Abstract
The well defined, immature murine dendritic cell (DC) line D1 was used to study the role of DC maturation in CTL induction in vitro and in vivo. Maturation of D1 cells, characterized by markedly increased expression of MHC and costimulatory molecules, was induced by incubation with lipopolysaccharide, agonistic CD40 antibody, or specific CD4+ T helper (Th) cells. Activated, but not immature, D1 cells efficiently primed alloreactive T cell responses in vitro. Similarly, priming of CTL immunity in vivo in CD4-depleted mice was only observed if these mice were immunized with activated D1 cells. This study provides formal evidence that activation of DCs, induced by Th-independent as well as Th-dependent stimuli, is essential for efficient induction of CTL responses.
Keywords: dendritic cells, CD40 ligation, lipopolysaccharide, Th cells, cytotoxic T lymphocyte induction
Introduction
Dendritic cells (DCs) comprise a family of professional APCs that are present in low numbers in many body tissues 1. DCs are crucial for the initiation of primary immune responses of both helper and cytotoxic T lymphocytes, and thus act as “nature's adjuvant”. DCs can differentiate from immature to mature stages. Maturation of DCs is characterized by a decreased antigen processing capacity and an increased cell surface expression of MHC and costimulatory molecules 1 2 3. In addition, rearrangement of cytoskeleton 4, adhesion molecules 5, and cytokine receptors 6 upon maturation allows DCs to migrate to lymphoid organs, where they can induce primary T cell responses.
An important cognate event in the development of cell-mediated immunity is the interaction between CD40 and CD40 ligand (CD40L). CD40 is fairly widely distributed and is expressed on B lymphocytes, monocytes, and DCs, but also on endothelial and epithelial cells 7. The ligand of CD40 (CD40L or CD154) has a more restricted distribution, being mainly expressed by activated CD4+ T lymphocytes 8. In vivo, use of CD40-stimulatory antibodies has shown that CD40 signaling provides help for the induction of CTL responses 9 10. The CD40-specific antibody FGK45 was capable of replacing T cell help in CD4-depleted mice for cross-priming adenovirus- and OVA-specific CTLs 9 10. The fact that the role of B cells was excluded implied the involvement of DCs. Still, activation and functional antigen presentation by DCs was not formally proven in these in vivo experiments. In related studies, DC populations were used for activation by CD40 antibodies in vitro and tested for their CTL priming capacity in vitro and in vivo 11. However, these DCs were not phenotypically immature, as indicated by their very high expression levels of B7.2. No studies have been reported in which highly purified immature and mature DC preparations have been compared with respect to their CTL-inducing capacity in vitro and/or in vivo. It is indeed very difficult, if not impossible, to obtain such pure populations. We have therefore used the well characterized growth factor–dependent, long term DC line D1 to examine the role of activation of DCs by different stimuli in the induction of T cell responses. This nontransformed homogeneous DC population is of C57BL/6 origin and is spleen derived 4 12. Immature D1 cells are characterized by proliferative capacity, high antigen uptake ability, and low T cell–stimulatory efficiency, thus behaving as “classical” immature DCs. Moreover, expression levels of costimulatory molecules and cell surface MHC class I molecules are low, whereas cell surface MHC class II expression is intermediate. Upon activation by living bacteria or the bacterial component LPS, D1 cells can be activated to full maturation. This results in cell growth arrest, low antigen uptake, and high expression of costimulatory and MHC molecules at the cell surface 12.
Although the concept of requirement of DC maturation for CTL induction is well established, no formal evidence bearing out this concept has been published thus far. Here we report that functional maturation of DCs is essential for effective CTL priming capacity in vitro and in vivo. In the absence of CD4+ T cells, this can be achieved independently by either of two signals: CD40 triggering or LPS stimulation.
Materials and Methods
Mice.
Female C57BL/6 (B6; H-2b) and BALB/c (H-2d) mice were obtained from IFFA Credo, and BALB/c × C57BL/6 F1 (CB6 F1, H-2dxb) mice were from Charles River Laboratories. Mice were maintained under specific pathogen–free conditions and used at 6–10 wk of age.
Cell Lines and Reagents.
Adenovirus type 5 E1–transformed B6 mouse embryo cells (Ad5E1-MECs) were generated as described 10. P815 is a DBA/2 (H-2d)-derived mastocytoma cell line. Cell lines were cultured in IMDM (BioWhittaker) containing 8% heat-inactivated FCS (Greiner), 100 IU/ml penicillin, 2 mM l-glutamine, and 20 μM 2-ME. Th1 cells were obtained from DO11.10 TCR-transgenic mice 13 on BALB/c background (H-2d). These Th cells can efficiently recognize OVA helper peptide as presented in I-Ab. LPS of Escherichia coli (serotype 0111:B4) was from Difco Labs. The FGK45 hybridoma 14 was provided by Dr. A. Rolink (Basel Institute for Immunology, Basel, Switzerland) and used as concentrated hybridoma supernatant with endotoxin levels below detection (Limulus Amebocyte Lysate COATEST® for endotoxin). Synthetic peptides used were: E7CTL (HPV16 E7 49–57), RAHYNIVTF; E1ACTL (E1A 234–243), SGPSNTPPEI; and OVATh (OVA 323–339), ISQAVHAAHAEINEAGR.
DCs.
D1 cell line, a long term growth factor–dependent immature splenic DC line derived from B6 (H-2b) mice, was cultured as described 4. Both floating and adherent cells (detached using 2 mM EDTA) were collected and used.
Antibodies and Cell Surface Immunofluorescence.
The following antibodies were purchased from PharMingen: FITC-coupled CD86/B7.2 antibody (GL1), FITC-coupled CD8 antibody (Ly2), and PE-conjugated anti–class II (I-Ab,d/Ed) antibody (2G9). PE-coupled CD40 antibody (3/23) was obtained from Serotec. Anti–class I (Kb) mAb (B8-24-3) was purified and biotinylated. D1 cells were incubated with antibodies in the presence of 30% 2.4G2 supernatant (rat anti–mouse FcγRIII/II) to block FcR binding. PE-conjugated, E1ACTL-loaded H-2Db tetramers were provided by T. Schumacher (Netherlands Cancer Institute, Amsterdam, The Netherlands). Staining for tetramer complexes was carried out as described 15. Flow cytometry was performed with FACScan™ (Becton Dickinson).
Induction of Allospecific Responses In Vitro.
Immature D1 cells or D1 cells that were treated with 10 μg/ml LPS or 30 μg/ml FGK45 for 48 h were irradiated and incubated at graded doses with allogeneic BALB/c spleen cells in 96-well flat-bottomed plates. Syngeneic B6 spleen cells were used as control. Allospecific proliferation was measured after 4 d. 18 h before termination, 0.5 μCi [3H]thymidine was added per well. To induce allospecific CTLs, 3 × 106 BALB/c spleen cells were incubated with 104 irradiated immature D1 cells or LPS- or FGK45-treated D1 cells in 24-well plates. After 6-d incubation at 37°C, cells were harvested and used as effectors in a cytotoxicity assay. 51Cr-labeled cells of H-2b haplotype (RMA) or H-2d haplotype (P815) were used as targets. Percent specific lysis of triplicate wells was calculated 10.
Induction of CTL Responses In Vivo.
To induce CTL responses in vivo, untreated D1 cells or D1 cells treated for 48 h with 10 μg/ml LPS, 30 μg/ml FGK45, or Th1 cells (DC/Th = 10:1, in the presence of 5 μM OVATh peptide) were loaded with E1ACTL peptide for 2 h at 37°C and washed five times. 106 D1 cells were injected intravenously into B6 mice (LPS- and FGK45-treated D1 cells) or CB6 F1 mice (Th1-treated D1 cells) in PBS with 0.5% BSA. CB6 F1 mice were used to avoid alloresponses (Th1 cells are BALB/c derived). Mice were depleted of CD4+ cells by intraperitoneal injection of 100 μg of purified CD4 antibody GK1.5 in PBS at day 5, 3, and 1 before and at day 1 and 7 after injection of D1 cells. Depletion was performed to prevent endogenous CD4+ Th cells from activating the D1 cells in vivo (our unpublished results). After 10 d, spleen cells (5 × 106 per well) were restimulated with irradiated Ad5E1-MECs (5 × 105 per well) in 2-ml cultures in 24-well plates in the absence of additional cytokines. After 6 d, lymphocyte cultures were tested for cytotoxicity against Eu3+-labeled RMA cells loaded with E1ACTL peptide or control E7CTL peptide.
IL-12 Production.
D1 cells (106) were seeded in 24-well plates with OVATh-specific Th1 cells (D1/Th = 10:1) in the presence or absence of 5 μM OVATh peptide. After 48-h culture at 37°C, supernatants were tested for IL-12 p40 content using a standard sandwich ELISA. Coating antibody was rat anti–mouse IL-12 p40/p70 mAb (clone C15.6; PharMingen). Detection antibody was biotinylated rat anti–mouse IL-12 p40/p70 (clone C17.8; PharMingen). Streptavidin–horseradish peroxidase and ABTS (Sigma-Aldrich) were used as enzyme and substrate, respectively.
Results
Agonistic CD40 Antibody or LPS Treatment Induces Phenotypic Maturation of Murine DCs.
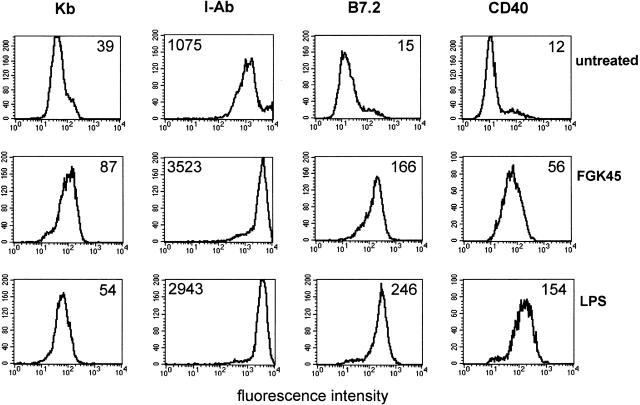
To study the effect of maturation on DC function, we used the well established murine DC line D1 12. D1 cells can be maintained in culture in an immature state, as indicated by very low levels of costimulatory molecules (B7.2 [CD86] and CD40) and low to intermediate levels of MHC class I (Kb) and II (I-Ab), respectively (Fig. 1). When incubated with the CD40-specific agonistic antibody FGK45 or the CD40-independent stimulus LPS for 48 h, D1 cells exhibit strongly elevated levels of the costimulatory molecules B7.2 and CD40 as well as MHC class I and II (Fig. 1). This demonstrates that triggering of CD40 on D1 cells using the FGK45 mAb induced maturation of these cells similar to LPS.
Figure 1.
Both agonistic CD40 antibody and LPS treatment induce phenotypic maturation of D1 cells. D1 cells were treated with LPS or CD40-stimulating antibody FGK45 or left untreated (immature) for 48 h and stained with antibodies against the indicated markers. Data from one representative experiment out of three experiments performed are shown. Numbers indicate the median fluorescence intensity.
Improved Induction of Allospecific T Cell Responses by FGK45- and LPS-treated D1 Cells.
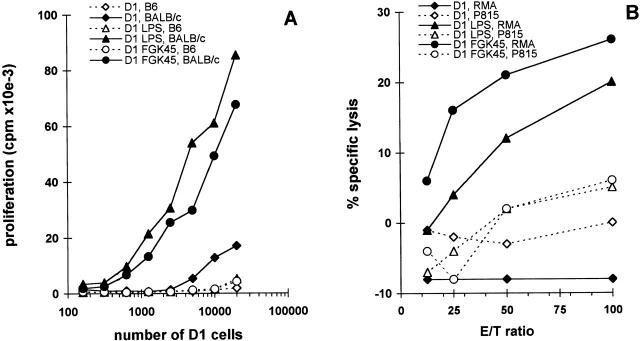
We observed that LPS- or FGK45-matured D1 cells are more efficient in presenting both MHC class I and class II binding peptides derived from OVA to peptide-specific MHC class I– or class II–restricted T cell hybridomas (data not shown). This indicates that the induced MHC class I and class II molecules are functional and that the elevated expression of these molecules is associated with more efficient antigen presentation. We therefore investigated whether FGK45- or LPS-treated D1 cells are more efficient in induction of a primary MLR. Immature D1 cells and LPS- or FGK45-treated D1 cells were used as allogeneic stimulators for unprimed BALB/c spleen cells. B6 spleen cells were used as syngeneic control. D1 cells were titrated and cultured with a fixed number of spleen cells for 4 d. Both FGK45- and LPS-activated D1 cells induced much higher alloproliferation than nontreated D1 cells (Fig. 2 A). In the same experiment, we tested the presence of allospecific cytotoxic T cells in the MLR. After 6 d, the cultured cells were tested for specific cytotoxicity. CTL induction in vitro by both FGK45- and LPS-activated D1 cells was much more efficient than by immature D1 cells (Fig. 2 B). Thus, treatment of D1 cells with FGK45 or LPS led to functional maturation, which resulted in acquisition of the capacity to induce strong alloproliferative and allocytotoxic T cell responses.
Figure 2.
Efficient induction of primary allospecific responses in vitro by mature D1 cells. D1 cells (H-2b), treated with agonistic CD40 antibody FGK45 or LPS for 48 h, were used as stimulator cells in a proliferation assay with 105 BALB/c (H-2d) or control syngeneic B6 (H-2b) responder cells (A) or as stimulator cells in bulk cultures for the induction of allospecific CTLs (B). For CTL induction, 104 D1 cells were incubated with 3 × 106 BALB/c spleen cells for 6 d. CTL activity was measured in a cytotoxicity assay using targets of H-2b haplotype (RMA) or H-2d haplotype (P815). Values represent means of triplicates from one representative experiment out of three performed.
In Vivo Priming of Antigen-specific CTLs by CD40- or LPS-stimulated D1 Cells in CD4-depleted Mice.
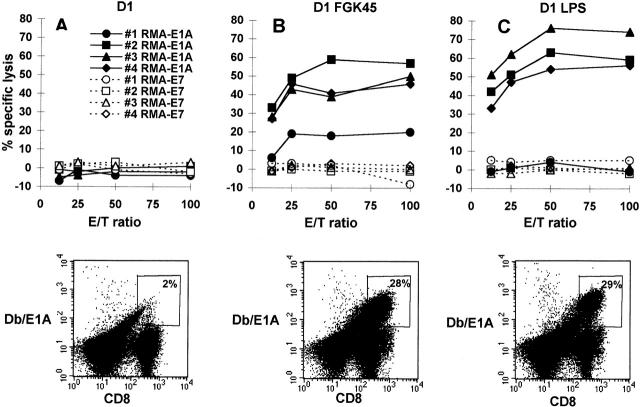
Next, we investigated whether the phenotypic and functional differences between immature and activated D1 cells as observed in vitro had consequences for their capacity to prime specific CTL immunity in vivo. Immature D1 cells, exogenously loaded with a human adenovirus CTL epitope (E1ACTL), were injected into CD4-depleted mice. 10 d after immunization, spleens were harvested and splenocytes were restimulated in vitro with Ad5E1-MECs. After 6 d, epitope-specific CTL activity was measured in a cytotoxicity assay. E1ACTL peptide–loaded immature D1 cells did not prime peptide-specific CTLs in CD4-depleted mice (Fig. 3 A and 4 C), in agreement with our recent study 15 describing the lack of Ad5E1A-specific CTL priming in the absence of CD4+ T cell help. However, in this study, CTL priming was restored by in vivo CD40 triggering, which is thought to activate bone marrow–derived APCs in vivo. Therefore, we studied whether in vitro–activated D1 cells, using agonistic CD40 antibody or LPS as stimuli 16, were able to induce priming of peptide-specific CTLs in vivo. In contrast to immature D1 cells (Fig. 3 A, top panel), D1 cells activated in vitro by FGK45 (Fig. 3 B, top panel) or LPS (Fig. 3 C, top panel) indeed primed E1ACTL-specific CTLs in vivo in CD4-depleted mice. The induction of E1ACTL-specific cytotoxicity correlated with elevated numbers of CD8+ T cells in the bulk cultures that stained with PE-conjugated H-2Db tetramers containing the E1ACTL peptide (Db/E1A) (Fig. 3, bottom panel).
Figure 3.
Induction of primary peptide-specific CTL responses in vivo by D1 cells treated with agonistic CD40 antibody or LPS. Immature D1 cells (A) and FGK45- (B) or LPS-treated (C) D1 cells were used for in vivo CTL induction by loading them with E1ACTL peptide and injecting them into CD4-depleted B6 mice. Three mice were injected with immature D1 cells, and four mice were injected with FGK45- or LPS-treated D1 cells. After 10 d, 5 × 106 spleen cells were restimulated in vitro using 5 × 105 Ad5E1-MECs. After 6 d, cells were used in a cytotoxicity assay using targets loaded with E1ACTL peptide or E7CTL peptide as control. Data shown in the top panels are means of triplicates from one representative experiment out of three experiments performed. The bottom panels show detection of E1ACTL-specific CD8+ cells in bulk cultures. Bulk cultures were analyzed for the presence of CD8+ cells capable of interacting with the H-2Db–E1ACTL tetrameric complexes. Indicated are percentages of the CD8+ cells staining with H-2Db–E1ACTL tetramers.
In Vivo Priming of Antigen-specific CTLs in CD4-depleted Mice by D1 Cells Stimulated with CD4+ Th Cells Plus Antigen.
The physiological activation signal through CD40 is thought to be delivered by CD40L+CD4+ Th cells. We therefore investigated whether in vitro incubation of D1 cells with peptide-specific Th cells resulted in functional activation of these APCs. Phenotypic maturation (Fig. 4 A) and IL-12 p40 production by D1 cells (Fig. 4 B) was induced by incubation of D1 cells with OVA-specific Th cells in the presence but not the absence of OVATh peptide. As already shown in Fig. 3 A, immunization of CD4-depleted mice with peptide-loaded immature D1 cells failed to induce significant CTL immunity (Fig. 4 C). In contrast, five out of eight mice immunized with E1ACTL peptide–loaded D1 cells that were preincubated with the OVA-specific Th cells showed strong E1A-specific CTL immunity (Fig. 4 D). No detectable CTL activity against control E7CTL peptide–loaded target cells was observed for mice injected with immature (Fig. 4 E) or Th-treated (Fig. 4 F) D1 cells. Therefore, in vitro Th-mediated activation empowered the DCs to prime CTL immunity in vivo.
Figure 4.
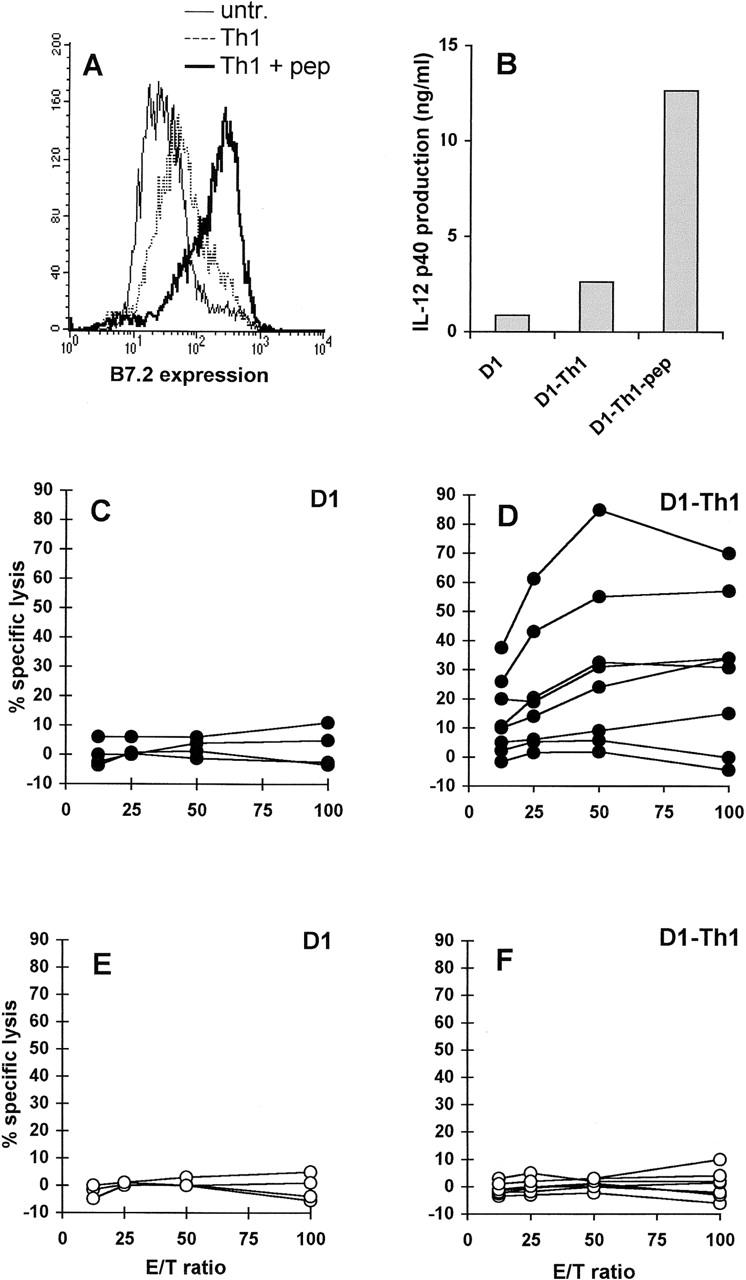
Priming of peptide-specific CTLs in vivo by D1 cells stimulated in vitro by specific CD4+ Th cells. B7.2 (CD86) expression of D1 cells was measured after 48-h incubation with Th1 cells in the presence or absence of 5 μM OVATh peptide (A). IL-12 p40 production was measured in supernatants of cultures containing D1 cells and OVATh-specific Th1 cells in the presence or absence of OVATh peptide (B). Immature D1 cells (C and E) and D1 cells incubated with OVATh-specific Th1 cells and OVATh peptide (D and F) were used for in vivo CTL induction after loading with E1ACTL peptide and injecting them into CD4-depleted mice. Four mice were injected with E1ACTL-loaded immature D1 cells (C and E); eight mice were injected with E1ACTL-loaded D1 cells that had been preincubated for 48 h with OVATh-specific Th cells in the presence of 5 μM OVATh peptide (D and F). 10 d after immunization, spleen cells were restimulated with Ad5E1-MECs and tested in a cytotoxicity assay using E1ACTL peptide–loaded (C and D) or control E7CTL peptide–loaded (E and F) RMA cells as targets. Data shown are means of triplicates. Each line represents one mouse.
Discussion
These experiments provide formal evidence that CTL priming in vivo depends on DC activation. The required DC activation state can be induced by CD4+ Th cells or by triggering of DCs with agonistic CD40 antibody or LPS.
Although the concept that DCs require maturation for efficient induction of T cell responses is widely recognized 1 2 4 16, it has been mainly shown for induction of proliferative responses. In the “licence to kill” model 17 18 supported by recent papers from three groups 9 10 11, Th cells activate APCs, thereby licensing them to directly activate CTLs. CD40-mediated maturation of mouse and human DCs by soluble 16 19 and transfected CD40L- 20 or CD40-specific antibody 11 has been reported. However, in studies where immature and mature DCs are compared for CTL induction or tumor protection, DCs in the “immature” state express already quite high levels of costimulatory and MHC molecules 11 19 21. These studies therefore allow no firm conclusions on the DC phenotype required for CTL induction. Our experience with culturing, for example, bone marrow–derived DCs in the presence of GM-CSF is that the purity and activation status of the resulting cells varies considerably between experiments, precluding firm conclusions on correlations between phenotype and function. By using the spleen-derived immature DC line D1, we were able to obtain a constant and reproducible source of pure and immature DCs that could be induced to mature by physiological (anti-CD40) and bacterial (LPS) stimuli in a well controlled fashion.
The anti-CD40 antibody FGK45 was used successfully in recent in vivo studies, illustrating the importance of the CD40–CD40L interaction in CTL induction 9 10. Antibody-induced CD40 activation even caused a therapeutic effect of a peptide-based CTL-inducing vaccine against established HPV16-induced mouse tumors, whereas this vaccine had only preventive activity in the absence of CD40 triggering 15. However, in these studies, no formal proof was obtained that the in vivo FGK45 treatment directly stimulated DCs and that this was causally related to the observed effects. In this study, we show that the antibody FGK45 directly activates the DC line D1, endowing these DCs with powerful CTL-inducing capacity in vitro and in vivo.
FGK45- and LPS-treated D1 cells showed elevated levels of MHC class I and II and strongly increased levels of the costimulatory molecules B7.2 and CD40. Incubation with CD40L–CD8 fusion protein led to a similar level of phenotypic maturation of D1 cells (data not shown), indicating that FGK45 triggers CD40 in a physiological way. Mature D1 cells were capable of effective induction of both MHC class I– and class II–directed alloresponses in vitro and peptide-specific CTL responses in vivo. The acquisition of the capacity to induce CTLs most likely is the result of both the increase in the expression of MHC class I and costimulatory molecules and the profile of cytokine production by mature DCs. Our data suggest that activation of the DCs is more important than the way in which the cells are activated for induction of CTL responses. The efficiency of CTL induction in vivo in CD4-depleted mice by in vitro LPS-matured DCs suggests that even CD40-independent activation of DCs leads to efficient CTL induction. This indicates the possibility that CD8+ CTL responses can be induced both by CD4+ Th-dependent (CD40-mediated) and CD4+ Th-independent pathways of DC activation. CD40 ligation by CD40L expressed on CD4+ Th cells might be the most relevant maturation condition for DCs in case of the lack of inflammatory conditions, such as many instances of tumor growth or responses to minor histocompatibility antigens, both of which are profoundly CD4+ Th cell dependent 22 23. In a recent study, CD40-independent, Th-dependent CD8+ T cell priming was reported, although a major CD40-dependent pathway of CTL activation was shown 21. In the case of strong inflammatory viruses or bacteria, however, DCs can apparently become activated in a CD4+ Th cell–independent way 24 25 26. In addition, antiviral CD8+ T cells were shown to be capable of inducing functional maturation of DCs in the absence of CD4+ Th cells 27. Clearly, the extent of DC maturation and/or polarization is a major determinant of the CD4+ helper dependence of CTL responses against infectious agents and tumor cells.
Acknowledgments
We thank Dr. D. Roelen for critical reading of this manuscript.
D.H. Schuurhuis and M.J. Kleijmeer are supported by the Dutch Organization for Research (grant NWO 901-09-241). F. Ossendorp is financed by the Netherlands Cancer Foundation (grant 97-1451).
References
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Cella M., Engering A., Pinet V., Pieters J., Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- Pierre P., Turley S.J., Gatti E., Hull M., Meltzer J., Mirza A., Inaba K., Steinman R.M., Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- Winzler C., Rovere P., Rescigno M., Granucci F., Penna G., Adorini L., Zimmermann V.S., Davoust J., Ricciardi-Castagnoli P. Maturation stages of mouse dendritic cells in growth factor–dependent long-term cultures. J. Exp. Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roake J.A., Rao A.S., Morris P.J., Larsen C.P., Hankins D.F., Austyn J.M. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 1995;181:2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Schaerli P., Loetscher P., Schaniel C., Lenig D., Mackay C.R., Qin S.X., Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Van Kooten C., Banchereau J. Functions of CD40 on B cells, dendritic cells and other cells. Curr. Opin. Immunol. 1997;9:330–337. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- Roy M., Waldschmidt T., Aruffo A., Ledbetter J.A., Noelle R.J. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J. Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- Bennett S.R.M., Carbone F.R., Karamalia F., Flavell R.A., Miller J.F.A.P., Heath W.R. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- Schoenberger S.P., Toes R.E.M., van der Voort E.I.H., Offringa R., Melief C.J.M. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- Ridge J.P., Di Rosa F., Matzinger P. A conditional dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- Rescigno M., Winzler C., Delia D., Mutini C., Lutz M., Ricciardi-Castagnoli P. Dendritic cell maturation is required for initiation of the immune response. J. Leukoc. Biol. 1997;61:415–421. [PubMed] [Google Scholar]
- Ria F., Penna G., Adorini L. Th1 cells induce and Th2 inhibit antigen-dependent IL-12 secretion by dendritic cells. Eur. J. Immunol. 1998;28:2003–2016. doi: 10.1002/(SICI)1521-4141(199806)28:06<2003::AID-IMMU2003>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Rolink A., Melchers F., Andersson J. The SCID but not the RAG-2 gene product is required for Su-Se heavy chain class switching. Immunity. 1996;5:319–330. doi: 10.1016/s1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- Diehl L., Den Boer A.T., Schoenberger S.P., Van der Voort E.I.H., Schumacher T.N.M., Melief C.J.M., Offringa R., Toes R.E.M. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat. Med. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- Cella M., Scheidegger D., Palmer-Lehman K., Lane P., Lanzavecchia A., Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacityT–T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerder S., Matzinger P. A fail-safe mechanism for maintaining self-tolerance. J. Exp. Med. 1992;176:553–564. doi: 10.1084/jem.176.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Immunology-licence to kill. Nature. 1998;393:413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- Labeur M.S., Roters B., Pers B., Mehling A., Luger T.A., Schwarz T., Grabbe S. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J. Immunol. 1999;162:168–175. [PubMed] [Google Scholar]
- Caux C., Massacrier C., Vanbervliet B., Dubois B., Van Kooten C., Durand I., Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Yuan L., Zhou X., Sotomayor E., Levitsky H.I., Pardoll D.M. CD40-independent pathways of T cell help for priming of CD8+ cytotoxic T lymphocytes. J. Exp. Med. 2000;191:541–550. doi: 10.1084/jem.191.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E., Roopenian D., Goulmy E. Much ado about minor histocompatibility antigens. Immunol. Today. 1998;19:108–112. doi: 10.1016/s0167-5699(97)01213-9. [DOI] [PubMed] [Google Scholar]
- Melief C.J.M., Toes R.E.M., Medema J.P., van der Burg S.H., Ossendorp F., Offringa R. Strategies for immunotherapy of cancer. Adv. Immunol. 2000;75:235–281. doi: 10.1016/s0065-2776(00)75006-1. [DOI] [PubMed] [Google Scholar]
- Cella M., Salio M., Sakakibara Y., Langen H., Julkunen I., Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J. Exp. Med. 1999;189:821–829. doi: 10.1084/jem.189.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R.M.L., Holmes K.L., Hugin A., Frederickson T.N., Morse H.C.I. Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987;328:77–79. doi: 10.1038/328077a0. [DOI] [PubMed] [Google Scholar]
- Vasilakos J.P., Michael J.G. Herpes simplex virus class I-restricted peptide induces cytotoxic T lymphocytes in vivo independent of CD4+ T cells. J. Immunol. 1993;150:2346–2355. [PubMed] [Google Scholar]
- Ruedl C., Kopf M., Bachmann M.F. CD8+ T cells mediate CD40-independent maturation of dendritic cells in vivo. J. Exp. Med. 1999;189:1875–1883. doi: 10.1084/jem.189.12.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]