Abstract
The role played by antigenic peptides bound to major histocompatibility complex (MHC) molecules is evaluated with H2-DMα−/− mice. These mice have predominantly class II–associated invariant chain peptide (CLIP)-, not antigenic peptide–bound, MHC class II. H2-DMα−/− donor heart grafts survived three times longer than wild-type grafts and slightly longer than I-Aβ b−/− grafts. Proliferative T cell response was absent, and cytolytic response was reduced against the H2-DMα−/− grafts in vivo. Residual cytolytic T cell and antibody responses against intact MHC class I lead to eventual rejection. Removal of both H2-DMα and β2-microglobulin (β2m) in cardiac grafts lead to greater (8–10 times) graft survival, whereas removal of β2m alone did not have any effect. These results demonstrate the significance of peptide rather than just allogeneic MHC, in eliciting graft rejection.
Keywords: major histocompatibility complex class II, H2-DM, class II–associated antigenic peptide, alloreactivity, transplantation
Introduction
Allogeneic MHC represents a major barrier to transplant acceptance 1 2. Donor MHC antigens can trigger rejection through two pathways: (a) by interacting directly with recipient T cells, or (b) indirectly, as donor MHC-derived peptides expressed on recipient MHC molecules 3. Although both MHC class I and class II molecules are targets of allospecific responses, class II antigens play the predominant role in cardiac graft rejection, as elimination of class II expression significantly enhances cardiac graft survival, whereas eliminating class I has very little effect 4 5 6 7.
T cells recognize nominal antigen during an immune response as peptide complexed to self-MHC. However, it is unclear how recipient T cells interact with allogeneic donor MHC. The three main theories developed to explain alloreactivity differ in the posited role of the MHC-bound peptide 8 9 10 11 12. The first is the peptide-specific (or molecular mimicry) model, in which alloantigen is thought to mimic the structure of antigen complexed to self-MHC. Crystallographic analysis of alloreactive T cell clones has indicated that some molecular mimicry occurs. However, the TCR–MHC interactions in the self-MHC–X complex are substantially different from the interactions in the allo-MHC–Y complex 13 14 15. For example, there was an increase in the number of direct contacts between the TCR and polymorphic regions of the allo-MHC. This has the effect of decreasing TCR specificity for peptide. Alloreactive clones have, in general, been found to respond to a range of degenerate peptides 13 15 16 17 18 19 20. These observations are consistent with the second model, which is peptide-dependent allorecognition. Peptide-dependent alloreactive T cells respond to peptide-induced conformational changes in the MHC, but do not interact specifically with the peptide. According to the third model, alloreactive T cells contact polymorphic regions of allo-MHC in a peptide-independent fashion 8. In this case, polymorphisms in the MHC molecule itself are recognized by T cells, eliciting a strong alloresponse even in the absence of bound peptide. This possibility has been formally demonstrated in some instances of class I allorecognition 21 22 23, and in one case, peptide-independent CTLs were sufficient to reject allogeneic skin grafts 23. However, peptide-independent recognition has yet to be detected during an MHC class II–directed response.
Most of the evidence addressing the role of peptide in alloimmune responses could only be performed in simple in vitro systems using T cell clones. The recent development of mouse models expressing a single peptide–MHC class II complex has provided an experimental model to test this issue in vivo using vascularized organ grafts. One such mouse was generated by the inactivation of the H2-DMα gene, also called H2-Ma 24 25 26. H2-DM dimers are found in the antigen processing and presenting compartments of the class II pathway 27. There, it catalyzes the removal of the invariant chain–derived class II–associated invariant chain peptide (CLIP) from newly formed class II molecules 28 29 30. H2-DM further enhances peptide loading by facilitating the loading of foreign peptides onto MHC class II molecules 31 32 33. However, the dependence on H2-DM for peptide exchange varies between peptides and class II alleles. For example, I-Ak molecules are perhaps the least dependent upon H2-DM for peptide loading, and can spontaneously disassociate from CLIP and bind antigenic peptide. I-Ab molecules, on the other hand, are extremely dependent upon H2-DM expression for disassociation from CLIP and peptide loading 34 35 36 37.
H2-DMα−/− mice lack functional H2-DM dimers. As a result, most class II molecules on the surface of H2-DMα−/− cells are bound to the invariant chain–derived CLIP peptide 24 25 26. Despite restricting the peptide repertoire to a predominant peptide, CLIP, the level of class II expression is normal on cells isolated from these mice. However, it is clear that some non-CLIP peptides are bound with class II on H2-DMα−/− mice, and that some of the CD4+ T cells in H2-DMα−/− mice are selected by these non-CLIP peptides 38.
In this report, H2-DMα−/− mice were used to examine the role of peptide in allorecognition. H2-DMα−/− donor hearts grafted into fully allogeneic recipients display a reduced rate of rejection comparable to that observed for class II null donor grafts. The data presented suggest that targeting the peptides presented by allo-MHC may be an effective way of preventing graft rejection. This is the first report that takes advantage of these mice to demonstrate the significant role of peptide in an allograft response.
Materials and Methods
Animals.
(Balb/c × DBA/2)F1 (also known as CBYD2F1/J, H2d, hereafter referred to as CBY), C57BL/6 (B6, H2b), and β2-microglobulin (β2m)−/− (H2b) mice on the B6 background were purchased from The Jackson Laboratory. H2-DMα2/− mice were backcrossed onto the B6 (H2b) background for nine generations, and I-Aβ b−/− mice have been backcrossed onto the B6 background for >6 generations. B6-backcrossed H2-DMα2/− mice were also bred with β2m−/− mice and further intercrossed to produce double knockout (DKO) mice lacking expression of H2-DMα and β2m (H2-DMα2/− × β2m−/−). H2-DMα2/−, I-Aβ b−/−, and DKO mice were bred and maintained at the University of North Carolina at Chapel Hill. All mice were housed and maintained under specific pathogen-free conditions.
Mouse Heart Transplantation.
Vascularized heterotopic cardiac grafts were performed as described previously 39. In brief, donor and recipient animals were anesthetized with isoflurane, and the donor heart was harvested. The donor aorta and pulmonary artery were anastamosed to the recipient abdominal aorta and vena cava, respectively. The graft was monitored daily for the presence of palpable contractions, and was considered rejected when contractions ceased.
Control allografts (n = 14) consisted of donor hearts from B6 mice (H2b) transplanted into fully allogeneic CBY animals (H2d). For control isografts (n = 8), hearts from CBY mice were transplanted into CBY recipients. The experimental groups consisted of either H2-DMα2/− (n = 8), I-Aβ b−/− (n = 12), β2m−/− (n = 7), or DKO mice (H2-DMα2/− × β2m−/−, n = 6) donor hearts (H2b) transplanted into fully allogeneic CBY recipients (H2d). Statistical significance was assessed using the Mann-Whitney U Test.
In Vitro T Cell Responses.
MLRs were performed by incubating 2.5 × 105 T cell–enriched responders with titrated numbers of irradiated (2,500 rads) spleen cell stimulators in 96-well plates for 4–6 d at 37°C and 5% CO2. Cells were cultured in complete DMEM containing 10% FCS, 25 mM Hepes, 2 mM l-glutamine, 1% nonessential amino acids, 50 μM 2-ME, 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate. Cultures were pulsed with 1 μCi [3H]thymidine per well 12–18 h before harvest.
Cell-mediated alloimmunity was assessed by the DNA fragmentation assay (JAM test) 40. Heart graft recipient spleen cells were harvested and cocultured with equal numbers of irradiated (2,500 rads) allogeneic spleen cells from the same mouse strain as the original donor heart graft in complete DMEM for 6 d. Effectors were then recovered, washed, and incubated with labeled targets (10,000 per well) at the indicated E/T ratios for 3–4 h before being harvested and counted. Targets consisted of B6 and CBY Con A blasts that were labeled with [3H]thymidine (5 μCi/ml) for 3–6 h before use. Percent cytotoxicity was determined according to the formula [(S−E)/S] × 100, where E is the average experimental release of triplicate samples and S is the average spontaneous release of numerous samples.
Cytokine RNA Analysis.
At the time of harvest, a portion of the recipient animals' hearts, both native and donor, were snap frozen in LN2. At a later date, Trizol (GIBCO BRL) was used to extract total RNA from these tissues. Cytokine RNA levels were determined by RNase protection analysis following the kit manufacturer's protocol (RNA probe sets mCK-1 and mCK-3b; BD PharMingen). Approximately 2 μg total RNA was analyzed per sample. Protected bands were quantitated by phosphoimaging (Molecular Dynamics), and were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Immunohistochemistry.
Donor hearts were harvested 7 d after grafting. A portion of the grafted heart was frozen in OCT embedding compound (Tissue-Tek) before sectioning (5 μm) and staining. Acetone-fixed frozen sections were stained overnight at 4°C with biotinylated antibody against either CD4, CD8, or CD11b (Mac-1; BD PharMingen). The sections were then washed in 1× PBS and developed using the ABC and DAB-Ni reagent kits according to the manufacturers' instructions (Vector Laboratories).
Allospecific Antibody Quantitation and Flow Cytometry.
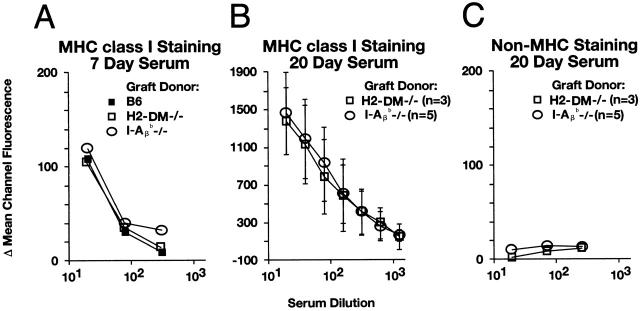
Allospecific antibodies were detected by indirect, two-color flow cytometry. At the time of graft failure, serum samples were collected and frozen for later analysis. On the day of the assay, splenic lymphocytes were collected from CBY mice, class II–deficient (I-Aβ b−/−) mice, and mice lacking expression of both class II and class I (I-Aβ b−/− × β2m−/−). CBY splenic lymphocytes served as a negative control, and MHC-negative (I-Aβ b−/− × β2m−/−) lymphocytes were used to demonstrate the MHC specificity of recipient serum alloantibodies. I-Aβ b−/− spleen cells, which lack surface MHC class II expression, were used to detect alloantibodies directed against donor MHC class I. Splenic lymphocytes were resuspended in HBSS containing 3% FCS. Red blood cells were lysed by incubation with 155 mM ammonium chloride for 5 min at 4°C. Cell surface staining was performed by preincubating 106 cells with FcBlock (BD PharMingen) for 30 min at 4°C. Diluted recipient serum or normal CBY mouse serum, as a negative control, was then added to the cells and further incubated for 1 h at 4°C. The cells were then washed three times and incubated with both a FITC-labeled goat anti–mouse Ig antibody (BD PharMingen), to detect Ig bound to the cell surface, and a PE-labeled rat anti–mouse B220 antibody (BD PharMingen), to identify B cells. Lymphocytes were identified according to their forward and side scatter profiles, and a total of 10,000 events was collected using a Becton Dickinson FACScan™. B220+ cells were selected and analyzed for alloantibody staining using the Cicero software program (Cytomation). Alloantibody staining is presented as the change in mean channel fluorescence (ΔMCF = MCF sample dilution − MCF normal mouse serum control).
For surface MHC class II analysis, cell suspensions were prepared and cell staining was performed essentially as described above for alloantibody quantitation. The anti–I-Ab antibodies M5/114.15.2, AF6-120.1, KH74, and their corresponding isotype controls were purchased from BD PharMingen. The anti–class II antibody NIMR-4 and its isotype control were purchased from Southern Biotechnology Associates, Inc. Cells were analyzed using a FACScan™ flow cytometer and CELLQuest™ software (Becton Dickinson).
Results
Naive Allogeneic T Cells Do Not Respond to H2-DMα−/− APCs In Vitro.
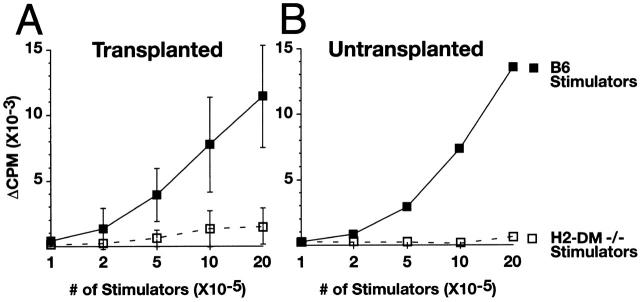
Alloreactive T cells have been suggested to represent 1–10% of the total T cell population 11. This observation is supported by the strong in vitro proliferation typically associated with an MLR. Two previous reports found H2-DMα−/− spleen cells are defective in stimulating the proliferation of allogeneic T cells 24 25, whereas a third group reported that H2-DMα−/− APCs induce allogeneic T cell proliferation 26. Each of these groups used H2-DMα−/− mice on a mixed genetic background. For this study, all of the H2-DMα−/− mice have been backcrossed to B6 mice for nine generations. This is particularly critical for these experiments because (a) background genes greatly influence the outcome of transplant acceptance and/or rejection; (b) other transgenic mice used in this report are backcrossed onto the same genetic background; and (c) H2-DM has the most dramatic role in loading peptides onto class II from H2b mice 34 35 36 37. To evaluate the ability of these H2-DMα−/− mice to stimulate allogeneic T cells, a one-way MLR was performed. Allogeneic T cells proliferate minimally in response to B6-backcrossed H2-DMα−/− APCs, even though they responded well to B6 APCs (Fig. 1 A). As expected, allogeneic T cells also failed to react against H2-DMα−/− cells from the mixed B6 × 129 background (Fig. 1 B). Thus, allogeneic CLIP–I-Ab complexes do not trigger significant allospecific proliferation in vitro.
Figure 1.

Naive CBY T cells do not respond to H2-DMα−/− APCs. Splenocytes from H2-DMα−/− and B6 mice were used to stimulate T cells isolated from naive CBY mice that were not implanted with allografts. Mixed lymphocyte reactions were cultured for 5–6 d. Cell proliferation was determined by measuring uptake of [3H]thymidine added 12–18 h before harvest. Splenocytes from (A) B6-backcrossed H2-DMα−/− mice and (B) mixed-background B6 × 129 H2-DMα−/− mice are inefficient activators of allogeneic T cell proliferation. (C) The reduced proliferative response is not due to a decrease in surface MHC class II expression. As expected, both mixed-background and inbred H2-DMα−/− mice express a level of MHC class II equivalent to that of wild-type B6 animals, as detected by three anti–MHC class II antibodies. KH74 detects MHC class II–CLIP complexes poorly, and as expected, produced a smaller signal when used to label H2-DMα−/− cells.
The inability to respond to H2-DMα−/− cells is not due to a decrease in the amount of surface MHC class II expressed by our inbred animals. The MHC class II staining profile of spleen cells from inbred H2-DMα−/− animals is identical to that displayed by the mixed-background animals. Both mixed-background and inbred H2-DMα−/− mice express as much surface MHC class II as wild-type B6 animals when detected with the anti–class II antibodies AF6-120.1, NIMR-4, and M5/114 (Fig. 1 C). The anti–I-Ab antibody KH74 stains H2-DMα−/− cells poorly, consistent with this antibody's previously suggested low affinity for CLIP–I-Ab complexes 24.
Elimination of H2-DMα Inhibits Allospecific Recognition In Vivo.
Allogeneic class II is the major contributor to the rejection of vascularized cardiac grafts 4 5 6 7. In these previous studies, the use of I-Aβ b−/− mice as donors significantly prolonged the survival of the graft, and in some donor/recipient strain combinations led to indefinite graft survival. However, the role of the MHC-bound peptide has not been evaluated in vivo. H2-DMα−/− mice allow this question to be addressed because, although their level of class II expression is normal, virtually all the class II molecules expressed by these animals are bound to the CLIP peptide.
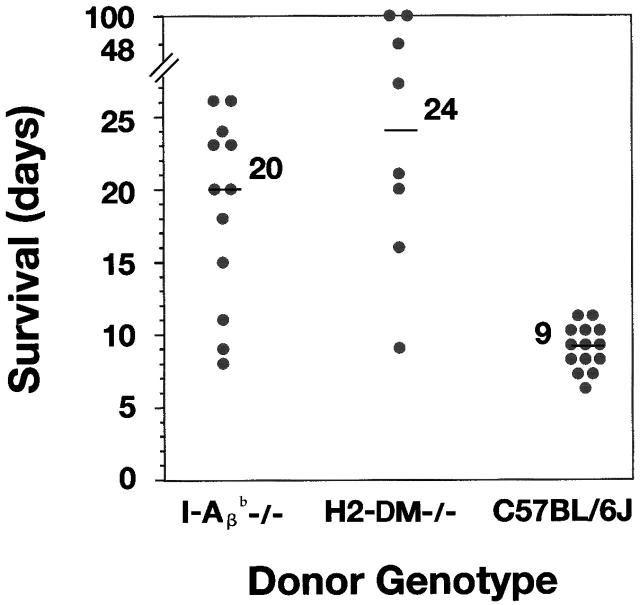
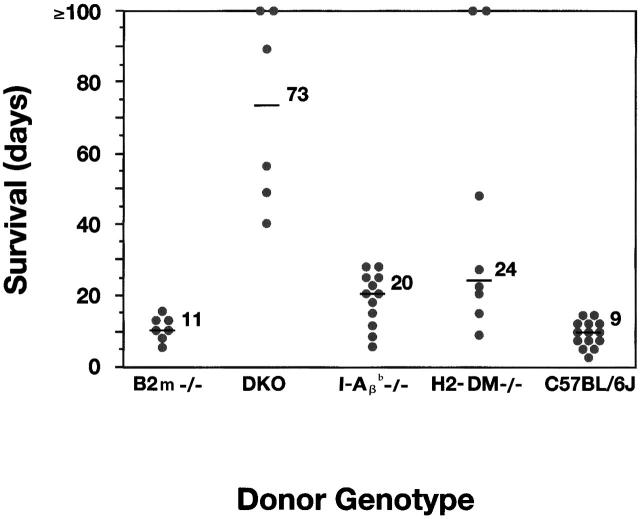
As donors, H2-DMα−/− heart grafts survived for a significantly prolonged time compared with B6 controls when transplanted into allogeneic recipient mice. The median survival of H2-DMα−/− grafts is 24 d, with an average survival of >40 d (Fig. 2). The median and average survival time of control B6 grafts is 9 d, which is similar to our previous experience. The median survival of H2-DMα−/− grafts was slightly greater than that observed for allogeneic class II negative control I-Aβ b−/− grafts, with a median survival of 20 d. Therefore, targeting peptide presentation effectively eliminated recognition of allogeneic class II.
Figure 2.
(A) Cardiac grafts from inbred H2-DMα−/− mice, as well as inbred I-Aβ b−/− mice, exhibit enhanced survival in our allogeneic hosts. Cardiac grafts from the indicated donors (H2b) were grafted into the abdomen of CBY (H2d) recipient mice. Graft survival was monitored daily by palpation of the abdomen. Each point represents an individual, and the line in each group represents the median survival. The median survival of cardiac grafts from B6 wild-type allogeneic controls was 9 d (n = 14), from H2-DMα−/− mice, 24 d (n = 8, P < 0.0007), and from I-Aβ b−/− mice, 20 d (n = 12, P < 0.0006). Control isografts were not rejected (n = 8, data not shown). Statistical significance was determined using the Mann-Whitney U test compared with B6 control donors.
Even more surprising, three of the H2-DMα−/− grafts survived for a greatly prolonged period of time, with two grafts surviving for >100 d, and one graft surviving for 48 d (Fig. 2). Although indefinite survival of I-Aβ b−/− cardiac grafts has been seen with some donor/recipient strain combinations, the donor/recipient strains used in this study result in the rejection of I-Aβ b−/− cardiac grafts, with a median survival of 20 d, and no I-Aβ b−/− grafts survived beyond 30 d. The prolonged survival of some H2-DMα−/− grafts suggests that the elimination of peptide presentation may be even more effective at delaying or preventing graft rejection than the elimination of donor class II expression.
In Vivo Stimulation with H2-DMα−/− Grafts Failed to Expand Alloreactive T Cells.
One possible explanation for the delayed rejection of H2-DMα−/− grafts is that the precursor frequency of CLIP–I-Ab–specific T cells is low in the recipients, and it simply takes longer for these cells to expand and efficiently mediate graft rejection. If this were the case, allogeneic T cells that recognize H2-DMα−/− cells should be expanded after in vivo immunization with the cardiac allograft. To determine if donor-specific T cells had developed in recipients of H2-DMα−/− grafts, an MLR analysis of spleen cells was performed from animals that had received cardiac allografts. The spleens from graft recipients were harvested 7 d after transplant, enriched for T cells, and stimulated with irradiated allogeneic cells. Primed allogeneic T cells from recipients of H2-DMα−/− hearts remained refractory to stimulation by H2-DMα−/− APCs, whereas these same cells responded well to B6 stimulators (Fig. 3 A). The same pattern was observed for T cells from naive animals (Fig. 3 B). Although H2-DMα−/− cells express normal levels of class II, the recipients of H2-DMα−/− hearts failed to develop significant CD4+ T cell reactivity to CLIP– I-Ab complexes.
Figure 3.
Primed CBY lymphocytes, which reject H2-DMα−/− grafts, do not respond to H2-DMα−/− stimulator cells in a secondary MLR. Total splenocytes from H2-DMα−/− and B6 mice were isolated, irradiated, and combined with spleen cells isolated from recipients of H2-DMα−/− grafts. (A) These cells responded to irradiated stimulator cells from B6 controls (▪), but not H2-DMα−/− animals (□). Data are means ± SD of four animals. (B) The same pattern is observed from naive animals. Mixed lymphocyte reactions were cultured for 5–6 d. Cell proliferation was determined by measuring uptake of [3H]thymidine added 12–18 h before harvest.
T Cells from H2-DMα−/− Graft Recipients Generate Type 1 and Type 2 Cytokine RNA.
To explore the underlying mechanism for the enhanced survival of H2-DMα−/− grafts, cytokine RNA profile was analyzed by isolating RNA from the donor heart and performing an RNase protection analysis. Those cytokines with detectable levels of RNA are presented in Fig. 4, and all the cytokines tested are listed in Table . Increases in the type 1 cytokine IFN-γ as well as IL-15, IL-6, and IL-10 were observed in all grafted hearts (Fig. 4). H2-DMα−/− donor grafts from two time points were studied: day 7 approximates the time wild-type grafts are typically rejected, and day 20 represents the time when H2-DMα−/− grafts are rejected. However, there were no differences in the level of RNA expression between the different donor groups. There were also increases in the tissue of toxic cytokines TNF-α and lymphotoxin β, but again their levels did not appear to differ between donor groups or to correlate with the rate of rejection. This is not entirely surprising, as enhanced graft survival may not be associated with cytokine expression, although an association between rejection episodes and Th1 cytokine expression is common 41 42. Based on the results presented here, the enhanced survival of H2-DMα−/− grafts is not due to a deviation of the immune response to a different cytokine profile.
Figure 4.
Graft recipients express both Th1 and Th2 cytokine RNA. Donor hearts were recovered from recipient animals 7 d after transplantation or at the time of graft failure. Total RNA was isolated and analyzed by RNase protection analysis for the presence and level of various cytokine transcripts. No significant difference in the level of any cytokine was observed between groups. The cytokine profile displayed by each recipient group was identical. Data are means ± SD of RNA samples from the indicated number of animals. MIF, macrophage migration inhibitory factor.
Table 1.
Cytokine RNA Expression of Donor Grafts
| Donor | |||
|---|---|---|---|
| Cytokine | B6 | H2-DMα2/− | I-Ab−/− |
| IFN-γ | + | + | + |
| IL-15 | +/− | +/− | +/− |
| IL-6 | +/− | +/− | +/− |
| IL-10 | +/− | +/− | +/− |
| MIF | +++ | +++ | +++ |
| LTβ | + | ++ | + |
| TGF-β | ++ | ++ | ++ |
| TNF-α | + | + | + |
| TNF-β | +/− | +/− | +/− |
| IL-2 | − | − | − |
| IL-4 | − | − | − |
| IL-5 | − | − | − |
| IL-9 | − | − | − |
| IL-13 | − | − | − |
| IFN-β | − | − | − |
Cytokine RNA levels were determined by RNase protection analysis of donor tissue harvested 7 d after graft or at the time of rejection. Both type 1 and type 2 cytokines were elevated to an equal extent in each recipient group (shown in Fig. 4). MIF, macrophage migration inhibitory factor; LTβ, lymphotoxin β.
CTL Production Is Reduced in Recipients of H2-DMα−/− Grafts.
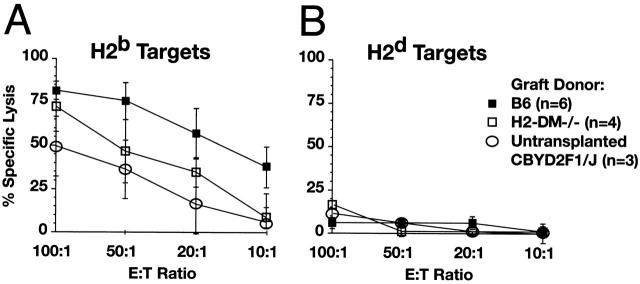
The H2-DMα defect only affects peptide loading on class II. Class I peptide diversity is normal in these mice. To determine if recipients of H2-DMα−/− hearts produce normal cytotoxic T cell activity, the presence of cytotoxic T cells in the recipients of H2-DMα−/− cardiac grafts was evaluated by CTL assay. In this case, recipients of H2-DMα−/− hearts are less effective than recipients of B6 hearts at lysing labeled H2b targets; however, CTL activity is clearly detectable (Fig. 5). Activation of alloreactive CTLs can occur through direct recognition of allo–class I complexes by recipient CTLs, and this class I–directed response may be largely responsible for the rejection of H2-DMα−/− and I-Aβ b−/− grafts. This reduced, albeit detectable, CTL response parallels the delayed but present allograft rejection and may reflect a decrease in CD4+ T cell help.
Figure 5.
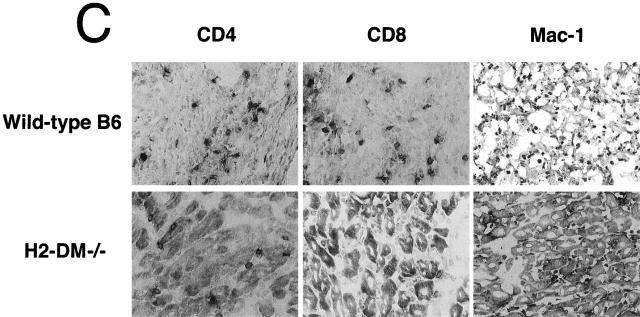
Recipients of H2-DMα−/− cardiac grafts have reduced CTL activity compared with recipients of B6 grafts. Splenocytes from recipients of H2-DMα−/− or B6 grafts were harvested 7 d after transplant. Isolated splenocytes were restimulated with irradiated spleen cells from the same donor strain used for the original graft. After 7 d in culture, the restimulated recipient cells were harvested and assayed for the ability to lyse labeled targets. (A) CTLs from recipients of H2-DMα−/− (□) or B6 (▪) hearts efficiently lysed allogeneic H2b targets, but (B) did not lyse syngeneic H2d control targets. Data are means ± SD of at least three animals. (C) Consistent with the reduced CTL response, immunohistochemistry of 7-d grafts reveals a clear decrease in the number of infiltrating CD8+ T cells, as well as decreases in the number of CD4+ and Mac-1+ cells infiltrating H2-DMα−/− grafts.
H2-DMα−/− Grafts have Fewer Infiltrating Leukocytes.
Decreased proliferative and CTL responses may reflect a reduction in the number of cells infiltrating the graft. Immunohistochemistry of donor cardiac tissue harvested 7 d after grafting identified a decrease in the number of infiltrating CD4+ T cells, CD8+ T cells, and Mac-1+ cells in H2-DMα−/− grafts compared with wild-type B6 grafts (Fig. 5 C). In general, H2-DMα−/− grafts had a small number of CD4+ T cells and CD8+ T cells within the myocardium, and only slightly more cells within the epicardium. However, a substantial number of Mac-1+ cells are present throughout H2-DMα−/− grafts. In contrast, sections of wild-type B6 grafts stained for CD4+, CD8+, and Mac-1+ cells more uniformly throughout the section. The level of tissue destruction and the degree of staining for CD4+ and CD8+ cells was much greater in wild-type B6 grafts than in H2-DMα−/− grafts. Mac-1+ cells were also increased in wild-type B6 grafts. Consistent with a reduced CTL response, there is a clear decrease in the number of CD8+ T cells infiltrating H2-DMα−/− grafts. However, recipients of H2-DMα−/− grafts still develop significant CTL activity against the graft, which likely contributes to eventual rejection.
Recipients of H2-DMα−/− Grafts Generate Class I–specific Alloantibodies.
Recipients of H2-DMα−/− grafts generated CTL activity against class I MHC. To determine if alloantibodies against class I MHC are also generated, the serum from graft recipients was assessed for alloreactivity. Alloantibody levels were quantitated by FACS® analysis (as described in the Materials and Methods). Recipient serum did not stain B cells from CBY mice (data not shown) or B cells from mice lacking expression of both MHC class I and class II (β2m−/− × I-Aβ b−/−; Fig. 6 C). This result indicates that the main target of recipient alloantibodies is donor MHC. Splenic B cells from I-Aβ b−/− mice, which lack surface expression of MHC class II, were used to detect alloantibodies directed against MHC class I. Comparable levels of class I–directed alloantibodies were detected in H2-DMα−/−, I-Aβ b−/−, and B6 controls. When day 7 time-matched samples were compared, each recipient group had similar levels of alloantibodies (Fig. 6 A). IgM alloantibodies were a substantially higher proportion of 7-d serum alloreactivity than of H2-DMα−/− and I-Aβ b−/− samples collected at later time points, after isotype switching had occurred (data not shown). Even higher levels of alloantibodies were detected by 20 d, the point at which H2-DMα−/− and I-Aβ b−/− grafts were rejected (Fig. 6 B). This suggests that substantial seroreactivity against class I MHC molecules is being produced in these recipients, and likely contributes to their rejection.
Figure 6.
Recipients of H2-DMα−/− grafts are efficient producers of alloantibodies. The presence and level of serum alloantibodies was determined by FACS®. (A) All three recipient groups produce nearly equal levels of alloantibodies directed against allogeneic class I 7 d after transplant. (B) Serum collected at the time of rejection from recipients of I-Aβ b−/− or H2-DMα−/− grafts, ∼20 d, have an elevated level of class I–specific alloantibodies. (C) Recipient sera did not stain B cells from mice lacking MHC expression (β2m−/− × I-Aβ b−/−). In B and C, data are means ± SD of serum samples from at least three animals.
Reduction of Class I Expression Further Enhances H2-DMα−/− Cardiac Graft Survival.
The data presented thus far suggest that rejection of H2-DMα−/− grafts may be directed against class I differences between donor and recipient animals. To further explore this possibility, H2-DMα−/− mice were bred with β2m−/− mice to generate donor animals deficient for class I expression, but which expressed CLIP–I-Ab (H2-DMα−/− × β2m−/−). Grafts from donor animals deficient only for β2m were rejected similarly to wild-type B6 controls, with median survival 11 vs. 9 d, respectively (Fig. 7). In contrast, donor grafts from H2-DMα−/− × β2m−/− DKO animals survived significantly longer than control grafts and even longer than H2-DMα−/− grafts, with a median survival of >70 d. All the DKO grafts survived >40 d. Decreasing class I expression alone did not alter graft survival, but in combination with the removal of H-2DMα−/− greatly enhanced graft survival. Therefore, the antigraft response seen in H2-DMα−/− mice was mainly directed against class I and not CLIP–I-Ab.
Figure 7.
Rejection of cardiac grafts from mice lacking both β2m and H2-DMα (β2m−/− × H2-DMα−/−, DKO). Cardiac grafts from the indicated donors (H2b) were grafted into the abdomen of CBY (H2d) recipient mice. Graft survival was monitored by palpation of the abdomen. Each point represents an individual recipient, and the line in each group represents the median survival. Syngeneic grafts from CBY mice were not rejected (data not shown). The median survival of cardiac grafts from β2m−/− controls was 11 d (n = 7), and from DKO donors was >70 d (n = 6, P = 0.0027). Statistical significance was determined using the Mann-Whitney U test compared with β2m−/− control donors.
Discussion
Previous work established that direct recognition of allo-MHC may occur through peptide-specific, peptide-dependent, and/or peptide-independent mechanisms. One group has even shown that peptide-independent CD8+ T cell lines reject skin grafts 23. However, it has not been possible to determine the relative contribution of each of these responses to the rejection of a primary graft. Here, we demonstrate the importance of peptide, not just allogeneic MHC, in the rejection of vascularized cardiac grafts. Our results are also supported by a recent report that failed to detect the generation of any allo-MHC–CLIP–specific T cells in mice primed with H2-DMα−/− spleen cells 43.
Based on our results, peptide-independent allorecognition of class II is unlikely to contribute to the acute rejection of H2-DMα−/− grafts. However, it is more difficult to determine the relative contribution of peptide-specific versus peptide-dependent responses. Here, the distinction is based on the extent of peptide specificity. Even alloreactive T cell clones that initially seemed to support the peptide-specific model have been shown to cross-react with a related set of degenerate peptides 13 15 16 17 18 19 20. Of course, TCR cross-reactivity is not unique to allorecognition 44 45 46 47 48 49. One estimation has each T cell capable of responding to 3 × 105 different peptides 50.
One school of thought is that peptide-dependent responses are directed toward peptide-induced conformational changes in MHC, with little or no actual interaction between the TCR and peptide. Because distinct peptides induce different conformational changes, each peptide will stimulate distinct sets of peptide-dependent T cells. This paper clearly proves that one specific peptide, CLIP, is insufficient to globally activate peptide-dependent T cell responses. Peptide-dependent recognition does not occur in recipients of H2-DMα−/− grafts at a level strong enough to mediate rapid rejection when only CLIP–I-Ab is provided. The most straightforward explanation is that the enhanced survival of the H2-DMα−/− grafts is primarily due to the inhibition of the peptide-specific response.
Direct recognition of allo-MHC by peptide-specific and/or peptide-dependent recipient T cells provides a major contribution to the rejection of vascularized heart grafts. This interaction is dependent upon a proper structure of the class II molecule. Because CLIP–I-Ab molecules from H2-DMα−/− mice are unstable in SDS and are recognized by some, but not all, class II–specific antibodies, it is possible that the CLIP– I-Ab complex has an unusual structure that makes it unsuitable as a model for allorecognition. Although the structure of mouse CLIP–I-Ab has not been reported, the structure of human CLIP–DR3 is known and has been shown to be almost identical to the structure of the influenza virus hemagglutinin peptide (306–318)–DR1 complex 51. It is unlikely that the binding of CLIP to mouse class II is fundamentally different than the binding of CLIP to human DR3. Therefore, the CLIP–I-Ab complex should be reflective of the structure of other peptide–mouse class II complexes. Although SDS instability does suggest a low-affinity interaction between class II and peptide, it does not indicate a structural abnormality. In fact, SDS-unstable complexes have been shown to be capable of inducing T cell responses in other systems 52.
It is also likely that some of the MHC molecules from the H2 DMα−/− animals are complexed with peptides other than CLIP. Although the I-Ab haplotype is the most dependent upon H2-DM for removal of CLIP, some mouse haplotypes (e.g., I-Ak, I-Ad) can exchange peptide in the absence of H2-DM 34 35 36 37. Although peptide loading experiments showed significant impairment of class II peptide exchange in the H2-DMα−/− animals 25 26 43, it is clear that not all class II molecules are bound to CLIP 38 53. This suggests that either the level of expression of non-CLIP peptides is too low to induce a response, that the non-CLIP peptides are nonactivating, or that the CLIP–MHC complex is acting similarly to an altered peptide ligand, not only failing to induce a response but actually inhibiting responses directed toward other peptide–MHC complexes expressed on the same cell. It has been shown that an abundance of an altered peptide ligand on a cell can reduce or remove positive responses to activating peptides present on the same cell 54.
Allogeneic MHC has been suggested to be the major mediator of graft rejection, either through the direct interaction and stimulation of alloreactive T cells, or indirectly as a source of allogeneic peptides that are acquired and presented by recipient APCs. I-Aβ b−/− grafts, which lack MHC class II expression, can neither stimulate alloreactive T cells directly nor provide MHC class II peptides to stimulate alloreactive T cells indirectly. On the other hand, H2-DMα−/− grafts do not appear to interact directly with alloreactive T cells, but could provide MHC class II peptides to stimulate alloreactive T cells indirectly. When the survival of I-Aβ b−/− and H2-DMα−/− grafts is compared, a complete absence of class II expression is no more effective at prolonging graft survival than removing peptide diversity. Therefore, indirectly presented MHC class II–derived peptides are not primarily responsible for the accelerated rejection of B6 grafts by CBY hosts.
The absence of class II does not prolong the survival of all grafts. For example, class I–deficient but not class II–deficient islet grafts are accepted indefinitely 55 56, whereas skin grafts are rapidly rejected regardless of MHC expression 57 58. Also, the importance of minor antigens in some instances of graft rejection cannot be ignored. Consider that certain strain combinations acutely reject cardiac grafts, even though they are matched at both MHC loci 59 60 61. Nevertheless, the importance of the MHC in graft rejection is indisputable, and is viewed as a potential point of therapeutic intervention.
The bulk of our evidence strongly favors the interpretation that peptide plays a crucial role in the recognition and rejection of vascularized cardiac grafts. Direct recognition by peptide-specific and/or peptide-dependent T cells is the key mediator of antigraft responses. However, indirect recognition of donor peptides may, in some cases, generate an antigraft response of equal intensity. The data presented here not only validate targeting donor class II expression as a means of reducing graft rejection, but allow this approach to be taken one step further. Dictating which peptide(s) donor class II molecules can express may enhance graft survival more effectively than eliminating donor class II expression.
Acknowledgments
This work was supported by National Institutes of Health grants AI-41580, AI-29564, AI-41751, and DK-38108, and National Multiple Sclerosis Society grant RG1725 to J.P.-Y. Ting; National Institutes of Health grant DK-38108 to T. Coffman; a National Kidney Foundation Fellowship to W.J. Brickey; and a National Institutes of Health predoctoral fellowship to N.J. Felix.
Footnotes
Abbreviations used in this paper: β2m, β2-microglobulin; B6, C57BL/6; CBY, CBYD2F1/J; CLIP, class II–associated invariant chain peptide; DKO, double knockout.
References
- Gorer P.A. The genetic and antigenic basis of tumor transplantation. J. Pathol. Bacteriol. 1937;44:691. [Google Scholar]
- Snell G.D. Methods for the study of histocompatibility genes. J. Genet. 1948;49:87. doi: 10.1007/BF02986826. [DOI] [PubMed] [Google Scholar]
- Auchincloss H., Jr., Sultan H. Antigen processing and presentation in transplantation. Curr. Opin. Immunol. 1996;8:681–687. doi: 10.1016/s0952-7915(96)80086-0. [DOI] [PubMed] [Google Scholar]
- Lim S.M., White D.J., Calne R.Y. Minor and class I MHC incompatibilities do not cause rejection of heart grafts but influence the rejection of skin grafts. Transplant. Proc. 1987;19:4229–4230. [PubMed] [Google Scholar]
- Stepkowski S.M., Raza-Ahmad A., Duncan W.R. The role of class I and class II MHC antigens in the rejection of vascularized heart allografts in mice. Transplantation. 1987;44:753–759. doi: 10.1097/00007890-198712000-00006. [DOI] [PubMed] [Google Scholar]
- Campos L., Naji A., Deli B.C., Kern J.H., Kim J.I., Barker C.F., Markmann J.F. Survival of MHC-deficient mouse heterotopic cardiac allografts. Transplantation. 1995;59:187–191. [PubMed] [Google Scholar]
- Qian S., Fu F., Li Y., Lu L., Rao A.S., Starzl T.E., Thomson A.W., Fung J.J. Impact of donor MHC class I or class II antigen deficiency on first- and second-set rejection of mouse heart or liver allografts. Immunology. 1996;88:124–129. doi: 10.1046/j.1365-2567.1996.d01-633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M.J. High determinant density may explain the phenomenon of alloreactivity. Immunol. Today. 1984;5:128. doi: 10.1016/0167-5699(84)90233-0. [DOI] [PubMed] [Google Scholar]
- Oldstone M.B. Molecular mimicry and autoimmune disease Cell 50 1987. 819 820[published erratum at 51:878] [DOI] [PubMed] [Google Scholar]
- Kourilsky P., Claverie J.M. MHC-antigen interactionwhat does the T cell receptor see? Adv. Immunol. 1989;45:107–193. doi: 10.1016/s0065-2776(08)60693-8. [DOI] [PubMed] [Google Scholar]
- Sherman L.A., Chattopadhyay S. The molecular basis of allorecognition. Annu. Rev. Immunol. 1993;11:385–402. doi: 10.1146/annurev.iy.11.040193.002125. [DOI] [PubMed] [Google Scholar]
- Tallquist M.D., Pease L.R. Alloreactive 2C T cells recognize a self peptide in the context of the mutant Kbm3 molecule. J. Immunol. 1995;155:2419–2426. [PubMed] [Google Scholar]
- Daniel C., Horvath S., Allen P.M. A basis for alloreactivityMHC helical residues broaden peptide recognition by the TCR. Immunity. 1998;8:543–552. doi: 10.1016/s1074-7613(00)80559-2. [DOI] [PubMed] [Google Scholar]
- Speir J.A., Garcia K.C., Brunmark A., Degano M., Peterson P.A., Teyton L., Wilson I.A. Structural basis of 2C TCR allorecognition of H-2Ld peptide complexes. Immunity. 1998;8:553–562. doi: 10.1016/s1074-7613(00)80560-9. [DOI] [PubMed] [Google Scholar]
- Zhao R., Loftus D.J., Appella E., Collins E.J. Structural evidence of T cell xeno-reactivity in the absence of molecular mimicry. J. Exp. Med. 1999;189:359–370. doi: 10.1084/jem.189.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr M., Slanetz A.E., Boyd L.F., Jelonek M.T., Khilko S., al-Ramadi B.K., Kim Y.S., Maher S.E., Bothwell A.L., Margulies D.H. T cell receptor-MHC class I peptide interactionsaffinity, kinetics, and specificity. Science. 1994;265:946–949. doi: 10.1126/science.8052850. [DOI] [PubMed] [Google Scholar]
- Sykulev Y., Brunmark A., Jackson M., Cohen R.J., Peterson P.A., Eisen H.N. Kinetics and affinity of reactions between an antigen-specific T cell receptor and peptide-MHC complexes Immunity. 1 1994. 15 22a [DOI] [PubMed] [Google Scholar]
- Sykulev Y., Brunmark A., Tsomides T.J., Kageyama S., Jackson M., Peterson P.A., Eisen H.N. High-affinity reactions between antigen-specific T-cell receptors and peptides associated with allogeneic and syngeneic major histocompatibility complex class I proteins Proc. Natl. Acad. Sci. USA 91 1994. 11487 11491b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist M.D., Yun T.J., Pease L.R. A single T cell receptor recognizes structurally distinct MHC–peptide complexes with high specificity. J. Exp. Med. 1996;184:1017–1026. doi: 10.1084/jem.184.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist M.D., Weaver A.J., Pease L.R. Degenerate recognition of alloantigenic peptides on a positive-selecting class I molecule. J. Immunol. 1998;160:802–809. [PubMed] [Google Scholar]
- Rojo S., Lopez D., Calvo V., Lopez de Castro J.A. Conservation and alteration of HLA-B27-specific T cell epitopes on mouse cells. Implications for peptide-mediated alloreactivity. J. Immunol. 1991;146:634–642. [PubMed] [Google Scholar]
- Villadangos J.A., Galocha B., Lopez de Castro J.A. Unusual topology of an HLA-B27 allospecific T cell epitope lacking peptide specificity. J. Immunol. 1994;152:2317–2323. [PubMed] [Google Scholar]
- Smith P.A., Brunmark A., Jackson M.R., Potter T.A. Peptide-independent recognition by alloreactive cytotoxic T lymphocytes (CTL) J. Exp. Med. 1997;185:1023–1033. doi: 10.1084/jem.185.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung-Leung W.P., Surh C.D., Liljedahl M., Pang J., Leturcq D., Peterson P.A., Webb S.R., Karlsson L. Antigen presentation and T cell development in H2-M-deficient mice. Science. 1996;271:1278–1281. doi: 10.1126/science.271.5253.1278. [DOI] [PubMed] [Google Scholar]
- Martin W.D., Hicks G.G., Mendiratta S.K., Leva H.I., Ruley H.E., Van Kaer L. H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation, and T cell repertoire selection. Cell. 1996;84:543–550. doi: 10.1016/s0092-8674(00)81030-2. [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Wolf P., Tourne S., Waltzinger C., Dierich A., Barois N., Ploegh H., Benoist C., Mathis D. Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell. 1996;84:531–541. doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- Lindstedt R., Liljedahl M., Peleraux A., Peterson P.A., Karlsson L. The MHC class II molecule H2-M is targeted to an endosomal compartment by a tyrosine-based targeting motif. Immunity. 1995;3:561–572. doi: 10.1016/1074-7613(95)90127-2. [DOI] [PubMed] [Google Scholar]
- Denzin L.K., Robbins N.F., Carboy-Newcomb C., Cresswell P. Assembly and intracellular transport of HLA-DM and correction of the class II antigen-processing defect in T2 cells. Immunity. 1994;1:595–606. doi: 10.1016/1074-7613(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Morris P., Shaman J., Attaya M., Amaya M., Goodman S., Bergman C., Monaco J.J., Mellins E. An essential role for HLA-DM in antigen presentation by class II major histocompatibility molecules. Nature. 1994;368:551–554. doi: 10.1038/368551a0. [DOI] [PubMed] [Google Scholar]
- Fling S.P., Arp B., Pious D. HLA-DMA and -DMB genes are both required for MHC class II/peptide complex formation in antigen-presenting cells. Nature. 1994;368:554–558. doi: 10.1038/368554a0. [DOI] [PubMed] [Google Scholar]
- Denzin L.K., Cresswell P. HLA-DM induces CLIP dissociation from MHC class II alpha beta dimers and facilitates peptide loading. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- Sloan V.S., Cameron P., Porter G., Gammon M., Amaya M., Mellins E., Zaller D.M. Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature. 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- Sherman M.A., Weber D.A., Jensen P.E. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- Wolf P.R., Tourne S., Miyazaki T., Benoist C., Mathis D., Ploegh H.L. The phenotype of H-2M-deficient mice is dependent on the MHC class II molecules expressed. Eur. J. Immunol. 1998;28:2605–2618. doi: 10.1002/(SICI)1521-4141(199809)28:09<2605::AID-IMMU2605>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Stebbins C.C., Peterson M.E., Suh W.M., Sant A.J. DM-mediated release of a naturally occurring invariant chain degradation intermediate from MHC class II molecules. J. Immunol. 1996;157:4892–4898. [PubMed] [Google Scholar]
- Stebbins C.C., Loss G.E., Jr., Elias C.G., Chervonsky A., Sant A.J. The requirement for DM in class II–restricted antigen presentation and SDS-stable dimer formation is allele and species dependent. J. Exp. Med. 1995;181:223–234. doi: 10.1084/jem.181.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A.G., Campbell P.L., Reynolds P., Gautam A.M., McCluskey J. Antigen presentation and assembly by mouse I-Ak class II molecules in human APC containing deleted or mutated HLA DM genes. J. Immunol. 1994;153:5382–5392. [PubMed] [Google Scholar]
- Grubin C.E., Kovats S., deRoos P., Rudensky A.Y. Deficient positive selection of CD4 T cells in mice displaying altered repertoires of MHC class II-bound self-peptides. Immunity. 1997;7:197–208. doi: 10.1016/s1074-7613(00)80523-3. [DOI] [PubMed] [Google Scholar]
- Corry R.J., Winn H.J., Russell P.S. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- Wunderlich J., Shearer G., Livingstone A. Assays for T cell function Coligan J., Kruisbeek A., Margulies D., Shevach E., Strober W. Current Protocols in Immunology 1997. 3 John Wiley & Sons, Inc. New York: 11.1–3.11.20. [Google Scholar]
- Dallman M.J. Cytokines and transplantationTh1/Th2 regulation of the immune response to solid organ transplants in the adult. Curr. Opin. Immunol. 1995;7:632–638. doi: 10.1016/0952-7915(95)80069-7. [DOI] [PubMed] [Google Scholar]
- Nickerson P., Steurer W., Steiger J., Zheng X., Steele A.W., Strom T.B. Cytokines and the Th1/Th2 paradigm in transplantation. Curr. Opin. Immunol. 1994;6:757–764. doi: 10.1016/0952-7915(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Mendiratta S.K., Kovalik J.P., Hong S., Singh N., Martin W.D., Van Kaer L. Peptide dependency of alloreactive CD4+ T cell responses. Int. Immunol. 1999;11:351–360. doi: 10.1093/intimm/11.3.351. [DOI] [PubMed] [Google Scholar]
- Bhardwaj V., Kumar V., Geysen H.M., Sercarz E.E. Degenerate recognition of a dissimilar antigenic peptide by myelin basic protein-reactive T cells. Implications for thymic education and autoimmunity. J. Immunol. 1993;151:5000–5010. [PubMed] [Google Scholar]
- Reay P.A., Kantor R.M., Davis M.M. Use of global amino acid replacements to define the requirements for MHC binding and T cell recognition of moth cytochrome c (93-103) J. Immunol. 1994;152:3946–3957. [PubMed] [Google Scholar]
- Evavold B.D., Sloan-Lancaster J., Wilson K.J., Rothbard J.B., Allen P.M. Specific T cell recognition of minimally homologous peptidesevidence for multiple endogenous ligands. Immunity. 1995;2:655–663. doi: 10.1016/1074-7613(95)90010-1. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K.W., Strominger J.L. Molecular mimicry in T cell-mediated autoimmunityviral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliyaperumal A., Mohan C., Wu W., Datta S.K. Nucleosomal peptide epitopes for nephritis-inducing T helper cells of murine lupus. J. Exp. Med. 1996;183:2459–2469. doi: 10.1084/jem.183.6.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersh G.J., Allen P.M. Structural basis for T cell recognition of altered peptide ligandsa single T cell receptor can productively recognize a large continuum of related ligands. J. Exp. Med. 1996;184:1259–1268. doi: 10.1084/jem.184.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol. Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Amaya M., Mellins E., Wiley D.C. The structure of an intermediate in class II MHC maturationCLIP bound to HLA-DR3. Nature. 1995;378:457–462. doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- Wu S., Gorski J., Eckels D.D., Newton-Nash D.K. T cell recognition of MHC class II-associated peptides is independent of peptide affinity for MHC and sodium dodecyl sulfate stability of the peptide/MHC complex. Effects of conservative amino acid substitutions at anchor position 1 of influenza matrix protein 19-31. J. Immunol. 1996;156:3815–3820. [PubMed] [Google Scholar]
- Tourne S., Miyazaki T., Oxenius A., Klein L., Fehr T., Kyewski B., Benoist C., Mathis D. Selection of a broad repertoire of CD4+ T cells in H-2Ma0/0 mice. Immunity. 1997;7:187–195. doi: 10.1016/s1074-7613(00)80522-1. [DOI] [PubMed] [Google Scholar]
- Basu D., Williams C.B., Allen P.M. In vivo antagonism of a T cell response by an endogenously expressed ligand. Proc. Natl. Acad. Sci. USA. 1998;95:14332–14336. doi: 10.1073/pnas.95.24.14332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markmann J.F., Bassiri H., Desai N.M., Odorico J.S., Kim J.I., Koller B.H., Smithies O., Barker C.F. Indefinite survival of MHC class I-deficient murine pancreatic islet allografts. Transplantation. 1992;54:1085–1089. doi: 10.1097/00007890-199212000-00025. [DOI] [PubMed] [Google Scholar]
- Osorio R.W., Ascher N.L., Jaenisch R., Freise C.E., Roberts J.P., Stock P.G. Major histocompatibility complex class I deficiency prolongs islet allograft survival. Diabetes. 1993;42:1520–1527. doi: 10.2337/diab.42.10.1520. [DOI] [PubMed] [Google Scholar]
- Grusby M.J., Auchincloss H., Jr., Lee R., Johnson R.S., Spencer J.P., Zijlstra M., Jaenisch R., Papaioannou V.E., Glimcher L.H. Mice lacking major histocompatibility complex class I and class II molecules. Proc. Natl. Acad. Sci. USA. 1993;90:3913–3917. doi: 10.1073/pnas.90.9.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchincloss H., Jr., Lee R., Shea S., Markowitz J.S., Grusby M.J., Glimcher L.H. The role of “indirect” recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice. Proc. Natl. Acad. Sci. USA. 1993;90:3373–3377. doi: 10.1073/pnas.90.8.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peugh W.N., Superina R.A., Wood K.J., Morris P.J. The role of H-2 and non-H-2 antigens and genes in the rejection of murine cardiac allografts. Immunogenetics. 1986;23:30–37. doi: 10.1007/BF00376519. [DOI] [PubMed] [Google Scholar]
- Wang Y.C., Mayne A., Sell K.W., Ahmed-Ansari A. The influence of MHC and non-MHC genes on the nature of murine cardiac allograft rejection. I. Kinetic analysis of mononuclear cell infiltrate and MHC-class I/class II expression in donor tissue. Transplantation. 1990;50:313–324. doi: 10.1097/00007890-199008000-00028. [DOI] [PubMed] [Google Scholar]
- Bishop D.K., DeBruyne L.A., Chan S., Xu S., Eichwald E.J. Dissociation of mouse cardiac transplant rejection and donor alloantigen-specific T cell responsiveness. Transpl. Immunol. 1995;3:222–228. doi: 10.1016/0966-3274(95)80028-x. [DOI] [PubMed] [Google Scholar]