Abstract
In this study, we show that a single intranasal dose of a plasmid encoding active transforming growth factor β1 (pCMV-TGF-β1) prevents the development of T helper cell type 1 (Th1)-mediated experimental colitis induced by the haptenating reagent, 2,4,6-trinitrobenzene sulfonic acid (TNBS). In addition, such plasmid administration abrogates TNBS colitis after it has been established, whereas, in contrast, intraperitoneal administration of rTGF-β1 protein does not have this effect. Intranasal pCMV-TGF-β1 administration leads to the expression of TGF-β1 mRNA in the intestinal lamina propria and spleen for 2 wk, as well as the appearance of TGF-β1–producing T cells and macrophages in these tissues, and is not associated with the appearances of fibrosis. These cells cause marked suppression of interleukin (IL)-12 and interferon (IFN)-γ production and enhancement of IL-10 production; in addition, they inhibit IL-12 receptor β2 (IL-12Rβ2) chain expression. Coadministration of anti–IL-10 at the time of pCMV-TGF-β1 administration prevents the enhancement of IL-10 production and reverses the suppression of IL-12 but not IFN-γ secretion. However, anti–IL-10 leads to increased tumor necrosis factor α production, especially in established colitis. Taken together, these studies show that TGF-β1 inhibition of a Th1-mediated colitis is due to: (a) suppression of IL-12 secretion by IL-10 induction and (b) inhibition of IL-12 signaling via downregulation of IL-12Rβ2 chain expression. In addition, TGF-β1 may also have an inhibitory effect on IFN-γ transcription.
Keywords: delivery, hapten, interferon γ, tumor necrosis factor α, β-galactosidase
Introduction
The recent development of various murine models of intestinal inflammation has provided new insights into the pathogenesis and therapy of the human inflammatory bowel diseases 1 2 3 4 5. Two such models have been particularly useful in the elucidation of the T cells that are involved in both the induction and resolution of experimental colitis. The first is Th1 T cell–mediated colitis developing in C.B-17 SCID mice after transfer of normal BALB/c CD45RBhigh T cells 6 7 8, and the second is the hapten-induced model of colonic inflammation in which either 2,4,6-trinitrobenzene sulfonic acid (TNBS) or oxazolone is delivered intrarectally to SJL/J or BALB/c mice to induce Th1 or Th2 T cell–mediated colitides, respectively 9 10 11. Both the “SCID transfer model” and the Th1 TNBS colitis models are characterized by transmural inflammation associated with severe weight loss as well as histopathologic features that mimic some characteristics of Crohn's disease 12 13 14.
Studies using these models have established that experimental colitis can be modulated by regulatory cytokines. Thus, in the SCID transfer model, it has been shown that cotransfer of CD4+ antigen-specific T cells producing high levels of IL-10 (Tr1 cells) at the time of transfer of CD45RBhigh cells can prevent the induction of colitis 15. In addition, it has been shown that oral administration of TNP-haptenated colonic protein before the time of intrarectal TNBS administration suppresses the ability of the latter to induce colitis 16. Such suppression after feeding was most likely due to oral tolerance induction, which had been shown to be due to TGF-β1–producing Th3 cells 17 18. This was consistent with the finding that suppression was associated with the generation of mucosal T cells producing TGF-β, and was abrogated by concomitant administration of anti–TGF-β 16. Taken together, these data on the regulation of experimental colitides by cytokines strongly support the concept that there is a reciprocal relation between IL-12 and IFN-γ and IL-10 and TGF-β in Th1 T cell–mediated inflammation, and that the outcome of the inflammation depends on which of these cytokine groups dominate in any given mucosal immune response.
One approach to the treatment of mucosal inflammation based on the above-described antiinflammatory roles of IL-10/TGF-β is the administration of naked plasmid DNA encoding one or the other cytokines via an intranasal route. This approach is based on previous studies that show that such administration leads to long-term expression of TGF-β or IL-10 in multiple tissues 19 20. We therefore determined the effect of TGF-β–encoding DNA on TNBS colitis using a plasmid engineered to produce an active form of TGF-β1 that does not require processing for its activity 21 22 23. We found that intranasal administration of the TGF-β1 plasmid resulted in expression of TGF-β1 in mucosal tissues, which both prevent induction of TNBS colitis and reverse established TNBS colitis. In addition, we showed that these effects were due at least in part to the capacity of TGF-β1 to induce high levels of IL-10 secretion, which in turn inhibits IL-12 and TNF-α production, as well as the capacity of TGF-β1 to inhibit IL-12 by downregulation of the IL-12Rβ2 chain.
Materials and Methods
Mice.
Specific pathogen-free, 5–6-wk-old male SJL/J mice obtained from the National Cancer Institute and maintained in the National Institute of Allergy and Infectious Diseases animal holding facilities were used in all studies. Animal use adhered to National Institutes of Health Laboratory Animal Care Guidelines.
Plasmid Preparation.
Porcine TGF-β1 cDNA that had mutations at 223Cys→gSer and 225Cys→gSer and that encoding biological active TGF-β1 was obtained from pPK9a (Dr. P. Kondaiah, Indian Institute of Science, India) by digestion with Bg1II at both 5′ and 3′ TGF-β1 cDNA sites 22. The mutant TGF-β1 cDNA thus obtained was subcloned into the BamHI site of the pSL1180 superlinker vector (Amersham Pharmacia Biotech), from which it was excised with EcoRI and EcoRV and ligated into the EcoRI and SmaI sites of the pCI vector under the control of CMV promotor (Promega). The vector obtained was designated pCMV-TGF-β. Using a similar approach, murine IL-10 cDNA (American Type Culture Collection) and β-galactosidase cDNA (CLONTECH Laboratories, Inc.) were inserted into the pCI vector and were designated pCMV-IL-10 and pCMV-GAL, respectively. A large quantity of purified plasmid was prepared by two cycles of CsC12 ultracentrifugation followed by extensive dialysis against TE buffer and two cycles of ethanol precipitation; the purified plasmid was stored at −70°C until use.
Intranasal Administration of Plasmid DNA.
20 μl of PBS containing 100 μg of plasmid DNA was administered intranasally to mice lightly anesthetized with metophane (methoxylflurance; Schering-Plough Animal Health). The quality of plasmid DNA was verified by electrophoresis on 1% agarose gel just before administration.
Induction of TNBS Colitis.
TNBS colitis was induced as described previously, with slight modifications. For the study of prevention of induction of TNBS colitis, plasmid was administered by intranasal transfer, and 1 h later, 2.5 mg of TNBS (Sigma-Aldrich) dissolved in 50% ethanol was administered per rectum as described previously 9 16. For the study of treatment of TNBS colitis, 1.5–2.0 mg of TNBS in 45% ethanol was administered per rectum, and 7 d later, mice losing weight continuously and/or showing other clinical features of chronic colitis were administered plasmid DNA by intranasal transfer. A control group consisted of mice receiving ethanol without TNBS. The weight of each mouse was monitored every 24 h, and mice were killed at the indicated time points for assessment of histologic findings and cytokine production.
Treatment of Mice with Anti–IL-10 mAb or rTGF-β1.
In some experiments, mice with established TNBS colitis were administered neutralizing anti–IL-10 mAbs mixture (SCX-1 and SCX-2, 1 mg/mouse; DNAX) 24, rat control IgM (2 mg/mouse) obtained from the National Cancer Institute, or rTGF-β1 (each mouse was administered 5 μg/d for 3 d; R&D Systems) intraperitoneally. Optimal doses of rTGF-β1 were determined by preliminary studies (100 ng–10 μg/mouse).
Histologic Assessment of Tissues.
Tissues obtained at indicated time points from mice treated in various ways and control mice were fixed in 10% buffered formalin phosphate (Sigma-Aldrich). They were then embedded in paraffin, cut into sections, and stained with hematoxylin and eosin (H and E). Stained colon sections were examined for evidence of colitis using histologic criteria described previously 9.
DNA-PCR and Reverse Transcription PCR Detection of pCMV-TGF-β1 in Tissues.
Tissue DNA was isolated by DNA isolation kit (Gentra Systems) and subjected to plasmid DNA-PCR. Tissue RNA was isolated by standard guanidinium isothiocyanate extraction method followed by the treatment with RQ-1 RNAse-free DNAse (Promega) and subjected to reverse transcription (RT)-PCR specific for plasmid TGF-β1 mRNA. Primers used were 5′ primer (22 mer), 5′-AGAAGTTGGTCGTGAGGCACTG-3′ derived from the 5′ sequence of synthesized splicing site upstream of TGF-β1 cDNA, and 3′ primer (22 mer) 5′-GAGCTCCGACGTGTTGAACAG-3′ derived from the sequence within the TGF-β1 cDNA in the plasmid. The splice site facilitated the distinction of the sizes between DNA-PCR and RT-PCR of pCMV-TGF-β1. For glyceraldehyde 3-phosphate dehydrogenase (GAPDH), the primer sequences are 5′ primer (24 mer) 5′-GTCTTCACCACCATGGAGAAGGCT-3′ and 3′ primer (23 mer) 5′-CATGCCAGTGAGCTTCCCGTTCA-3′. PCR was performed by thermal cycler for 30 cycles of 94°C for 45 s, 60°C for 60 s, and 72°C for 90 s, and final extension was performed at 72°C for 10 min. Amplified products were visualized by 2% agarose gel 25.
Confocal Immunofluorescence Studies.
Tissues as indicated were put into OCT compound on dry ice, and 5-μm cryosections were cut at standard procedure 9. Dual immunofluorescence was performed to determine colocalization of intracellular β-galactosidase and cell surface markers as follows: (a) sections were fixed in cold acetone and rehydrated in PBS; (b) sections were exposed to 5% goat serum and hamster anti-Fc blocking mAb (1:100 dilution; BD PharMingen) in PBS for 20 min to block nonspecific Abs of fluorochrome; (c) samples were treated with rabbit anti–β-galactosidase Ab (1:1,000 dilution; Chemicon) for 40 min, followed by Texas red–conjugated goat anti–rabbit Ab (Jackson ImmunoResearch Laboratories) and FITC-conjugated anti-CD3ε or anti-CD11b (BD PharMingen) for 40 min; and (d) finally, sections were mounted and analyzed by confocal immunofluorescence using on a Leica TCS-NT/SP confocal microscope using a 40× objective, numerical aperture 1.2. Fluorochromes were excited using an argon laser at 488 nm for FITC and a krypton laser at 568nm for Texas red. Differential interference contrast (DIC) images were collected simultaneously with the fluorescence images using the transmitted light detector. Images were processed using the Leica TCS-NT/SP software (v1.6.551).
Isolation and Culture of Lamina Propria T Cells and Macrophages.
Lamina propria (LP) mononuclear cells (LPMCs) were isolated from LPs using a modified technique of Van der Heijden and Stok 26 as described previously. The resultant LPMCs were plated on a plastic surface for separation into adherent and nonadherent cell populations. Nonadherent cells were further enriched by negative selection using mouse CD4+ T cell purification columns (R&D Systems). The resultant cell populations were shown by flow cytometry (FACScan™; Becton Dickinson) to contain >90% CD4+ T cells. LP T cells thus obtained were suspended in complete medium consisting of RPMI 1640 supplemented with 3 mM l-glutamine, 10 mM Hepes buffer, 10 μg/ml gentamycin, 100 U/ml each of penicillin and streptomycin (Amersham Pharmacia Biotech), 0.05 mM 2-ME, and 10% heat-inactivated FCS (Amersham Pharmacia Biotech), and were cultured at a concentration of 106 cells/ml in 24-well culture plates (Costar) with or without stimulation by plate-bound anti-CD3ε (clone 145-2C11; BD PharMingen) precoated at 10 μg/ml anti-CD3ε in carbonate buffer (pH 9.5) overnight at 4°C and soluble anti-CD28 (1 μg/ml, clone 37.51; BD PharMingen) for 48 h. The adherent cell population (LP macrophage-enriched cells, which were shown by flow cytometry to contain 70% F4/80-positive cells and a 25% N418-positive cell population). These cells were cultured at a concentration of 106 cells/ml and stimulated overnight (18 h) with IFN-γ (1,000 U/ml; R&D Systems) and then with Staphylococcus aureus Cowans (SAC)at a 1:10,000 dilution (Pansorbin®; Calbiochem) for an additional 24 h. For measurement of TGF-β1, secreting cells were cultured in serum-free medium supplemented with 1% nutridoma-SP (Roche Molecular Biochemicals) for 60 h as described previously 11.
Cytokine Assays.
Supernatants of cell cultures were collected and assayed for cytokine content (IFN-γ, IL-4, IL-12, TNF-α) by specific ELISA (mini-kit or duo paired cytokines; Endogen). TGF-β1 concentration was also determined by ELISA (Max TGFβ1 assay kit; Promega), but in this case after the treatment of sera- or serum-free supernatants with 1 M HCl, followed by the NaOH neutralization. Optical densities were measured on an ELISA reader (MR 5000; Dynatech Labs) at a wavelength of 490 nm as described previously 9 11 16.
Flow Cytometric Analysis for the Expression of IL-12Rβ2 Chain.
Isolated LP T cells were first treated with hamster anti–IL-12Rβ2 chain mAb (HAM 10B9; gift of Dr. R. Ehrhardt, Protein Design Laboratories, Palo Alto, CA) 27 or hamster IgG control (BD PharMingen). The cells were then washed and incubated with biotinylated anti–hamster IgG (H+L; Jackson ImmunoResearch Laboratories) followed by PE-conjugated streptavidin and FITC-conjugated rat anti-CD4 mAb (BD PharMingen). The analysis was performed on a Becton Dickinson FACScan™ with CELLQuest™ II software.
Results
Prevention of Induction of TNBS Colitis by Intranasal Administration of pCMV-TGF-β1 Plasmid.
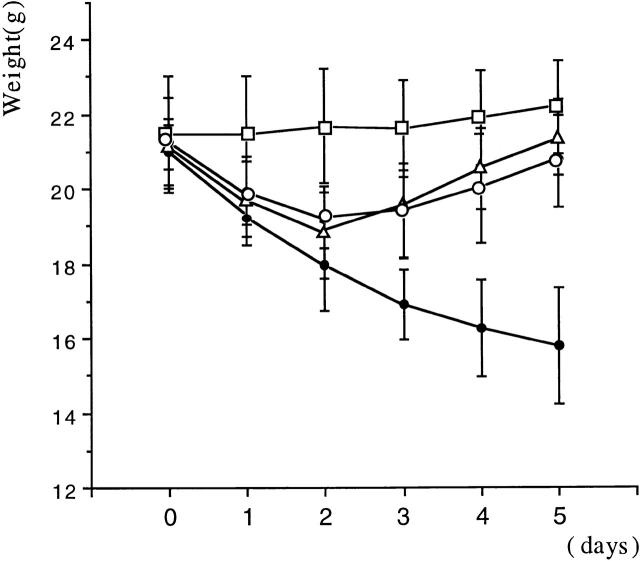
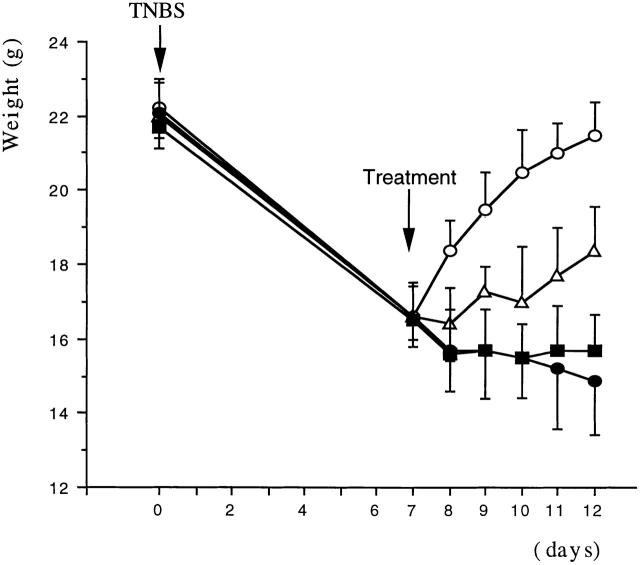
In initial studies, we determined the capacity of a plasmid expressing DNA encoding an active form of TGF-β (pCMV-TGF-β1 plasmid) to prevent induction of TNBS colitis. As reported earlier, rectal instillation of TNBS (2.5 mg in 50% ethanol) induces colitis in >95% of SJL/J mice and, as shown in Fig. 1, mice treated in this way manifest a major symptom of such colitis, a profound and sustained weight loss. In contrast, mice undergoing colitis induction and also administered a single intranasal dose of pCMV-TGF-β1 plasmid on day 0 of induction showed a transient loss of weight followed after day 3 by rapid weight gain to a weight approaching that of control mice who had received the pCMV-TGF-β1 plasmid in the absence of TNBS colitis induction. This transient loss of weight is similar to that seen previously and in this study in mice receiving 50% ethanol alone (9 16; data not shown). Intranasal administration of pCMV-TGF-β1 plasmid did not itself cause weight loss (Fig. 1), and administration of control plasmid not containing a TGF-β1 DNA cassette (pCI) did not prevent TNBS colitis–associated weight loss (not shown).
Figure 1.
Intranasal administration of pCMV-TGF-β1 prevented TNBS-induced colitis in SJL/J mice. Weight changes (mean ± SD) of one experiment representative of three independent experiments is shown. Each group consists of 10 mice treated intranasally on day 0 with pCMV-TGF-β1 (100 μg/mouse). Alone, □; pCMV-TGF-β1 (100 μg intranasally) plus rectal instillation of TNBS (2.5 mg/mouse), ○; pCMV-TGF-β1 (100 μg intranasally) plus rectal instillation of TNBS (2.5 mg) and anti–IL-10 mAbs (SXC-1 and SXC-2 intraperitoneally 1 mg each/mouse), ▵; rectal instillation of TNBS alone (2.5 mg), •.
The preventive effect of intranasal pCMV-TGF-β1 plasmid administration was confirmed by histologic examination of colonic tissue. Thus, as shown in Fig. 2A and Fig. B, mice administered TNBS per rectum alone exhibited severe colitis marked by transmural leukocyte infiltration, loss of goblet cells, and severe thickening of the colonic wall compared with mice administered pCMV-TGF-β1 alone. In contrast, as shown in Fig. 2 C, mice given TNBS per rectum plus intranasal pCMV-TGF-β1 manifested normal colonic histology except for a slight thickening of the serosal connective tissue. Importantly, the mice treated with intranasal pCMV-TGF-β1 alone (Fig. 2 A) or in combination with TNBS (Fig. 2 C) did not exhibit fibrotic changes in the colon or in multiple other tissues such as the lung, liver, spleen, and kidney when examined by H and E staining at 5, 14, 28, and 56 d after TNBS induction. In addition, no fibrosis was detected in these tissues when they were stained with Masson's trichrome stain, which is specific for collagen deposition (data not shown).
Figure 2.
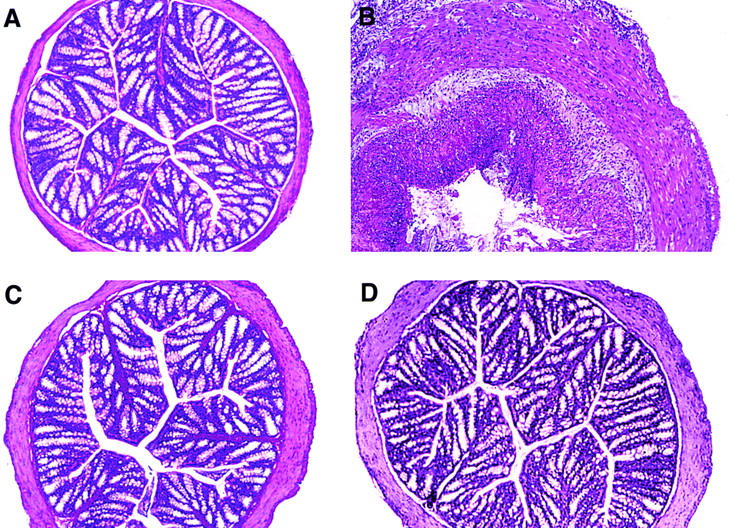
Histologic analysis of the colon from SJL/J mice at day 5 after initial intranasal administration of pCMV-TGF-β1 in the TNBS colitis induction. H and E–stained cross section of colon (original magnification: ×100) from a mouse administrated (A) pCMV-TGF-β1 (intranasally) alone showing normal appearance; (B) TNBS alone showing severe transmural colitis; (C) pCMV-TGF-β1 and rectal instillation of TNBS showing normal histology except for minimal increase in thickness of the colon wall; and (D) pCMV-TGF-β1 and rectal instillation of TNBS plus anti–IL-10 mAbs showing normal histology except for minimal increase in thickness of the colon wall.
TGF-β1 in Serum and Tissues after Intranasal Administration of pCMV-TGF-β1 Plasmid.
We next conducted a series of studies focusing on the mechanism of the preventive effect of intranasal pCMV-TGF-β1 plasmid administration on TNBS colitis. In a first study, we determined serum levels of TGF-β1 in tissues and cellular localization of the plasmid-derived TGF-β1 after intranasal administration of the plasmid. To study serum levels, we initially determined serum TGF-β1 titers of the active form of TGF-β1 (as defined by TGF-β1 detected without acid treatment; described in Materials and Methods) in the serum of mice administered pCMV-TGF-β1 plasmid intranasally 5 d after instillation of TNBS/ethanol per rectum or ethanol alone per rectum. We found that the serum level was increased in both groups (13.2 ± 0.4 ng/ml, n = 5 and 12.2 ± 0.3 ng/ml, n = 5, respectively) compared with similar mice not administered intranasal plasmid (3.5 ± 0.2 ng/ml, n = 5 and 3.4 ± 0.2 ng/ml, n = 5, respectively). In contrast, total TGF-β1 levels in serum (defined as TGF-β1 detectable after acid treatment; described in Materials and Methods) were much higher and did not differ among the various groups tested, i.e., those administered intranasal pCMV-TGF-β1 plasmid or not administered plasmid (range: 87.9–109.2 ng/ml).
To study tissue distribution of TGF-β1 DNA and mRNA after intranasal administration of pCMV-TGF-β1, we performed PCR and RT-PCR on DNA and mRNA, respectively, extracted from various tissues. Here, we took advantage of the fact that the pCMV-TGF-β1 plasmid possesses a splicing site at the 5′ end of the TGF-β1 DNA, which gave rise to RNA that could be distinguished by size from both contaminating plasmid TGF-β1 DNA in RNA extracts that persisted after treatment with DNAse and endogenous TGF-β1 mRNA. As shown in Fig. 3, pCMV-TGF-β1 DNA could be detected in the DNA extracted from lung, spleen, and colons of mice administered intranasal pCMV-TGF-β1 plasmid at day 5 and 4 wk after administration of plasmid. However, such DNA could not be detected 2 mo after such administration (not shown). In contrast, TGF-β1 mRNA could be detected at day 14 after administration of plasmid in all tissues, but was not detected in colon and spleen tissues and only faintly in lung tissue at 28 d.
Figure 3.
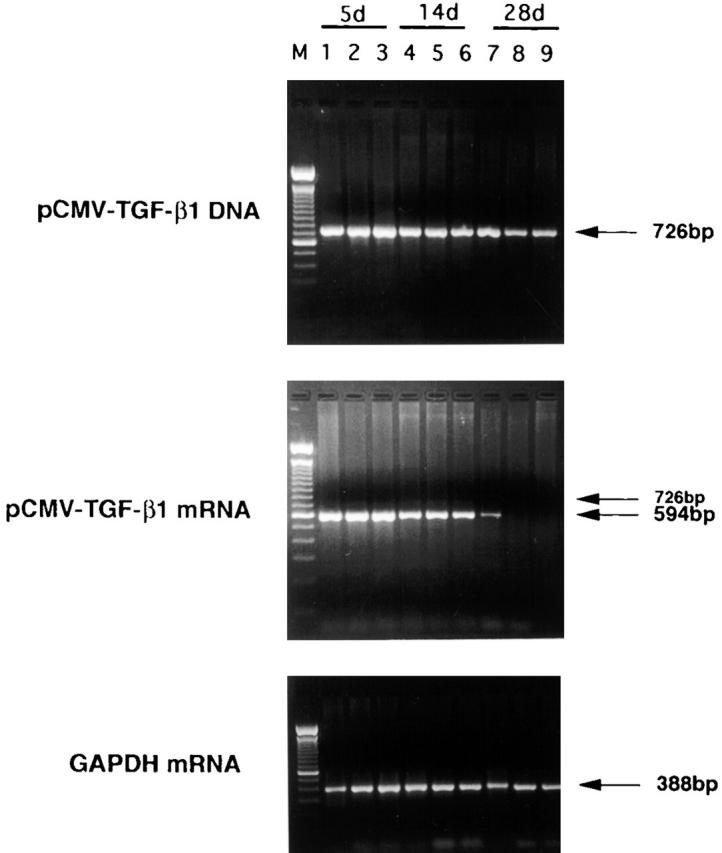
Analysis of pCMV-TGF-β1 DNA and mRNA in tissues by PCR and RT-PCR, respectively, at 5, 14, and 28 d after intranasal administration of pCMV-TGF-β1 and TNBS treatment. RT-PCR detects spliced mRNA message that differs in size from DNA and is thus not due to plasmid DNA contamination of endogenous TGF-β1 mRNA (described in text). Lane M, marker; lanes 1, 4, and 7, lung; lanes 2, 5, and 8, colon; lanes 3, 6, and 9, spleen; lanes 1–3, day 5; lanes 4–6, day 14; lanes 7–9, day 28. In TGF-β1 mRNA, a faint band of 726 bp was also detected because of the presence of unspliced message. One experiment representative of three independent experiments is shown.
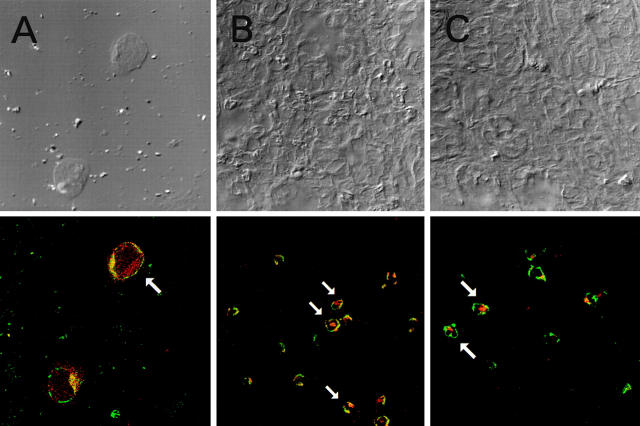
In further studies to determine if pCMV-TGF-β1 plasmid is expressed in T cells and/or macrophages in mice administered this plasmid and undergoing induction of TNBS colitis, we administered to such mice an equivalent (surrogate) plasmid in which the TGF-β1 DNA cassette was replaced by a β-galactosidase DNA cassette, as β-galactosidase but not TGF-β1 can be reproducibly detected in cells as a marker for the presence of a plasmid DNA. In these studies, we used immunostaining with anti–β-galactosidase to detect β-galactosidase rather than the X-gal reaction, as such immunostaining has been shown to be the more sensitive detection method 28 29. Accordingly, tissues harvested from mice 5 d after intranasal administration of pCMV-GAL and intrarectal administration of TNBS were stained with FITC-labeled anti-CD3 or anti-CD11b and Texas red–labeled rabbit anti–β-galactosidase, then were examined by confocal microscopy to detect cells colocalizing the cell surface markers with cytoplasmic β-galactosidase. As shown in Fig. 4A and Fig. B, cells in tissues exhibiting both green cell surface staining (CD3+ cells) as well as red cytoplasmic staining (β-galactosidase–containing cells) were found in CD4+-enriched cell cytospin samples from spleen and inflamed colon tissues. In addition, as shown in Fig. 4 C, CD11b+/β-galactosidase colocalizing cells were also found in inflamed colon tissue. One CD11b+/β-galactosidase+ binucleated cell with arrow could be a granulocyte, as CD11b is also positive in granulocytes. Scanning of multiple fields showed that CD11b+/β-galactosidase colocalizing cells were more numerous than CD3+/β-galactosidase colocalizing cells, and both types of cells tended to occur as focal accumulations. Finally, as shown in Table , after administration with pCMV-TGF-β1, the splenic CD4+ T cell–enriched cell population as well as the adherent cell population produced increased the amount of TGF-β1 in vitro. Taken together, these studies show that pCMV-TGF-β DNA is taken up by both CD3+ T cells and CD11b+ adherent cells that are present in inflamed tissues of mice administered TNBS.
Figure 4.
Confocal immunofluorescence studies indicating that intranasally administered CMV-GAL is taken up by CD3+ T cells as well as by CD11b+ cells. Representative confocal microscopy fields from (A) cytospin preparation of spleen CD4+ cells (2 × 104 cells per slide) enriched by immunocolumn (original magnification: ×1,000) and (B) and (C) colon sections obtained from mice with mild TNBS colitis (×400). Differential interference contrast (phase contrast) images (top) were collected simultaneously with the fluorescence images using the transmitted light detector. Two-color immunofluorescence (bottom) was performed with rabbit anti–β-galactosidase Ab followed by Texas red–goat anti–rabbit IgG Ab and FITC–anti-CD3 mAb (A) and (B) or FITC–anti-CD11b (C). In staining of cytospin preparation, cells were first stained with FITC anti-CD3 and fixed with 4% paraformaldehyde, then were stained with rabbit anti–β-galactosidase Ab followed by Texas red–goat anti–rabbit IgG Ab. 9 ± 5 cells per slide were CD3+ β-galactosidase+ in five cytospin slides.
Table 1.
TGF-β1 and IL-10 Production of Spleen Cells after Intranasal Transfer of pCMV-TGF-β1 Plasmid or Intraperitoneal Administration of Recombinant TGF-β1
| Cell population | TGF-β1 | IL-10 | |
|---|---|---|---|
| (pg/ml) | (pg/ml) | ||
| None | T cells (total) | 45 | 140 |
| Adherent cells | 40 | 124 | |
| TGF-1 plasmid | T cells (total) | 812 | 1,238 |
| CD8 T cells | 673 | 1,483 | |
| CD4 T cells | 935 | 745 | |
| Adherent cells | 796 | 438 | |
| rTGF-β1 | T cells (total) | 112 | 106 |
| CD8 T cells | 98 | 70 | |
| CD4 T cells | 118 | 120 | |
| Adherent cells | 72 | 597 |
Cells were obtained from groups of five mice killed on day 4. Adherent cells were separated by adherence to plastic dishes, and T cells were separated from nonadherent cells by passing through a T cell column. CD4-enriched and CD8-enriched T cells were further separated by magnetic beads after incubation with anti-CD8 or anti-CD4 mAb. T cells were stimulated in vitro with anti-CD3/anti-CD28, whereas adherent cells were stimulated with SAC plus IFN-γ. TGF-β1 and IL-10 production was determined by ELISA in duplicate.
In additional studies, we also sought insight into means by which intranasally administered pCMV-TGF-β plasmid gains access to intestinal tissue. In these studies, we showed first that intranasally administered plasmid as detected by PCR is present in gastric contents soon after intranasal administration, indicating that one possible mode of access is inadvertent swallowing of plasmid DNA followed by direct entry of DNA into LP macrophages and T cells. In addition, we also observed that intranasal administration of plasmid is followed by the appearance of plasmid DNA, again detected by PCR, in draining cervical lymph nodes but not in the blood (serum or formed elements) at 24 h (data not shown). Because it has been shown that cells originating in cervical nodes can migrate via lymphatics to other mucosal tissues, lymphatic migration of cells from nasal and/or cervical areas to the gastrointestinal tract is a second possible mode of access.
Effect of Intranasal Administration of pCMV-TGF-β1 Plasmid on Cytokine Production in Mice Undergoing Induction of TNBS Colitis.
In the next series of studies, we determined the effect of intranasal pCMV-TGF-β1 administration on cytokine production in mice subjected to induction of TNBS colitis. In these studies, LPMCs were obtained from colons of mice 5 d after the induction of TNBS colitis both treated and not treated with intranasal pCMV-TGF-β1 plasmid or control plasmid. Then, after culture with anti-CD3/anti-CD28 (described in Materials and Methods), TGF-β1, IFN-γ, and IL-10 levels were measured in the culture supernatant.
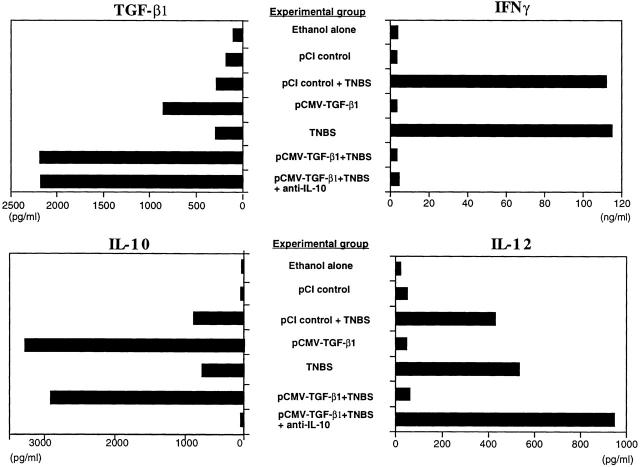
As shown in Fig. 5, anti-CD3/anti-CD28–stimulated LPMCs obtained from mice given intrarectal TNBS and also administered intranasal pCMV-TGF-β1 produced greatly increased amounts of TGF-β1 compared with cells obtained from mice induced with TNBS alone. In addition, similarly stimulated LPMCs from mice administered intranasal pCMV-TGF-β1 and not given TNBS also produced increased amounts of TGF-β1, but not to the extent seen in the TNBS-induced mice. Thus, the data is consistent with the view that T cells expressing the pCMV-TGF-β1 plasmid are nonspecifically induced to produce TGF-β1 via the CMV promotor in the context of an inflammation. This view is supported by additional studies that showed that although unstimulated LPMCs and spleen mononuclear cells obtained from mice induced with intrarectal TNBS do not produce significant amounts of TGF-β1, the same cells stimulated by a variety of polyclonal stimulants (anti-CD3/anti-CD28, PMA/ionomycin, and Con A) produce very substantial amounts of TGF-β1 (data not shown). Finally, it should be noted that these ex vivo studies are likely to reflect in vivo TGF-β1 production because, as noted above, mice administered intranasal pCMV-TGF-β1 exhibited increased levels of circulating TGF-β1 in the circulation.
Figure 5.
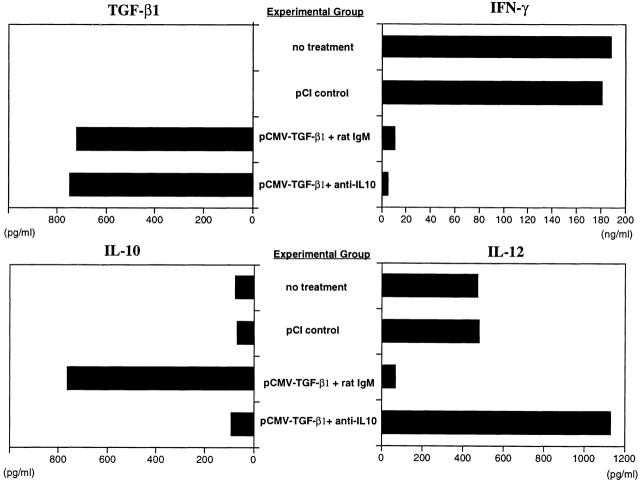
Cytokine production by LPMCs from mice administered intranasal pCMV-TGF-β1 and control plasmid under various conditions. LPMCs were isolated from pooled colon cell populations (n ≧ 6) obtained at day 5 after induction of TNBS colitis and intranasal administration of pCMV-TGF-β1 or pCI control plasmid, with or without coadministration of intraperitoneal anti–IL-10 mAbs (SXC-1 and SXC-2). LPMCs were stimulated with anti-CD3ε and anti-CD28 mAb or SAC and IFN-γ as described in Materials and Methods. All four cytokines in the supernatant were determined by ELISA in duplicate wells, and SD was within 5%. Values from one representative study out of three independent experiments with consistent results are shown. These data are obtained from the same experiment shown in Fig. 1.
As further shown in Fig. 5, anti-CD3/anti-CD28–stimulated LPMCs from mice given TNBS per rectum that were administered pCMV-TGF-β1 intranasally also produced markedly decreased levels of IFN-γ. This was in striking contrast to the LPMCs from mice given TNBS per rectum and not administered pCMV-TGF-β1 plasmid or mice administered control plasmid (pCI), which produced high levels of IFN-γ. Taken together, these results suggest that there is a reciprocal relation between TGF-β and IFN-γ secretion, and that TGF-β secreted by cells expressing the pCMV-TGF-β1 plasmid suppresses IFN-γ secretion.
In parallel studies, we determined the production of IL-10 in the same anti-CD3/anti-CD28–stimulated LPMC populations. Thus, as shown in Fig. 5, although TNBS colitis induction with or without intranasal control plasmid administration was associated with a moderate rise in IL-10 production, such induction with intranasal administration of pCMV-TGF-β1 was associated with a striking increase in IL-10 production. In view of the dramatic effect of pCMV-TGF-β1 administration on IL-10 production and thus the possibility that TGF-β was acting through IL-10 to prevent TNBS colitis, we also measured the secretion of cytokines in mice treated with anti–IL-10 at the time of TNBS colitis induction (Materials and Methods). As shown in Fig. 5, although anti–IL-10 administration completely reversed the stimulatory effect of pCMV-TGF-β1 on IL-10 secretion, it had no effect on either TGF-β1 production or IFN-γ production. This, plus the fact that anti–IL-10 administration to the whole mouse had no effect on the ability of pCMV-TGF-β1 to prevent TNBS colitis as shown by weight change or histologic analysis (Fig. 1 and Fig. 2 D), strongly suggests that the plasmid is not acting solely through induction of IL-10 secretion.
The Effect of Intranasal pCMV-TGF-β1 Plasmid Administration on IL-12 Production and Signaling during Induction of TNBS Colitis.
Given the effect of intranasal pCMV-TGF-β1 administration on IFN-γ secretion after TNBS colitis induction, it was logical to determine the effect of such plasmid administration on IL-12 secretion and signaling. In this case, purified LPMCs obtained from mice 5 d after TNBS administration were stimulated with SAC and IFN-γ (described in Materials and Methods) rather than anti-CD3/anti-CD28, and IL-12 p70 was measured in cultured supernatants. As again shown in Fig. 5, IL-12 secretion was substantially increased in cultures of cells from mice induced with TNBS per rectum and either treated or untreated with control pCI plasmid compared with mice induced with ethanol alone. In contrast, IL-12 secretion was reduced to baseline levels in mice induced with TNBS per rectum and treated with intranasal pCMV-TGF-β1 plasmid. However, perhaps more importantly, administration of anti–IL-10 at the time of TNBS colitis induction as described above reverses the suppressive effect on IL-12 secretion exerted by intranasal pCMV-TGF-β1 plasmid administration. This was especially noteworthy because, as already noted, anti–IL-10 production did not relieve the suppressive effect of plasmid administration on IFN-γ secretion.
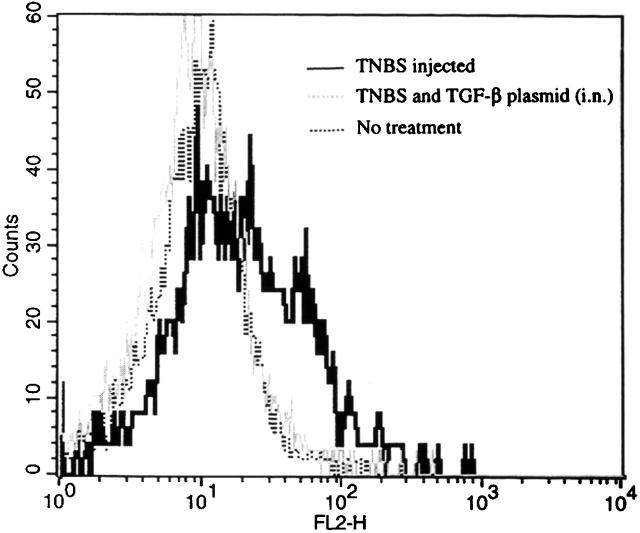
The above results showing that intranasal pCMV-TGF-β plasmid administration when associated with anti–IL-10 administration gives rise to an increased IL-12 response yet has no effect on a IFN-γ response suggests that TGF-β secretion interferes with IL-12 signaling. To test this possibility, we determined the expression of the β2 chain of the IL-12R by flow cytometry using a mAb specific for the β2 chain 27. As shown in Fig. 6, induction of TNBS colitis followed by isolation of LPMCs at 3 d after induction led to increased β2 chain expression; in contrast, concomitant intranasal administration of pCMV-TGF-β1 plasmid at the time of TNBS induction led to LPMCs that exhibited decreased β2 chain expression. These data were corroborated by in vitro studies in which spleen T cells were isolated from mice that had undergone TNBS colitis induction and were then stimulated in vitro with Con A alone or in the presence of exogenous TGF-β or IL-10. Although TGF-β1 downregulated β2 chain expression, IL-10 did not (data not shown). Taken together, these data established that the production of TGF-β1 by cells in mice given intranasal pCMV-TGF-β1 plasmid leads to abrogation of the Th1 response (at least in part) by blocking IL-12 signaling.
Figure 6.
Intranasal administration of pCMV-TGF-β1 to mice with TNBS colitis decreases the expression of IL-12Rβ2 chain expression on LP CD4+ T cells. Mice were administered TNBS on day 0 with or without intranasal administration of pCMV-TGF-β1. LPMCs were isolated at day 5 and stained with FITC–anti-CD4, streptavidin-PE, and IL-12β2R mAb (described in Materials and Methods). The flow cytometric profile of IL-12β2R expresssion after gating CD4+ T cell population is shown. One experiment representative of three independent experiments is shown.
Effect of Intranasal Administration of pCMV-TGF-β1 Plasmid on Established TNBS Colitis.
In further studies, we determined if pCMV-TGF-β1 plasmid administration could reverse established TNBS colitis in addition to preventing its development as described above. Accordingly, SJL/J mice were administered TNBS per rectum to induce TNBS colitis, and at day 7 after colitis was established, they were administered a single dose of intranasal pCMV-TGF-β1 plasmid along with an intraperitoneal injection of anti–IL-10 or a control rat IgM; alternatively, the mice were administered rTGF-β1 (5 μg/d for 3 d). As shown in Fig. 7, mice treated with pCMV-TGF-β1 plus control rat IgM manifested a striking reversal of weight loss, accompanied by an improvement in coat appearance and activity and, as shown in Fig. 8A, Fig. B, and Fig. D, almost complete resolution of macroscopic and microscopic evidence of colitis (data not shown). In some contrast, as also shown in Fig. 7, mice treated with intranasal pCMV-TGF-β1 plus anti–IL-10 had a less dramatic effect on weight gain and, as shown in Fig. 8 C, only a moderate resolution of macroscopic evidence of colitis. Finally, intraperitoneal administration of rTGF-β1 had no affect on the course of established TNBS colitis, i.e., the mice did not recover weight and did not reverse colitis at either the microscopic or macroscopic levels (data not shown). As shown in Table , the splenic T cell population exhibited only a small increase in TGF-β1 production and no increase in IL-10 production after such administration, whereas adherent cells (macrophages) produced an increased amount of IL-10 but not TGF-β1.
Figure 7.
Intranasal administration of pCMV-TGF-β1 abrogates established TNBS colitis in SJL/J mice. Weight changes of mice in one experiment (mean ± SD) representative of three independent experiments is shown. Each group consisted of 15 mice administrated TNBS (1.5–2.0 mg/mouse) at day 0 and treated with pCMV-TGF-β1 (100 μg/mouse intranasally) and rat control IgM (2 mg/mouse intraperitoneally) at day 7, ○; pCMV-TGF-β1 and anti-IL-10 mAbs (SXC-1 and SXC-2, 1 mg/mouse intraperitoneally) at day 7, ▵; rTGF-β1 (5 μg/mouse intraperitoneally each day on days 7–9), ▪; no treatment, •.
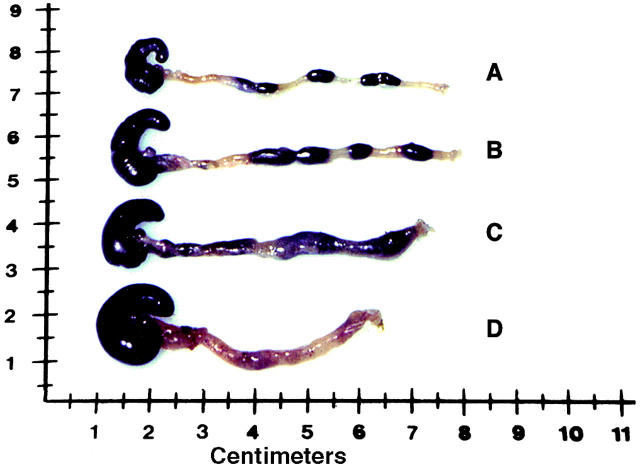
Figure 8.
Gross appearance of colons from SJL/J mice at 12 d after administration of TNBS on day 0, and after treatment with intranasal administration of pCMV-TGF-β1 and intraperitoneal administration of anti–IL-10 mAbs or rat control IgM on day 7. Representative colons from (A) a normal control mouse, (B) a mouse treated with pCMV-TGF-β1 (intranasally) and rat control IgM (intraperitoneally), (C) a mouse treated with pCMV-TGF-β1 (intranasally) and anti–IL-10 mAbs (intraperitoneally), and (D) nontreated established colitis.
Analysis of Cytokine Production in SJL/J Mice with Established TNBS Colitis Treated with pCMV-TGF-β1 with or without Concomitant Administration of Intraperitoneal Anti–IL-10.
As noted above, concomitant anti–IL-10 administration does not alter the capacity of pCMV-TGF-β plasmid given intranasally to prevent TNBS colitis, but it does diminish the capacity of the plasmid to treat established TNBS colitis. To determine the basis of this difference, we measured cytokine production by stimulated LP cells (in this case, LP T cells or purified adherent cells) extracted from mouse colons 5 d after intranasal plasmid and/or intraperitneal anti–IL-10 administration. As shown in Fig. 9, cells from mice treated with pCMV-TGF-β1 plasmid with and without intraperitoneal anti–IL-10 administration exhibited decreased IFN-γ production. Furthermore, as also shown in Fig. 9, administration of pCMV-TGF-β was again accompanied by downregulated IFN-γ–induced IL-12 production, and this was again reversed by administration of anti–IL-10 but not by administration of control rat IgM. In parallel with the situation obtained during administration of pCMV-TGF-β1 plasmid to mice during induction of TNBS colitis, such administration of the plasmid in established colitis led to increased IL-10 production by LP T cells (Fig. 9), but had little effect on production of IL-10 by LP adherent cells (data not shown).
Figure 9.
In vitro cytokine production by LP T cells or by LP adherent macrophages from mice with established TNBS colitis (day 12 after induction). Mice were treated with intranasal administration of pCMV-TGF-β1 or pCI control plasmid and intraperitoneal administration of anti–IL-10 mAbs or rat control IgM on day 7. LPMCs were isolated from pooled colon cell population (n ≧ 6), then separated into LP T cells and macrophages. LP T cells were stimulated with anti-CD3ε/anti-CD28 mAb for TGF-β1, IFN-γ, and IL-10 production, and LP macrophages were stimulated with SAC and IFN-γ for IL-12 production. All four cytokines in the supernatant were determined by ELISA in duplicate wells, and SD was within 5%. One experiment representative of three is shown; these data are obtained from the same experiment shown in Fig. 7.
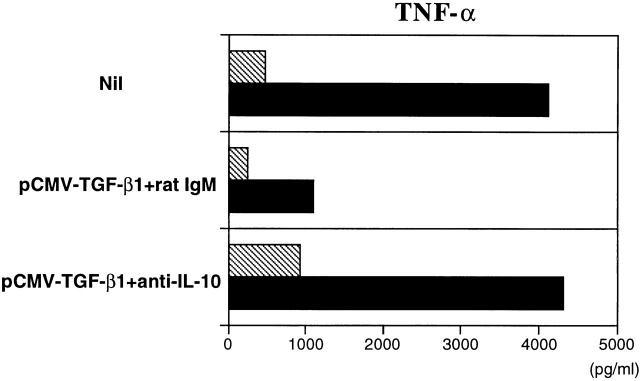
Finally, as shown in Fig. 10, intranasal pCMV-TGF-β1 administration plus intraperitoneal anti–IL-10 administration led to increased adherent cell TNF-α secretion, which was not seen in the absence of anti–IL-10 administration. Thus, it appears that the ability of anti–IL-10 administration to decrease the effect of intranasal pCMV-TGF-β1 plasmid administration on established TNBS colitis is due to loss of the downregulating effect of IL-10 on TNF-α secretion.
Figure 10.
In vitro TNF-α production by LP macrophages obtained from mice treated with intranasal administration of pCMV-TGF-β1 and coadministration intraperitoneally of anti–IL-10 mAbs or rat control IgM. Cells were obtained at day 5 during the induction phase (hatched bars) or at day 12 during the established phase (black bars) of TNBS colitis, and were cultured with SAC and IFN-γ for 24 h. Results of one representative experiment of three are shown. ELISA was performed in duplicate wells, and SD was within 5%.
Discussion
In this study, we explored the therapeutic potential of intranasal delivery of plasmid DNA encoding active TGF-β1 (pCMV-TGF-β1) in a murine model of experimental colitis (TNBS colitis), and showed that the administered plasmid was highly effective both in preventing induction of colitis and in ameliorating established colitis. In discussing the immunological mechanisms underlying this therapeutic effect and how they relate to other models of experimental mucosal inflammation, it is important to emphasize that most such models of inflammation are Th1 T cell–driven processes that are highly dependent on the production of IL-12 and the activation of the (signal transducer and activator of transcription 4 (STAT4) signaling pathway 30 31. Thus, in the TNBS colitis model, treatment of mice with anti–IL-12 leads to complete prevention of disease or resolution of already established disease. In addition, in the cell transfer model of colitis obtained by the reconstitution of Tgε26 mice with cells from wild-type mice, administration of anti–IL-12 prevents colitis, as does reconstitution of Tgε26 mice with cells from with STAT4−/− cells. Similarly, in the transfer model of colitis obtained by the reconstitution of recombination activating gene (RAG)−/− mice with CD45RBhi cells, although reconstitution with cells from wild-type mice causes severe colitis, reconstitution with cells from STAT4−/− mice causes only mild colitis 32. It is also important to emphasize that the inflammation in these and other models are conditional on the IL-12/STAT4 pathway but are not conditional on the induction of IFN-γ secretion. This is shown by the fact that TNBS colitis can be induced in IFN-γ−/− mice, and reconstitution of Tgε26 transgenic mice or RAG−/− mice with cells from IFN-γ−/− mice does not prevent the development of colitis 32. Thus, it is highly likely that other cytokines induced by the IL-12/STAT4 pathway, such as TNF-α, are sufficient to support the development of mucosal inflammation, although further study is required to determine how IL-12/STAT4 pathway–dependent TNF-α induction in T cells leads to the abundant TNF-α production in non-T cells known to be necessary for colitis 33 34 35. Taken together, these considerations support the view that IL-12 production and/or signaling is a key concomitant of many forms of experimental mucosal inflammation, and one can therefore predict that the suppressive effect of a plasmid causing the secretion of TGF-β1 acts by affecting the production of or signaling by IL-12.
Indeed, this prediction appears to hold true. First, intranasal pCMV-TGF-β plasmid administration suppressed IL-12 production, probably via the induction of IL-10 secretion, as no suppression was observed when the mice were coadministered anti–IL-10. Second, intranasal pCMV-TGF-β plasmid administration inhibited IL-12 signaling, as shown by the fact that although administration of the plasmid along with coadministration of anti-IL-10 negated the effect of the plasmid on IL-12 production, IFN-γ secretion remained low. In addition, plasmid administration was associated with downregulation in the capacity of T cells to express the β2 chain of the IL-12 receptor, which expression is critical for Th1 differentiation 27 36 37. On these bases, we postulate that the ability of intranasal pCMV-TGF-β plasmid administration to protect and/or treat TNBS colitis and, by extension, other Th1 colitides, is due to its indirect effect on IL-12 production via IL-10 production and its direct effect on IL-12 induction and IFN-γ secretion. The latter, in turn, occur via downregulation of IL-12Rβ2 chain expression or through an as yet poorly understood effect of TGF-β on IFN-γ transcription. In any case, the end result is that plasmid administration acts through IL-12 to inhibit secretion of any of a variety of downstream inflammatory cytokines.
Previous studies of experimental murine colitis have provided evidence that both TGF-β and IL-10 play counterregulatory roles in the Th1 pathway, leading to inflammation. With regard to TGF-β, it has been shown that TNBS colitis can be prevented by feeding TNP-haptenated colonic protein before the time of TNBS administration per rectum 16. Moreover, such prevention is accompanied by the appearance of TGF-β–producing T cells and is abrogated by administration of anti–TGF-β. Recently we have shown that in this setting TGF-β is produced by TGF-β–producing CD4+ T cells which, when transferred to naive recipients, prevent induction of TNBS colitis or colitis induced by concomitantly transferred IFN-γ–producing CD4+ T cells (our unpublished results). Similarly, in the SCID transfer model, it has been shown that coadministration of anti–TGF-β prevents the ability of CD45RBlo T cells to prevent colitis induced by CD45RBhi T cells 8 38 39. The above data relating to the suppressive effect of TGF-β are paralleled by evidence relating to the suppressive effect of IL-10. Thus, it has been shown that CD45RBlo T cells from IL-10−/− mice fail to protect SCID mice given CD45RBhi T cells from the development of colitis and, more importantly, administration of Ab to IL-10R also prevents the ability of CD45RBlo T cells to protect SCID mice from the development of colitis 40.
The above studies showing counterregulatory effects of both TGF-β and IL-10 secretion on the development of colitis do not necessarily imply that the effects of these cytokines are independent. On the contrary, it is possible that IL-10 is necessary for the development of TGF-β T cells or for TGF-β secretion; conversely, it is possible that TGF-β is necessary for development of IL-10–secreting T cells or IL-10 secretion.
The present data relate to this question because they show that in a situation where TGF-β production is due to the presence of a CMV promotor–driven plasmid, and thus is IL-10 independent, TGF-β does not require IL-10 to bring about suppression. In other words, TGF-β is a sufficient suppressor effector molecule. However, it remains possible that in normal mice development of TGF-β–producing T cells and/or TGF-β secretion is dependent on IL-10. In addition, it remains possible that in normal mice TGF-β secretion acts in part via IL-10, as we show in this study that TGF-β induces IL-10 secretion, and it is known that IL-10 inhibits IL-12 secretion 41. One last point concerning the effect of intranasal administration of a TGF-β1–encoding DNA on IL-10 production is that such administration leads to IL-10 production in both T cells and macrophages. In contrast, in both an earlier study and this study, it was shown that administration of rTGF-β1 induced IL-10 production by macrophages, but not T cells 42. This difference may explain the fact that administration of rTGF-β1 did not affect established TNBS colitis, as the suppressive effect of TGF-β1 may be dependent on T cell secretion of IL-10.
One caveat to the above sufficiency of TGF-β as a suppressor effector is that the effect of anti–IL-10 on the ability of pCMV-TGF-β plasmid to affect TNBS colitis depended somewhat on whether the anti–IL-10 was administered during induction of colitis or after colitis was established. Thus, although plasmid administration completely blocked the induction of colitis in the presence of anti–IL-10 administration, it only partially reversed established colitis under these circumstances. This partial effect of anti–IL-10 in established colitis cannot be ascribed to an incomplete ability of anti–IL-10 to neutralize IL-10, as the anti–IL-10 used consisted of an Ab mixture that had a profound effect on IL-10 production by CD4+ T cells at both the protein and mRNA level (our unpublished observations). A more likely possibility relates to the fact that IL-10 has downregulatory effects on TNF-α secretion 43 44 45, and that anti–IL-10 administration was associated with increased TNF-α production mainly by LP macrophages. Thus, it is possible that intranasal pCMV-TGF-β plasmid administration completely prevented induction of TNBS colitis even in the presence of anti–IL-10, because in this situation a large number of macrophages capable of producing TNF-α and mediating disease had not yet accumulated, whereas in established colitis a large number of macrophages had accumulated whose secretion of TNF-α was not immediately suppressed in the presence of anti–IL-10.
The results obtained here, in parallel with previous studies of intranasal TGF-β DNA delivery, indicate that such delivery ameliorates T cell–mediated inflammation whether the latter is mediated by viral infection or, in this case, by an antigen or antigens in the mucosal milieu 19. For these observations to translate into therapy for human inflammation, much more must be known about the persistence of the DNA in tissues and its possible harmful effects. In these studies, we showed using RT-PCR with TGF-β1–specific primers that mRNA arising from plasmid TGF-β1–encoding DNA could be detected in tissues for 2 wk, as in previous reports, but was not detected after this time. As for possible harmful effects of DNA expressing TGF-β, in earlier studies it was shown that intratracheal administration of TGF-β1 (in an active form) via an adenovirus vector led to severe lung fibrosis in a rat model 46. In addition, it was shown that transgenic mice carrying an active TGF-β1 DNA under the control of an albumin promotor expressed TGF-β in hepatocytes and developed hepatic and renal fibrosis as well as atrophy of the pancreas and testis 47 48. In contrast, in our studies, careful histologic follow-up of multiple tissues disclosed neither macroscopic nor microscopic signs of fibrosis. The reason for this discrepancy from previous studies is not yet known. However, it may relate to the difference in the TGF-β1 DNA delivery system, and thus the duration of TGF-β1 secretion.
Finally, the possibly unique characteristics of intranasal delivery of plasmid as used here deserve comment. In previous reports, plasmid DNA encoding native TGF-β or adenovirus encoding IL-4 were intraperitoneally administered to rats with TNBS colitis, and plasmid DNA encoding native TGF-β was administered by an intramuscular route to rats with streptococcal cell wall–induced arthritis 49 50 51. Although these plasmid preparations had therapeutic effects, they required multiple administration of plasmid. In contrast, the intranasal gene delivery in this study required only a single plasmid administration and was noninvasive. These differences may relate to recent data showing that nasal administration of antigen is a highly effective method of inducing oral tolerance, possibly because of the presence of cells in nodes draining nasal passages that readily populate mucosal tissues 52.
Acknowledgments
We thank Drs. Ryuta Nishikomori, Rolf O. Ehrdhardt, and Kevin Chua for valuable technical advice and support.
Footnotes
Abbreviations used in this paper: H and E, hematoxylin and eosin; LP, lamina propria; LPMC, lamina propria mononuclear cell; RT, reverse transcription; SAC, Staphylococcus aureus Cowans; STAT4, signal transducer and activator of transcription 4.
References
- Elson C.O. Experimental models of intestinal inflammationnew insights into mechanism of mucosal hoemostasis. In: Ogra P.L., Mestecky J., Lamm M.E., Strober W., Bienestock J., McGhee J., editors. Mucosal Immunology. 2nd ed. Academic Press; San Diego, CA: 1999. pp. 1007–1024. [Google Scholar]
- Elson C.O., Sartor R.B., Tennyson G.S., Riddell R.H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Strober W., Kelsall B., Marth T., Ludviksson B., Erhardt R., Neurath M. Reciprocal IFN-γ and TGF-β responses regulate the occurrence of mucosa inflammation. Immunol. Today. 1997;18:61–64. doi: 10.1016/s0167-5699(97)01000-1. [DOI] [PubMed] [Google Scholar]
- Groux H., Powrie F. Regulatory T cells and inflammatory bowel disease. Immunol. Today. 1999;20:442–445. doi: 10.1016/s0167-5699(99)01510-8. [DOI] [PubMed] [Google Scholar]
- Bhan A.K., Mizoguchi E., Smith R.N., Mizoguchi A. Colitis in transgenic and knockout animals as models of human inflammatory bowel disease. Immunol. Rev. 1999;169:195–207. doi: 10.1111/j.1600-065x.1999.tb01316.x. [DOI] [PubMed] [Google Scholar]
- Powrie F., Leach M.W., Mauze S., Caddle L.B., Coffman R.L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in CB-17 Scid mice. Int. Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- Morrissey P.J., Charrier K., Braddy S., Liggitt D., Watson J.D. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J. Exp. Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Leach M.W., Mauze S., Menon S., Caddle L.B., Coffman R.L. Inhibition of Th1 responses prevents inflammatory bowel disease in SCID mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Neurath M.F., Fuss I., Kelsall B.L., Stüber E., Strober W. Antibodies to IL-12 abrogate established experimental colitis in mice. J. Exp. Med. 1995;182:1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohi T., Fujihashi K., Rennert P.D., Iwatani K., Kiyono H., McGhee J.R. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2–type responses. J. Exp. Med. 1999;189:1169–1180. doi: 10.1084/jem.189.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirivant M., Fuss I.J., Chu A., Strober W. Oxazolone colitisa murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J. Exp. Med. 1998;188:1929–1939. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D.K. Inflammatory bowel disease. N. Engl. J. Med. 1991;325:928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- Strober W., Ludviksson B.R., Fuss I.J. The pathogenesis of mucosal inflammation in murine models of inflammatory bowel disease and Crohn disease. Ann. Intern. Med. 1998;128:848–856. doi: 10.7326/0003-4819-128-10-199805150-00009. [DOI] [PubMed] [Google Scholar]
- Fiocchi C. Inflammatory bowel diseaseetiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- Groux H., O'Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J.E., Roncarolo M.G. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Neurath M.F., Fuss I., Kelsall B.L., Presky D.H., Waegell W., Strober W. Experimental granulomatous colitis in mice is abrogated by induction of TGF-β–mediated oral tolerance. J. Exp. Med. 1996;183:2605–2616. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Inobe J., Marks R., Gonnella P., Kuchroo V.K., Weiner H.L. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- Fukaura H., Kent S.C., Pietrusewicz M.J., Khoury S.J., Weiner H.L., Hafler D.A. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor-β1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J. Clin. Invest. 1996;98:70–77. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklin N.A., Daheshia M., Chun S., Rouse B.T. Immunomodulation by mucosal gene transfer using TGF-β DNA. J. Clin. Invest. 1998;102:438–444. doi: 10.1172/JCI2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun S., Daheshia M., Lee S., Eo S.K., Rouse B.T. Distribution fate and mechanism of immune modulation following mucosal delivery of plasmid DNA encoding IL-10. J. Immunol. 1999;163:2393–2402. [PubMed] [Google Scholar]
- Barnard J.A., Lyons R.M., Moses H.L. The cell biology of transforming growth factor-β. Biochim. Biophys. Acta. 1990;1032:79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- Samuel S.K., Hurta R.A., Kondaiah P., Khalil N., Turley E.A., Wright J.A., Greenberg A.H. Autocrine induction of tumor protease production and invasion by a metallothionein-regulated TGF-β1 (Ser223, 225) EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:1599–1605. doi: 10.1002/j.1460-2075.1992.tb05205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S.M., Poczatek M., Schultz-Cherry S., Villain M., Murphy-Ullrich J.E. The activation sequence of thrombospondin-1 interacts with the latency-associated peptide to regulate activation of latent transforming growth factor-β. J. Biol. Chem. 1999;274:13586–13593. doi: 10.1074/jbc.274.19.13586. [DOI] [PubMed] [Google Scholar]
- Mosmann T.R., Schumacher J.H., Fiorentino D.F., Leverah J., Moore K.W., Bond M.W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J. Immunol. 1990;145:2938–2945. [PubMed] [Google Scholar]
- Kitani A., Strober W. Differential regulation of Cα1 and Cα2 germ-line and mature mRNA transcripts in human peripheral blood B cells. J. Immunol. 1994;153:1466–1477. [PubMed] [Google Scholar]
- Van der Heijden P.J., Stok W. Improved procedure for the isolation of functionally active lymphoid cells from the murine intestine. J. Immunol. Methods. 1987;103:161–167. doi: 10.1016/0022-1759(87)90285-7. [DOI] [PubMed] [Google Scholar]
- Nishikomori R., Ehrhardt R.O., Strober W. T helper 2 cell differentiation occurs in the presence of interleukin 12 receptor β2 chain expression and signaling. J. Exp. Med. 2000;191:847–858. doi: 10.1084/jem.191.5.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couffinhal T., Kearney M., Sullivan A., Silver M., Tsurumi Y., Isner J.M. Histochemical staining following LacZ gene transfer underestimates transfection efficiency. Hum. Gene. Ther. 1997;8:929–934. doi: 10.1089/hum.1997.8.8-929. [DOI] [PubMed] [Google Scholar]
- Cheng G., Thompson R.P., Gourdie R.G. Improved detection reliability of β-galactosidase in histological preparations. Biotechniques. 1999;27:438–440. doi: 10.2144/99273bm08. [DOI] [PubMed] [Google Scholar]
- Jacobson N.G., Szabo S.J., Weber-Nordt R.M., Zhong Z., Schreiber R.D., Darnell J.E., Jr., Murphy K.M. Interleukin 12 signaling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J. Exp. Med. 1995;181:1755–1762. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierfelder W.E., van Deursen J.M., Yamamoto K., Tripp R.A., Sarawar S.R., Carson R.T., Sangster M.Y., Vignali D.A., Doherty P.C., Grosveld G.C., Ihle J.N. Requirement for Stat-4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–174. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- Simpson S.J., Shah S., Comiskey M., de Jong Y.P., Wang B., Mizoguchi E., Bhan A.K., Terhorst C. T cell–mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/Signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon gamma expression by T cells. J. Exp. Med. 1998;187:1225–1234. doi: 10.1084/jem.187.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M.F., Fuss I., Pasparakis M., Alexopoulou L., Haralambous S., Meyer zum Buschenfelde K.H., Strober W., Kollias G. Predominant pathogenic role of tumor necrosis factor-α in experimental colitis in mice. Eur. J. Immunol. 1997;27:1743–1750. doi: 10.1002/eji.1830270722. [DOI] [PubMed] [Google Scholar]
- Corazza N., Eichenberger S., Eugsterand H.P., Mueller C. Nonlymphocyte-derived tumor necrosis factor is required for induction of colitis in recombination activating gene (RAG)2−/− mice upon transfer of CD4+CD45RBhi T cells. J. Exp. Med. 1999;190:1479–1492. doi: 10.1084/jem.190.10.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontoyiannis D., Pasparakis M., Pizarro T.T., Cominelli F., Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elementsimplications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- Szabo S.J., Dighe A.S., Gubler U., Murphy K.M. Regulation of the interleukin (IL)-12Rβ2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J. Exp. Med. 1997;185:817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogge L., Barberis-Maino L., Biffi M., Passini N., Presky D.H., Gubler U., Sinigaglia F. Selective expression of an interleukin 12 receptor component by human T helper 1 cells. J. Exp. Med. 1997;185:825–831. doi: 10.1084/jem.185.5.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Carlino J., Leach M.W., Mauze S., Coffman R.L. A critical role for transforming growth factor-β but not interleukin 4 in the suppression of T helper type 1–mediated colitis by CD45RBlow CD4+ T cells. J. Exp. Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrie F., Correa-Oliveira R., Mauze S., Coffman R.L. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J. Exp. Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseman C., Mauze S., Leach M.W., Coffman R.L., Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal B.M., Dwyer B.K., Shevach E.M. An interleukin (IL)-10/IL-12 immunoregulatory circuit controls susceptibility to autoimmune disease. J. Exp. Med. 1998;187:537–546. doi: 10.1084/jem.187.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H., Kuwahara H., Ichimura Y., Ohtsuki M., Kurakata S., Shiraishi A. TGF-β enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J. Immunol. 1995;155:4926–4932. [PubMed] [Google Scholar]
- Clarke C.J., Hales A., Hunt A., Foxwell B.M. IL-10-mediated suppression of TNF-α production is independent of its ability to inhibit NF kappa B activity. Eur. J. Immunol. 1998;28:1719–1726. doi: 10.1002/(SICI)1521-4141(199805)28:05<1719::AID-IMMU1719>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Fiorentino D.F., Zlotnik A., Mosmann T.R, Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- Wanidworanun C., Strober W. Predominant role of tumor necrosis factor-α in human monocyte IL-10 synthesis. J. Immunol. 1993;151:6853–6861. [PubMed] [Google Scholar]
- Sime P.J., Xing Z., Graham F.L., Csaky K.G., Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-β induces prolonged severe fibrosis in rat lung. J. Clin. Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson N., Factor V., Nagy P., Kopp J., Kondaiah P., Wakefield L., Roberts A.B., Sporn M.B., Thorgeirsson S.S. Hepatic expression of mature transforming growth factor-β1 in transgenic mice results in multiple tissue lesions. Proc. Natl. Acad. Sci. USA. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp J.B., Factor V.M., Mozes M., Nagy P., Sanderson N., Bottinger E.P., Klotman P.E., Thorgeirsson S.S. Transgenic mice with increased plasma levels of TGF-β1 develop progressive renal disease. Lab. Invest. 1996;74:991–1003. [PubMed] [Google Scholar]
- Giladi E., Raz E., Karmeli F., Okon E., Rachmilewitz D. Transforming growth factor-β gene therapy ameliorates experimental colitis in rats. Eur. J. Gastroenterol. Hepatol. 1995;7:341–347. [PubMed] [Google Scholar]
- Hogaboam C.M., Vallance B.A., Kumar A., Addison C.L., Graham F.L., Gauldie J., Collins S.M. Therapeutic effects of interleukin-4 gene transfer in experimental inflammatory bowel disease. J. Clin. Invest. 1997;100:2766–2776. doi: 10.1172/JCI119823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X.Y., Gu M., Jin W.W., Klinman D.M., Wahl S.M. Plasmid DNA encoding transforming growth factor-β1 suppresses chronic disease in a streptococcal cell wall-induced arthritis model. J. Clin. Invest. 1998;101:2615–2621. doi: 10.1172/JCI2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolvers D.A., Coenen-de Roo C.J., Mebius R.E., van der Cammen M.J., Tirion F., Miltenburg A.M., Kraal G. Intranasally induced immunological tolerance is determined by characteristics of the draining lymph nodesstudies with OVA and human cartilage gp-39. J. Immunol. 1999;162:1994–1998. [PubMed] [Google Scholar]