Abstract
Long-lived humoral immunity is manifested by the ability of bone marrow plasma cells (PCs) to survive for extended periods of time. Recent studies have underscored the importance of BLyS and APRIL as factors that can support the survival of B lineage lymphocytes. We show that BLyS can sustain PC survival in vitro, and this survival can be further enhanced by interleukin 6. Selective up-regulation of Mcl-1 in PCs by BLyS suggests that this α-apoptotic gene product may play an important role in PC survival. Blockade of BLyS, via transmembrane activator and cyclophilin ligand interactor–immunoglobulin treatment, inhibited PC survival in vitro and in vivo. Heightened expression of B cell maturation antigen (BCMA), and lowered expression of transmembrane activator and cyclophilin ligand interactor and BAFF receptor in PCs relative to resting B cells suggests a vital role of BCMA in PC survival. Affirmation of the importance of BCMA in PC survival was provided by studies in BCMA−/− mice in which the survival of long-lived bone marrow PCs was impaired compared with wild-type controls. These findings offer new insights into the molecular basis for the long-term survival of PCs.
Keywords: B lymphocyte subsets, antibody formation, cell differentiation, cell lineage, immunophenotyping
Introduction
The persistence of plasma cells (PCs) within the BM is supported by soluble factors and cell–cell contact (1). The stroma contributes both soluble survival signals, such as IL-6, as well as cell contact–mediated signals (e.g., through the PC adhesion receptor, VLA-4; references 1, 2). In addition to stromal elements, PCs interact with various other BM resident cells, including developing lymphoid and myeloid lineage cells. The overall impact of these interactions is to support the longevity of the PC. Intrinsic qualities, such as increased expression of the antiapoptotic factors (e.g., Mcl-1, Bcl-xL, Bcl-2, Bim, and BCL-w) and decreased expression of the pro-apoptotic factors Bax and BID, may contribute to the long term survival of BM PCs (3). Deletion or enforced expression of certain factors, such as Bim and Bcl-2, by gene targeting have been shown to significantly alter PC survival (3–5).
A recently identified member of the TNF superfamily, BLyS (also referred to as BAFF, TALL-1, THANK, zTNF4, and TNFS13B), plays an integral role in the development of mature B cells (for review see reference 6). BLyS binds to three receptors of the TNF receptor family (B cell maturation antigen [BCMA], transmembrane activator and cyclophilin ligand interactor [TACI], and BAFF-R), all of which are expressed by mature B cells (6, 7). A deficiency in either BLyS or BAFF-R results in a severely diminished pool of mature B lymphocytes within the periphery (8–10), suggesting that the BLyS/BAFF-R axis is critical for B cell maturation. On the other hand, loss of the TACI receptor results in an enhanced number of peripheral B lymphocytes coupled with an inhibited T cell–independent (TI) response (6, 11). More recently, it has been shown that loss of TACI results in the development of autoimmunity, suggesting a negative regulatory role to this receptor (12). These observations have led to the idea that TACI functions as a negative regulator of the BLyS/BAFF-R B cell maturation axis. The function of the third receptor, BCMA, which is primarily restricted to the B cell lineage (6), remains unresolved. A comprehensive analysis of BCMA−/− mice by Xu and Lam have shown that loss of the BCMA receptor does not impact on the generation of mature peripheral B cells, alter the quality or magnitude of TI and T cell–dependent (TD) humoral immune responses, nor alter germinal center (GC) formation or the generation of short-lived PCs (13). Thus, previous analyses have not yet identified a function for the BCMA receptor.
Although BLyS clearly sustains the peripheral mature B cell pool, a requirement of BLyS for terminally differentiated B cells has not yet been fully resolved. Do et al. (14) have reported that BLyS may function in supporting GC B cell survival in vivo; however, it remains unclear whether BLyS affects the development and/or survival of memory B cells or long-lived PCs from GC B cells. However, a recent work implicates BLyS as a regulator of short-lived PC emergence resulting from TI responses within the marginal zone (MZ) of the spleen (15). Blockade of BLyS via TACI-Ig results in a loss of plasmablasts from MZ B cells in vitro and in vivo. Specialized CD11clow blood DCs provide MZ B cells with BLyS, which enhances B cell survival but not proliferation. These observations are consistent with the observations that activated macrophages and DCs express BLyS (16). The loss of plasmablast formation with TACI-Ig treatment reflects increased apoptosis of the MZ B cell population. Thus, BLyS is necessary for terminal differentiation of MZ B cells in TI responses. The importance of BLyS in supporting the terminal differentiation of B cells has been further evidenced by recent in vitro studies showing that BLyS selectively enhances the survival of plasmablasts generated from human memory cells (17). APRIL, a close relative of BLyS, also binds to BCMA and TACI, but not BAFF-R. Both BLyS and APRIL have been shown to signal through TACI and BCMA, and each ligand enhances Ig production from B cells (18). However, little is known about the function of APRIL in the regulation of humoral immune responses.
This paper shows that the survival of long-lived BM PCs can be sustained by recombinant BLyS or APRIL and IL-6 in vitro. Enhanced survival is correlated with an increase in Mcl-1 transcript levels in PCs stimulated with BLyS. Furthermore, in BM cultures containing PCs, the blockade by TACI-Ig of these ligands facilitates the decay of long-lived PCs. After the immunization and development of long-lived BM PCs, treatment with TACI-Ig causes a decay of this established PC population. Furthermore, we demonstrate that BM PCs express heightened mRNA for the BCMA receptor, but not TACI or BAFF-R compared with naive B cells. Confirmation that BCMA is the receptor on PCs critical for their survival is demonstrated by the reduced number of long-lived BM PCs in BCMA−/− mice compared with WT mice. We hypothesize that BLyS or APRIL expression within the BM, via triggering of BCMA on PCs, is critical for long-lived PC survival.
Materials and Methods
Mice and Reagents.
10–12-wk-old BALB/c and C57BL/6 (Jackson Laboratory) mice were maintained in the specific pathogen-free animal facility at Dartmouth Medical School. BCMA−/− mice were provided by J. Gross (Zymogenetics, Seattle, WA).
The following monoclonal antibodies were used for our studies: rat IgG2a anti–mouse B220 (clone RA3-6B2) and rat IgG2a anti–mouse CD138 (Syndecan-1, clone 281-2; BD Biosciences). The anti-Ly6C antibody was provided by T. Waldschmidt (University of Iowa, Iowa City, IA), and the soluble TACI-Ig was generated as described previously (19). To produce soluble BLyS, Flag-tagged human BLyS (amino acid 141-285) was expressed in Pichia pastoris (EasySelect™ Pichia Expression Kit; Invitrogen), and protein was purified on an anti-Flag affinity column. Streptavidin-CyChrome was obtained from BD Biosciences. Recombinant APRIL was purchased from US Biological.
Immunization.
Mice were challenged s.c. with 10 μg PE (Cyanotech Corp.) or KLH (Sigma-Aldrich) emulsified in CFA. For experiments in BCMA−/− mice, mice were immunized with 100 μg of alum-precipitated (4-hydroxy-3-nitrophenyl)acetyl chicken γ globulin (Biosearch Technologies) i.p. After 51 d, the animals were killed, and BM was isolated.
In Vitro Culture.
Cells were cultured in complete RPMI 1640 supplemented with 10% FBS containing IL-6 and/or soluble BLyS and/or soluble TACI-Ig for 4–10 d. At the end of the culture, cells were harvested and either enumerated for PCs by ELISPOTs or stained for PC markers (CD138 and Ly6C.)
FACS® Analysis and Sorting.
Cells were washed in 5% FCS in balanced salt solution (BSS) and resuspended in low-pH (pH 4.0) acetate buffer containing 0.05 M sodium acetate, 0.085 M NaCl, and 0.005 M KCl and 2% FCS in distilled H2O for removal of cytophilic Ig. Samples were incubated on ice for 1 min followed by the addition of an equal volume of 0.1 M Tris buffer, pH 8.0, containing 2% FCS. Samples were washed twice in 5% FCS BSS and stained. Chromatographically purified rat IgG was used as isotype control. For all studies, nonspecific staining was further reduced by the addition of heat-inactivated rat serum. Incubation with biotinylated antibodies was followed by incubation with streptavidin, peridinine chlorophyll protein, or CyChrome or allophycocyanin (BD Biosciences). Antibody incubations were for 30 min at 4°C followed by washing in BCS/BSS. A minimum of 500,000 events per sample were collected on a FACScan™, and dead cells were excluded based on forward and 90° light scatter. Data were analyzed with FlowJo software. In case of cell sorting, PCs (CD138+Ly6C+B220−) were sorted from CD138+ BM cells using the automatic cell–dispensing unit attached to the FACStarPLUS™ (Becton Dickinson).
ELISPOT Assay.
BM IgG-secreting cells were enumerated by an antigen-specific ELISPOT assay as described previously (20). In brief, BM PE or NP-specific IgG antibody-secreting cells (ASCs) were enumerated by an IgG ELISPOT assay. After isolation of BM cells, RBCs were lysed via incubation with ammonium chloride Tris, and T cells were depleted through anti–Thy-1.2 microbeads (Miltenyi Biotec). Of the remaining population, 2.4 × 106 cells/well were apportioned to PE or NP-BSA–coated Multiscreen® 96-well plates (Millipore), and threefold serial dilutions were made before incubation. Plates were incubated for 5 h at 37°C. After the incubation, plates were washed in 0.05% Tween 80 and in nano-pure water. ASCs detected by alkaline phosphatase–conjugated anti–mouse IgG (Southern Biotechnology Associates, Inc.). ELISPOTs were developed by a FAST BCIP/NBT (Sigma-Aldrich) chromagen substrate. ELISPOTs were enumerated via a ImmunoSpot® software (CTL Analyzers LLC) or by direct visual counting using a dual-axis light-dissecting microscope.
Real-time RT-PCR.
B cells were positively selected (>90% purity) using biotinylated anti-B220 Ab followed by streptavidin-coupled microbeads (Miltenyi Biotec). Purified B cells were stimulated with FGK45, an agonistic rat IgG2a anti–mouse CD40 antibody (17), in vitro for 60 h. Purified PCs were isolated by positive selection using αCD138 and magnetic beads, followed by electronic cell sorting for B220−Ly6c+CD138+ cells to a purity of >90%. Total RNA was isolated from either purified B cells, FGK blasts, or PCs using TRIzol (Invitrogen) followed by secondary purification over RNeasy columns (QIAGEN) with a DNase-I treatment step. 1 μg of DNA-free RNA was reverse transcribed to cDNA using Omniscript RT (QIAGEN). Real-time PCR was performed with the SYBR Green PCR Core Kit (Applied Biosystems) on an iCycler iQ instrument (Bio-Rad Laboratories). Amplification conditions were 95°C for 8 min, followed by 40 cycles of 94°C for 15 s, 63°C for 45 s, and 72°C for 15 s. Primers for the control gene mouse β-actin were as follows: forward, 5′-CCAATGTGTCCATGTCATTT-3′ and reverse, 5′-CAATAGTGATGACCTGGCCGT-3′. Other primers used were as follows: BCMA, forward, 5′-ATCTTCTTGGGGCTGACCTT-3′ and reverse, 5′-CTTTGAGGCTGGTCCTTCAG-3′; BAFF-R, forward, 5′-CCCCAGACACTTCAGAAGGA-3′ and reverse, 5′-AGGTAGGAGCTGAGGCATGA-3′; TACI, forward, 5′-GTGTGGCCACTTCTGTGAGA-3′ and reverse, 5′-CTGGTGCCTTCCTGAGTTGT-3′; Bcl2, forward, 5′-CTTAGAAAATACAGCATTGCGGAG-3′ and reverse, 5′-GGATGTGCTTTGCATTCTTGG-3′; Mcl1, forward, 5′-GCTCCGGAAA-CTGGACATTA-3′ and reverse, 5′-CCCAGTTTGTTACGCCATCT-3′; and Bclxl, forward, 5′-TTCGGGATGGAGTAA-ACTGG-3′ and reverse, 5′-TGCAATCCGACTCACCAATA-3′. Relative RNA expression was determined using the formula relative expression = 2−(ΔΔCT) × 1,000, where ΔΔCT = (cycle threshold [CT] gene of interest − CT β-actin in experimental sample) − (CT gene of interest − CT β-actin in a no-template control sample) (see the ΔΔCT method, Taqman® Bulletin 2: Applied Biosystems).
Online Supplemental Material.
Fig. S1 demonstrates that APRIL, also a ligand for TACI and BCMA, enhanced the survival of BM PCs in vitro. Fig. S2 shows that BCMA−/− mice have normal numbers of peripheral B cells, and generate normal TD immune responses, including GC formation and Ag-specific serum IgM and IgG titers compared to wild-type controls. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20031330/DC1.
Results and Discussion
BLyS Sustains BM PC In Vitro.
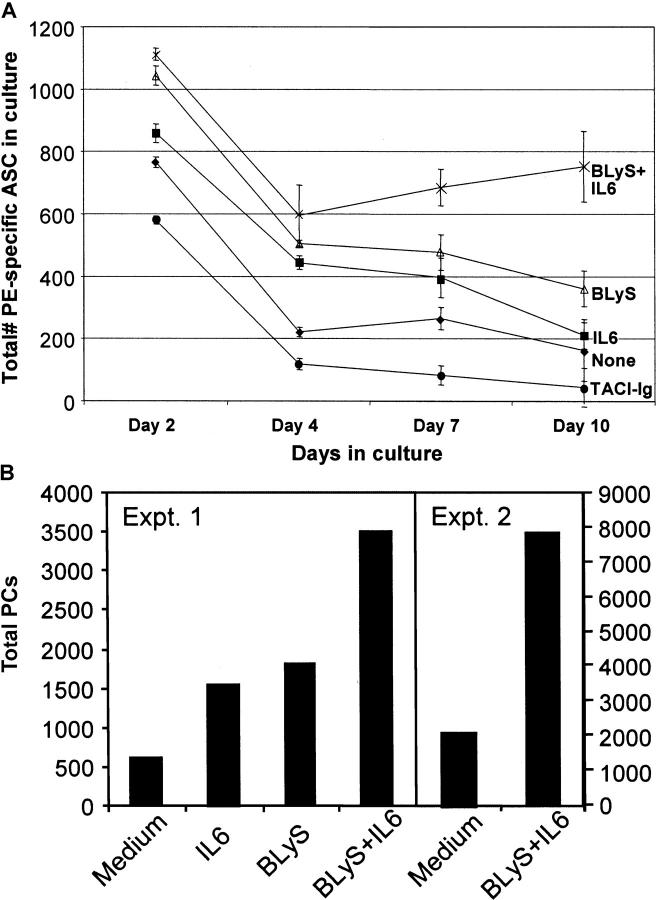
It has been shown that during the later stages of an immune response, the majority of PCs within the BM are long lived (21–23). Because BLyS has been shown to be a key survival factor for B cells in general, and recent transcriptional profiling has shown increased expression of BCMA on PCs, we asked whether BLyS could contribute to the survival of BM PCs (24). To this end, BM from mice 30 d after immunization with PE was isolated and cultured with IL-6 and/or BLyS for 10 d. As reported previously (2), and shown here, the addition of IL-6 can enhance Ag-specific PC survival over short time intervals (2–4 d); however, over extended time periods (10 d), IL-6 was not effective (Fig. 1 A). The addition of BLyS alone exerted a modest twofold effect on αPE ASC survival over 10 d, but in combination with IL-6, it enhanced the number of ASCs on day 10 over fourfold (from <200 to 800 ASCs).
Figure 1.
BLyS and IL-6 enhances BM PC survival in vitro. After a 6–8-wk immunization with 10 μg PE, whole BM samples were isolated from BALB/c and cultured in vitro with the indicated stimuli for a period of 10 d. (A) After isolation of whole BM samples, 8 × 106 BM cells were cultured in medium alone (♦), 200 ng/ml BLyS (▵), 500 pg/ml IL-6 (▪), IL-6 and BLyS (X), or 10 μg/ml TACI-Ig (•). At days 2, 4, 7, and 10 of in vitro culture, samples were isolated, and the number of PE-specific IgG BM ASCs was enumerated by ELISPOT. These data are representative of four experiments. (B) Immune BM was cultured as in A, and on day 7, samples were stained with αB220, αCD138, and αLy6C antibodies to estimate the number of total PCs (B220−CD138+Ly6C+) in culture. Two representative experiments out of four are shown.
Endogenous BLyS produced by BM cells, such as macrophages or DCs, could contribute to the maintenance of BM PCs. To analyze the impact of endogenous BLyS on BM PC survival, whole immune BM was cultured from immune mice in the presence or absence of TACI-Ig. Previous papers have demonstrated the ability of TACI-Ig to block BLyS ligand–receptor interactions (6, 19). The addition of TACI-Ig inhibited the maintenance of BM PCs in vitro (Fig. 1 A). TACI-Ig reduced the total number of PE-specific ASCs recovered on day 10 approximately fourfold compared with serum-only cultures. Moreover, there was a >17-fold decrease in the number of recovered ASCs on day 10 in TACI-Ig–treated cultures compared with the addition of IL-6 and BLyS, clearly showing a critical role of BLyS in PC survival. Confirmation that BLyS and IL-6 could sustain ASCs was provided by analysis of the total number of recovered B220−CD138+Ly6C+ from cultures on day 10 (Fig. 1 B). The two representative datasets show that compared with medium alone, BLyS and IL-6 enhances the recovery of PCs three to fourfold. Because APRIL is also a ligand for both TACI and BCMA, we addressed whether APRIL, like BLyS, could enhance the survival of BM PCs in vitro. As shown in Fig. S1 (available at http://www.jem.org/cgi/content/full/jem.20031330/DC1), APRIL enhanced the number of PCs in culture alone or in conjunction with IL-6.
TACI-Ig Impairs the Survival of PCs In Vivo.
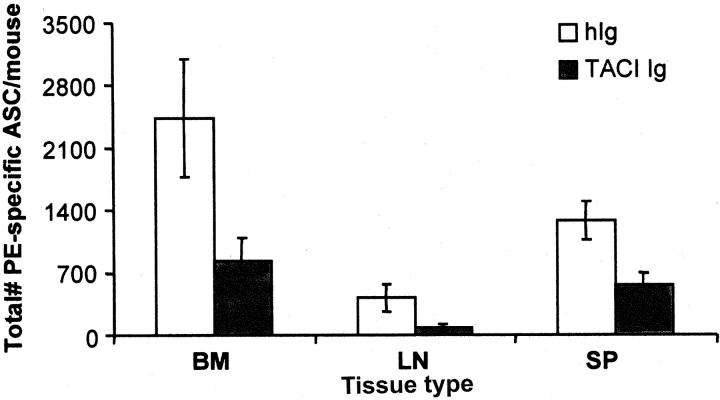
The importance of BLyS and APRIL to in vivo PC survival was addressed by blocking these ligands in vivo by the administration of TACI-Ig to immune mice. Immune mice, in which a stable population of long-lived BM PCs was present, were treated with TACI-Ig or control Ig for a period of 14 d. After treatment, the number of ASCs in the spleen, LN, and BM was determined. TACI-Ig treatment diminished the recovery of ASCs by 65% in the BM, with similar reductions in number in the spleen and LN (Fig. 2) . These data corroborate the in vitro data suggesting that BLyS and/or APRIL are important for the survival of long-lived PCs.
Figure 2.
Blockade of BLyS via TACI-Ig inhibits PC survival in vivo. Approximately 6–8 wk after immunization with PE-CFA, immune mice (four mice/group) were treated with 100 μg TACI-Ig or human Ig every 3 d for a period of 2 wk. After the 2-wk period of treatment, BM, lymph node (LN), or spleen (SP) tissue samples were isolated from treated mice, and the number of αPE IgG ASCs was determined by ELISPOT analysis. Standard error was calculated between values from four independent mice for each treatment group. Data are representative of three independent experiments.
PCs Bind BLyS and Selectively Up-regulate mRNA for BCMA but Not TACI or BAFF-R.
Data show that BLyS and/or APRIL can sustain PC survival in vitro, and contribute to PC persistence in vivo. To determine if PCs can bind BLyS, immune BM was incubated with biotinylated BLyS, and binding was ascertained by flow cytometry using fluorochrome-coupled avidin. As can be seen in Fig. 3 A, binding of biotinylated BLyS could be readily detected on B220−CD138+ cells in the BM. Only first-decade staining was observed with biotinylated irrelevant control protein (bio-Ig; unpublished data). Levels of binding to PCs were similar to those observed on mature B cells (B220+) in the spleen. We also observed higher levels of BLyS binding in the non–B cell compartment in the BM compared with the spleen. We suspect that this may be due to BLyS-R/TACI/BCMA expression by developing B cells present in the BM. Therefore, it is clear that long-lived PCs express a BLyS receptor.
Figure 3.
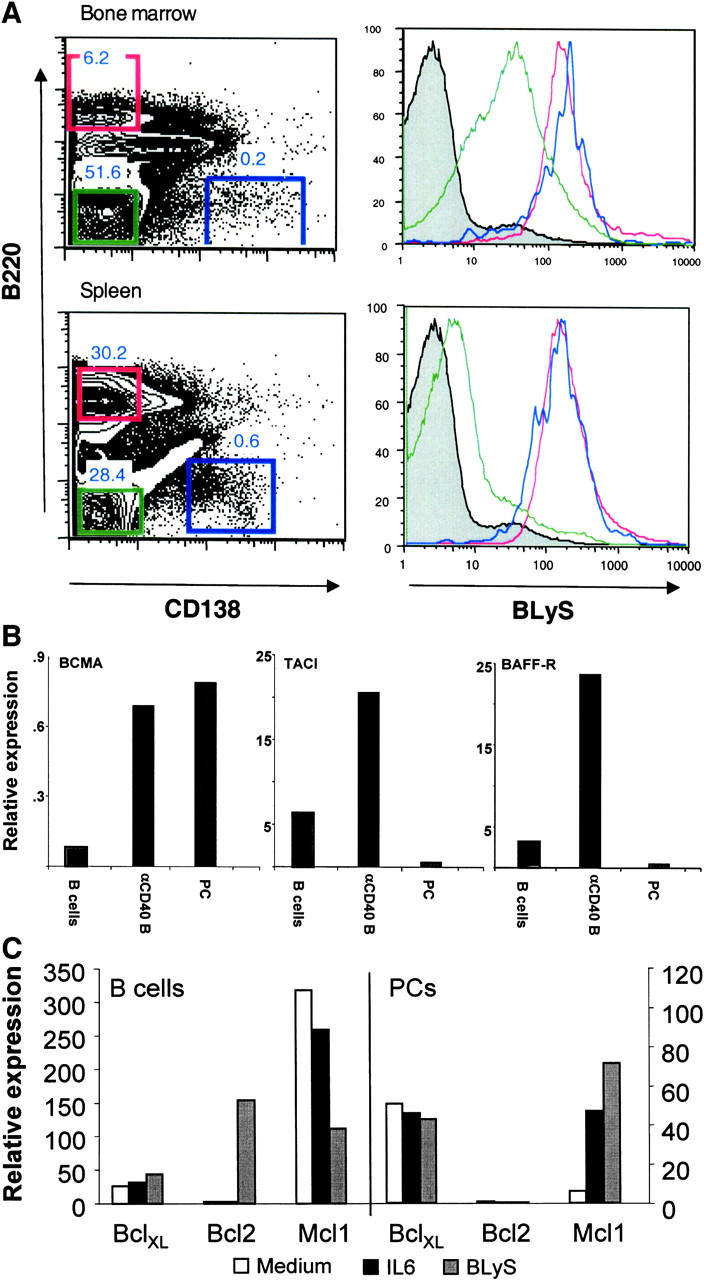
(A) PCs express BLyS receptor. Immune BM and spleen (SP) was isolated and stained with αCD138, αB220, and biotinylated BLyS. Histograms of BLyS receptor expression are shown for B220+CD138− cells (red); B220−CD138− cells (green); and B220−CD138+ cells (blue). Data are representative of three independent experiments. (B) BM PCs express heightened levels of BCMA mRNA but not BAFF-R or TACI. RNA from splenic B cells (B cells), αCD40-induced B cell blasts (αCD40 B), or BM PCs (PC) was isolated via the TRIzol method. Real-time RT-PCR was performed with each sample to evaluate the relative levels of mRNA expression for BCMA, TACI, and BAFF-R. Data are representative of three experiments. (C) BLyS enhances Mcl-1 expression in PCs but not B cells. Purified CD138+ BM PCs or splenic B cells were cultured with the indicated stimulus, as described in Fig. 1. After 18 h, RNA was isolated, and the relative expression of antiapoptotic genes was determined relative to β-actin.
To gain initial insights into which of these receptors may be functionally important in PC survival, real-time PCR analysis was use to evaluate receptor mRNA expression in PCs. BM PCs were purified via magnetic bead and FACS® sorting (>94% CD138+, B220−) and processed for real-time PCR analysis of mRNA. We observed that BM PCs express high levels of message for BCMA compared with resting B cells, and similar levels to that observed in αCD40-activated B cells (Fig. 3 B). In contrast, PCs expressed much lower levels of BAFF-R and TACI compared with both resting B cells and αCD40-activated B cells. These data confirm the heightened levels of BCMA expressed by PCs (or multiple myeloma) as deduced by transcriptional profiling (17, 24).
To begin to unravel the mechanism of BLyS-induced PC survival, the transcriptional expression of a number of α-apoptotic gene products was evaluated after BLyS stimulation or IL-6 stimulation of purified B cells and PCs. As can be seen in Fig. 3 C, BLyS up-regulated Bcl2 transcript expression in B cells but not in PCs. This is consistent with the observed increase of Bcl2 in B cells from the BLyS transgenic mice (25). In contrast, levels of Mcl-1 expressed in B cells was reduced by BLyS and increased by either BLyS or IL-6 in PCs, whereas Mcl-1 has been suggested important in PC survival (26, 27).
BCMA Is Critical for Long-Term Survival of BM PCs.
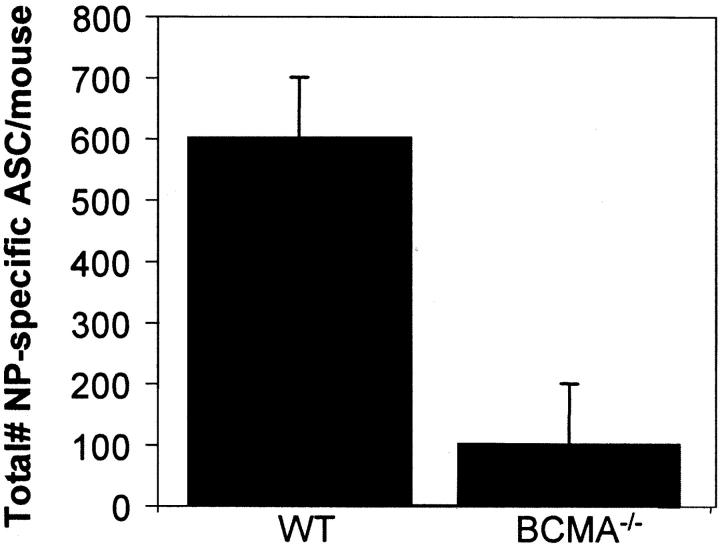
The heightened expression of BCMA and the capacity of BLyS to support PC survival suggested that BCMA may be critical for PC survival. To test this hypothesis, BCMA−/− and WT mice (BCMA+/−) were immunized, and the number of ASCs in the BM was evaluated 6–8 wk after immunization. As can be seen in Fig. 4 , compared with WT mice, the BCMA−/− mice had <20% of the number of ASCs and indicate a critical role of BLyS and BCMA in BM PC survival. The decrease of ASCs in BCMA−/− mice is not due to impaired TD humoral immune responses, as these mice showed normal numbers of peripheral B cells, GC responses, and Ag-induced serum IgM and IgG titers (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20031330/DC1).
Figure 4.
BCMA−/− mice have a reduced capacity to sustain long-lived BM PCs. BCMA+/− (WT) and BCMA−/− mice were immunized with 100 μg of alum-precipitated (4-hydroxy-3-nitrophenyl)acetyl chicken γ globulin for 7 wk. The number of NP-specific IgG ASCs in the BM was quantified by antigen-specific ELISPOT analysis. The determined p-value = 0.00094 and is representative of two experiments each with three mice/group.
Sustained humoral immune responses depend on the persistence of long-lived BM PCs. The studies discussed show that the BLyS–APRIL-BCMA pathway plays a role in this function. The data show that both BLyS and APRIL in conjunction with IL-6 can sustain the survival of PCs in vitro, and that these ligands are likely survival factors for PCs in vivo. Because TACI-Ig can functionally block both BLyS and APRIL, conclusive identification of the active ligand in vivo awaits additional studies. Be it BLyS or APRIL as the functional ligand in PC survival, the studies presented implicate BCMA as the receptor that controls the lifespan of BM PCs.
The data show that TACI-Ig treatment causes a decay in a preexisting population of long-lived PCs. Tangye et al. have recently shown that BLyS and APRIL can selectively enhance survival of plasmablasts generated from human memory B cells in vitro (28). We, too, have seen an effect on PC precursors (unpublished data); however, this effect on plasmablasts cannot account for the decay of ASCs seen in the studies presented. It is clear that BM PCs have an intrinsic half-life in months, so impairing precursor function cannot explain the precipitous decline in ASCs in TACI-Ig–treated mice. Furthermore, it is shown that PCs bind BLyS, suggesting that TACI-Ig impairs PC survival via a direct effect on PCs and not their precursors. Affirmation that APRIL can influence humoral immunity in vivo is provided by the enhanced humoral immune responses observed in APRIL transgenic mice (29).
We also demonstrate that BM PCs express heightened levels of mRNA for BCMA compared with B cells (Fig. 3 B). Our data corroborate earlier papers, which report heightened PC expression of BCMA compared with other B cell subsets by transcriptional profiling (30, 31). Very recently, Novak et al. reported the expression of BCMA on primary multiple myeloma and myeloma cell lines, but not on resting or memory peripheral B cell populations in humans, suggesting that the expression of BCMA occurs late in the course of B cell differentiation (32). Given the greatly reduced expression of BAFF-R and TACI in PCs compared with other B cell subsets and the heightened expression of BCMA, it suggests that BCMA may be the vital receptor for PC survival in the BM. Studies in BCMA−/− mice demonstrate that long-term PC survival was impaired in the absence of BCMA, providing conclusive functional importance of BLyS–APRIL-BCMA interactions. Previous studies in the BCMA−/− mice did not examine long-term PC survival. Interestingly, the BCMA−/− mice have been reported to show no defects in short-term production of Ig, GC formation, and immune architecture (13). We, too, evaluated many of these same parameters in the BCMA−/− mice and have concluded that B cell development, early humoral immune responses, and GC responses were indistinguishable from WT mice.
The manner through which BCMA ligation enhances BM PC survival is currently unknown; however, the up-regulation of Mcl-1 in PCs by BLyS is intriguing because Mcl-1 has been implicated in the survival of activated B cells and PCs (26, 27). As a survival receptor, structurally, BCMA is most similar to BAFF-R and not TACI (33, 34), consistent with the emerging functional similarities that both BAFF-R and BCMA function to promote B cell survival at distinct stages of B cell development. In contrast, TACI has been suggested to be a negative regulator of B cell activity and BAFF-R signaling.
In conclusion, BLyS or APRIL, two newly identified members of the TNF family, are necessary for the survival of long-lived BM PCs. Although BCMA appears to play little to no role in many of the events leading up to the formation of long-lived PCs, it certainly seems to play a role in their survival. Perhaps the most exciting aspect of the role of BCMA in PC survival is the recognition that therapeutic targeting of BCMA may be an effective way to selectively eliminate PCs in antibody-mediated autoimmunity.
Acknowledgments
This work was supported by National Center for Research Resources grants AI26296 (to R.J. Noelle), P20 RR16437 (to L.D. Erickson), and CA076274 (to R.J. Bram).
B.P. O'Connor, V.S. Raman, and L.D. Erickson contributed equally to this work.
The online version of this article includes supplemental material.
The present address of W.J. Cook is GlycoFi, Inc., Lebanon, NH 03756.
Abbreviations used in this paper: ASC, antibody-secreting cell; BCMA, B cell maturation antigen; BSS, balanced salt solution; GC, germinal center; MZ, marginal zone; PC, plasma cell; TACI, transmembrane activator and cyclophilin ligand interactor; TD, T cell–dependent; TI, T cell–independent.
References
- 1.Calame, K.L. 2001. Plasma cells: finding new light at the end of B cell development. Nat. Immunol. 2:1103–1108. [DOI] [PubMed] [Google Scholar]
- 2.Underhill, G.H., H.A. Minges Wols, J.L. Fornek, P.L. Witte, G.S. Kansas, and H.A. Minges-Wols. 2002. IgG plasma cells display a unique spectrum of leukocyte adhesion and homing molecules. Blood. 99:2905–2912. [DOI] [PubMed] [Google Scholar]
- 3.Strasser, A., S. Whittingham, D.L. Vaux, M.L. Bath, J.M. Adams, S. Cory, and A.W. Harris. 1991. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl. Acad. Sci. USA. 88:8661–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonnell, T.J., N. Deane, F.M. Platt, G. Nunez, U. Jaeger, J.P. McKearn, and S.J. Korsmeyer. 1989. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 57:79–88. [DOI] [PubMed] [Google Scholar]
- 5.Bouillet, P., D. Metcalf, D.C. Huang, D.M. Tarlinton, T.W. Kay, F. Kontgen, J.M. Adams, and A. Strasser. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 286:1735–1738. [DOI] [PubMed] [Google Scholar]
- 6.Mackay, F., P. Schneider, P. Rennert, and J. Browning. 2003. BAFF AND APRIL: a tutorial on B cell survival. Annu. Rev. Immunol. 21:231–264. [DOI] [PubMed] [Google Scholar]
- 7.Mackay, F., and J.L. Browning. 2002. BAFF: a fundamental survival factor for B cells. Nat. Rev. Immunol. 2:465–475. [DOI] [PubMed] [Google Scholar]
- 8.Thompson, J.S., S.A. Bixler, F. Qian, K. Vora, M.L. Scott, T.G. Cachero, C. Hession, P. Schneider, I.D. Sizing, C. Mullen, et al. 2001. BAFF-R, a novel TNF receptor that specifically interacts with BAFF. Science. 293:2108–2111. [DOI] [PubMed] [Google Scholar]
- 9.Schiemann, B., J.L. Gommerman, K. Vora, T.G. Cachero, S. Shulga-Morskaya, M. Dobles, E. Frew, and M.L. Scott. 2001. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 293:2111–2114. [DOI] [PubMed] [Google Scholar]
- 10.Lentz, V.M., M.P. Cancro, F.E. Nashold, and C.E. Hayes. 1996. Bcmd governs recruitment of new B cells into the stable peripheral B cell pool in the A/WySnJ mouse. J. Immunol. 157:598–606. [PubMed] [Google Scholar]
- 11.von Bulow, G.U., J.M. van Deursen, and R.J. Bram. 2001. Regulation of the T-independent humoral response by TACI. Immunity. 14:573–582. [DOI] [PubMed] [Google Scholar]
- 12.Seshasayee, D., P. Valdez, M. Yan, V.M. Dixit, D. Tumas, and I.S. Grewal. 2003. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 18:279–288. [DOI] [PubMed] [Google Scholar]
- 13.Xu, S., and K.P. Lam. 2001. B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol. Cell. Biol. 21:4067–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Do, R.K., E. Hatada, H. Lee, M.R. Tourigny, D. Hilbert, and S. Chen-Kiang. 2000. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J. Exp. Med. 192:953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balazs, M., F. Martin, T. Zhou, and J. Kearney. 2002. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 17:341–352. [DOI] [PubMed] [Google Scholar]
- 16.Nardelli, B., O. Belvedere, V. Roschke, P.A. Moore, H.S. Olsen, T.S. Migone, S. Sosnovtseva, J.A. Carrell, P. Feng, J.G. Giri, and D.M. Hilbert. 2001. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 97:198–204. [DOI] [PubMed] [Google Scholar]
- 17.Avery, D.T., S.L. Kalled, J.I. Ellyard, C. Ambrose, S.A. Bixler, M. Thien, R. Brink, F. Mackay, P.D. Hodgkin, and S.G. Tangye. 2003. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J. Clin. Invest. 112:286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsters, S.A., M. Yan, R.M. Pitti, P.E. Haas, V.M. Dixit, and A. Ashkenazi. 2000. Interaction of the TNF homologues BLyS and APRIL with the TNF receptor homologues BCMA and TACI. Curr. Biol. 10:785–788. [DOI] [PubMed] [Google Scholar]
- 19.Gross, J.A., J. Johnston, S. Mudri, R. Enselman, S.R. Dillon, K. Madden, W. Xu, J. Parrish-Novak, D. Foster, C. Lofton-Day, et al. 2000. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 404:995–999. [DOI] [PubMed] [Google Scholar]
- 20.Erickson, L.D, B.G. Durell, L.A. Vogel, B.P. O'Connor, M. Cascalho, T. Yasui, H. Kikutani, and R.J. Noelle. 2002. Short circuiting long-lived humoral immunity by heightened engagement of CD40. J. Clin. Invest. 109:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Slifka, M.K., R. Antia, J.K. Whitmire, and R. Ahmed. 1998. Humoral immunity due to long-lived plasma cells. Immunity. 8:363–372. [DOI] [PubMed] [Google Scholar]
- 22.Manz, R.A., and A. Radbruch. 2002. Plasma cells for a lifetime? Eur. J. Immunol. 32:923–927. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor, B.P., M. Cascalho, and R.J. Noelle. 2002. Short-lived and long-lived bone marrow plasma cells are derived from a novel precursor population. J. Exp. Med. 195:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claudio, J.O., E. Masih-Khan, H. Tang, J. Goncalves, M. Voralia, Z.H. Li, V. Nadeem, E. Cukerman, O. Francisco-Pabalan, C.C. Liew, et al. 2002. A molecular compendium of genes expressed in multiple myeloma. Blood. 100:2175–2186. [DOI] [PubMed] [Google Scholar]
- 25.Mackay, F., S.A. Woodcock, P. Lawton, C. Ambrose, M. Baetscher, P. Schneider, J. Tschopp, and J.L. Browning. 1999. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 190:1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spets, H., T. Stromberg, P. Georgii-Hemming, J. Siljason, K. Nilsson, and H. Jernberg-Wiklund. 2002. Expression of the bcl-2 family of pro- and anti-apoptotic genes in multiple myeloma and normal plasma cells: regulation during interleukin-6(IL-6)-induced growth and survival. Eur. J. Haematol. 69:76–89. [DOI] [PubMed] [Google Scholar]
- 27.Altmeyer, A., R.C. Simmons, S. Krajewski, J.C. Reed, G.W. Bornkamm, and S. Chen-Kiang. 1997. Reversal of EBV immortalization precedes apoptosis in IL-6-induced human B cell terminal differentiation. Immunity. 7:667–677. [DOI] [PubMed] [Google Scholar]
- 28.Tangye, S.G., D.T. Avery, E.K. Deenick, and P.D. Hodgkin. 2003. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J. Immunol. 170:686–694. [DOI] [PubMed] [Google Scholar]
- 29.Stein, J.V., M. Lopez-Fraga, F.A. Elustondo, C.E. Carvalho-Pinto, D. Rodriguez, R. Gomez-Caro, J. De Jong, A.C. Martinez, J.P. Medema, and M. Hahne. 2002. APRIL modulates B and T cell immunity. J. Clin. Invest. 109:1587–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Underhill, G.H., D. George, E.G. Bremer, and G.S. Kansas. 2003. Gene expression profiling reveals a highly specialized genetic program of plasma cells. Blood. 101:4013–4021. [DOI] [PubMed] [Google Scholar]
- 31.Tarte, K., F. Zhan, J. De Vos, B. Klein, and J. Shaughnessy, Jr. 2003. Gene expression profiling of plasma cells and plasmablasts: toward a better understanding of the late stages of B-cell differentiation. Blood. 102:592–600. [DOI] [PubMed] [Google Scholar]
- 32.Novak, A.J., J.R. Darce, B.K. Arendt, B. Harder, K. Henderson, W. Kindsvogel, J.A. Gross, P.R. Greipp, and D.F. Jelinek. 2003. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: a mechanism for growth and survival. Blood. 10.1182/blood-2003-06-2043. [DOI] [PubMed]
- 33.Karpusas, M., T.G. Cachero, F. Qian, A. Boriack-Sjodin, C. Mullen, K. Strauch, Y.M. Hsu, and S.L. Kalled. 2002. Crystal structure of extracellular human BAFF, a TNF family member that stimulates B lymphocytes. J. Mol. Biol. 315:1145–1154. [DOI] [PubMed] [Google Scholar]
- 34.Oren, D.A., Y. Li, Y. Volovik, T.S. Morris, C. Dharia, K. Das, O. Galperina, R. Gentz, and E. Arnold. 2002. Structural basis of BLyS receptor recognition. Nat. Struct. Biol. 9:288–292. [DOI] [PubMed] [Google Scholar]