Kohler H., Kaplan D.R., Strayer D.S. Clonal depletion in neonatal tolerance. Science. 1974;186:643–644. doi: 10.1126/science.186.4164.643. [DOI] [PubMed] [Google Scholar]
- Claflin J.L., Lieberman R., Davie J.M. Clonal nature of the immune response to phosphorylcholine. II. Idiotypic specificity and binding characteristics of anti-phosphorylcholine antibodies. J. Immunol. 1974;112:1747–1756. [PubMed] [Google Scholar]
- Herzenberg L.A., Kantor A.B. B-cell lineages exist in the mouse. Immunol. Today. 1993;14:79–83. doi: 10.1016/0167-5699(93)90063-Q. [DOI] [PubMed] [Google Scholar]
- Ochsenbein A.F., Fehr T., Lutz C., Suter M., Brombacher F., Hengartner H., Zinkernagel R.M. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- Kaplan D.R., Quintans J., Kohler H. Clonal dominanceloss and restoration in adoptive transfer. Proc. Natl. Acad. Sci. USA. 1978;75:1967–1970. doi: 10.1073/pnas.75.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masmoudi H., Mota-Santos T., Huetz F., Coutinho A., Cazenave P.A. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int. Immunol. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Herzenberg L.A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R.R., Stall A.M., Herzenberg L.A. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur. J. Immunol. 1986;16:1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- Forster I., Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur. J. Immunol. 1987;17:521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- Rothstein T.L., Kolber D.L. Anti-Ig antibody inhibits the phorbol ester-induced stimulation of peritoneal B cells. J. Immunol. 1988;141:4089–4093. [PubMed] [Google Scholar]
- Murakami M., Tsubata T., Okamoto M., Shimizu A., Kumagai S., Imura H., Honjo T. Antigen-induced apoptotic death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature. 1992;357:77–80. doi: 10.1038/357077a0. [DOI] [PubMed] [Google Scholar]
- Lalor P.A., Stall A.M., Adams S., Herzenberg L.A. Permanent alteration of the murine Ly-1 B repertoire due to selective depletion of Ly-1 B cells in neonatal animals. Eur. J. Immunol. 1989;19:501–506. doi: 10.1002/eji.1830190314. [DOI] [PubMed] [Google Scholar]
- Kantor A.B., Merrill C.E., Herzenberg L.A., Hillson J.L. V-gene usage and N-region insertions in B-1a, B-1b and conventional B cells. Semin. Immunol. 1996;8:29–35. doi: 10.1006/smim.1996.0005. [DOI] [PubMed] [Google Scholar]
- Benedict C.L., Kearney J.F. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity. 1999;10:607–617. doi: 10.1016/s1074-7613(00)80060-6. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Asano M., Shinton S.A., Gui M., Allman D., Stewart C.L., Silver J., Hardy R.R. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- Herzenberg L.A. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- Pospisil R., Young-Cooper G.O., Mage R.G. Preferential expansion and survival of B lymphocytes based on VH framework 1 and framework 3 expression“positive” selection in appendix of normal and VH-mutant rabbits. Proc. Natl. Acad. Sci. USA. 1995;92:6961–6965. doi: 10.1073/pnas.92.15.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berberian L., Goodglick L., Kipps T.J., Braun J. Immunoglobulin VH3 gene productsnatural ligands for HIV gp120. Science. 1993;261:1588–1591. doi: 10.1126/science.7690497. [DOI] [PubMed] [Google Scholar]
- Silverman G.J., Roben P., Bouvet J.-P., Sasano M. Superantigen properties of a human sialoprotein involved in gut-associated immunity. J. Clin. Invest. 1995;96:417–426. doi: 10.1172/JCI118051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuridor R., Lyles R.H., Pirofski L. Quantitative and qualitative differences in the serum antibody profiles of human immunodeficiency virus-infected persons with and without Cryptococcus neoformans meningitis. J. Infect. Dis. 1999;180:1526–1535. doi: 10.1086/315102. [DOI] [PubMed] [Google Scholar]
- Schneider R.F., Rosen M.J. Pneumococcal infections in HIV-infected adults. Semin. Respir. Infect. 1999;14:237–242. [PubMed] [Google Scholar]
- Claflin J.L., Lieberman R., Davie J.M. Clonal nature of the immune response to phosphorylcholine. I. Specificity, class, and idiotype of phosphorylcholine-binding receptors on lymphoid cells. J. Exp. Med. 1974;139:58–73. doi: 10.1084/jem.139.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]