Abstract
Psoriasis is a type I–deviated disease characterized by the presence of interferon (IFN)-γ and multiple IFN-related inflammatory genes in lesions. Because interleukin (IL)-23 is now recognized to play a role in the recruitment of inflammatory cells in a T helper cell (Th)1-mediated disease, we examined psoriasis skin lesions for production of this newly described cytokine. IL-23 is composed of two subunits: a unique p19 subunit and a p40 subunit shared with IL-12. We found a reliable increase in p19 mRNA by quantitative reverse transcription polymerase chain reaction in lesional skin compared with nonlesional skin (22.3-fold increase; P = 0.001). The p40 subunit, shared by IL-12 and IL-23, increased by 11.6-fold compared with nonlesional skin (P = 0.003), but the IL-12 p35 subunit was not increased in lesional skin. IL-23 was expressed mainly by dermal cells and increased p40 immunoreactivity was visualized in large dermal cells in the lesions. Cell isolation experiments from psoriatic tissue showed strong expression of p19 mRNA in cells expressing monocyte (CD14+ CD11c+ CD83−) and mature dendritic cell (DC) markers (CD14− CD11c+ CD83+), whereas in culture, the mRNAs for p40 and p19 were strongly up-regulated in stimulated monocytes and monocyte-derived DCs, persisting in the latter for much longer periods than IL-12. Our data suggest that IL-23 is playing a more dominant role than IL-12 in psoriasis, a Th1 type of human inflammatory disease.
Keywords: cytokines, gene expression, dendritic cell, IL-23, Th1 cells
Introduction
IL-23 was found during a genome scan for the IL-6/IL-12 cytokine family (1). IL-23 is a heterodimer, sharing a p40 subunit with IL-12 (1) but having a distinct p19 subunit. IL-23 binds to IL-12Rβ1 but not IL-12Rβ2. The receptor for this cytokine is heterodimeric and uses a novel second subunit, IL-23R, which is a member of the hematopoietin receptor family (2). IL-23 plays a role in type 1–polarized T cell immune responses. Although IL-12 strongly activates naive T cells, the initial description of IL-23 reported preferential actions on memory T cells to increase IFN-γ production and proliferation (1). Mice with p40-null mutations display reduced IFN-γ production and less resistance to a range of infectious pathogens compared with p35-null mice (3), suggesting an important role for IL-23 in controlling bacterial infections. More recent data indicate that IL-23 is a key cytokine controlling inflammation in peripheral tissues. In transgenic mice, overexpression of p19 produces inflammation in multiple organs and epithelial tissues, including the skin (4). In a recent study, the development of experimental autoimmune encephalitis occurred in mice lacking IL-12, but not in mice with targeted disruption of only IL-23 (5), and IL-23 was shown to be involved in the recruitment of monocytes into the inflamed nervous system.
Psoriasis vulgaris is a T cell–driven disease, with type I (IFN-γ–producing) T cells predominating in lesional skin and excess numbers of type 1 T cells in the peripheral circulation (6). Psoriasis affects ∼2.6% of the US population (7) and therefore might be the most common T cell–mediated inflammatory disease in humans. There is a complex cytokine network in psoriatic lesions that consists of elevated levels of IFN-γ, TNF-α, several interleukins (IL-1, IL-2, IL-6, IL-8, IL-12 [p40 subunit], IL-17, and IL-19), and multiple chemokines including MIG/CXCL9, IP-10/CXCL10, I-TAC/CXCL11, and MIP3α/CCL20 (8). A central role for IFN-γ as an inflammatory regulator is suggested from array-based gene expression studies that identify increased expression of STAT1 and more than 20 genes controlled by this transcription factor (9–11). IL-12, a 70-kD heterodimer formed from p40 and p35 subunits, also has the ability to stimulate IFN-γ production from T cells. Previously, IL-12 was thought to contribute to the type 1 T cell bias in psoriatic lesions because the p40 subunit is strongly up-regulated (12, 13) and because IL-12 receptor subunits (IL-12Rβ1 and IL-12Rβ2) are also up-regulated (13). However, we were puzzled by the failure to detect up-regulation of mRNA for the IL-12 p35 subunit in psoriasis lesions (13). Because the p40 subunit of IL-12 is shared with IL-23 (as is the β1 receptor subunit), we might have incorrectly assigned p40 overexpression to IL-12 rather than IL-23.
Accordingly, we studied expression of p19, the defining subunit for IL-23, in skin lesions of psoriasis vulgaris. We report consistent large increases in mRNAs encoding IL-23 subunits p19 and p40, but not p35 in psoriatic lesions. We visualized p40 protein in dendritic profiles within the dermis and showed that p19 was enriched in monocytes and DCs stimulated in the dermis and in culture. Therefore, IL-23 could be a key inflammatory cytokine in type 1–deviated human autoimmune and inflammatory diseases.
Materials and Methods
Subjects.
After obtaining informed consent, 22 adults with chronic plaque-type psoriasis had both lesional and nonlesional skin biopsies. The patients had active, untreated skin lesions. Biopsies were stored in liquid nitrogen until analysis.
RNA Isolation.
The biopsies were processed using the QIAGEN RNeasy kit. Using a rotor-stator tissue homogenizer (saw-tooth rotor; PowerGen 700), frozen specimens were homogenized in buffer (RLT; QIAGEN) for 30–60 s and spun in a microcentrifuge for 3 min at room temperature to pellet debris. The supernatant was loaded onto a QIAGEN minicolumn and spun for 15 s at high speed. A series of washes and DNase digestion was followed by final elution of RNA from the column using 30 μl RNase-free water (Sigma-Aldrich). RNA was quantitated by UV spectrophotometry.
TaqMan RT-PCR Quantitation of mRNA.
The primers and probes for the TaqMan RT-PCR assays for IL-12 p40, p19, and p35 were generated using the Primer Express algorithm version 1.0 from published sequences (National Center for Biotechnology Information). All primers and probes were synthesized by Applied Biosystems-PerkinElmer. RT-PCR reactions were performed according to the manufacturer's directions (EZ PCR Core Reagents; TaqMan and Applied Biosystems). We used an Applied Biosystems PRISM 7700 thermal cycler for 2 min at 50°C, 30 min at 60°C, 5 min at 95°C, and 40 cycles of 15 s at 95°C followed by 60 s at 60°C. The human acidic ribosomal protein gene (hARP), a housekeeping gene, was used to normalize each sample and each gene. The data were analyzed with Sequence Detection Systems version 1.7 software. Human IL-12 p40 (sequence data available from GenBank/EMBL/DDBJ under accession no. AY008847) forward primer: ACGGACAAGACCTCAGCCAC; reverse primer: GGGCCCGCACGCTAA; fluorescent probe: TCATCTGCCGCAAAAATGCCAGC. Human IL-12 p35 (sequence data available from GenBank/EMBL/DDBJ under accession no. AF404773): forward primer: CCACTCCAGACCCAGGAATG; reverse primer: GACGGCCCTCAGCAGGT; fluorescent probe: TCCCATGCCTTCACCACTCCCAA. Human IL-12 p19 (sequence data available from GenBank/EMBL/DDBJ under accession no. NM_016584) forward primer: GAGCCTTCTCTGCTCCCTGAT; reverse primer: AGTTGGCTGAGGCCCAGTAG; fluorescent probe: CCTGTGGGCCAGCTTCATGCCT. Murine IL-12 p40 (sequence data available from GenBank/EMBL/DDBJ under accession no. NM008352) forward primer: ACATCTACCGAAGTCCAATGCA; reverse primer: GGAATTGTAATAGCGATCC-TGAGC; fluorescent probe: TGCACGCAGACATTCCCG-CCT. Human hARP (sequence data available from GenBank/EMBL/DDBJ under accession no. NM_001002) forward primer: CGCTGCTGAACATGCTCAA; reverse primer: TGTCGAACACCTGCTGGATG; fluorescent probe: TCCCCCTTCTCCTTTGGGCTGG. All probes were conjugated with 6-FAM (dye) at the 5′-end and TAMRA (quench) at the 3′-end (Applied Biosystems).
Statistical Analysis.
Paired Student's t test was used to compare lesional and nonlesional gene expression levels. Bonferroni correction was performed on multivariate calculations of p-values for p19, p35, and p40 gene expression.
DC Generation and Maturation.
Mononuclear cells were isolated by density gradient centrifugation using Ficoll (Amersham Biosciences). Monocytes (purity >95%) were obtained and cultured to mature DCs as previously described (14).
Immunohistochemistry.
Monoclonal mouse anti–human antibodies to IL-12 p40 were obtained from R&D Systems (clone 31052.11). Binding of the mouse antibody was detected in skin samples using biotinylated horse anti–mouse IgG and an avidin–biotin complex used with a peroxidase visualization reaction according to the manufacturer's directions (Vectastain ABC elite; Vector Laboratories).
Split Skin Tissue Culture.
Shaved skin biopsy of lesional skin in two patients was obtained by scalpel to include an area of ∼2 cm2. The biopsy was placed in tissue culture medium (RPMI 1640, Hepes 10 mM) containing 0.5% dispase and incubated at 4°C overnight. The dermis was separated from the epidermis by forceps. For the RT-PCR assay of epidermis versus dermal p19 and p40 expression (see Fig. 2), the tissues were rinsed with PBS and then homogenized after separation. For the isolation of inflammatory cells from the dermis (see Fig. 3), the dermis was washed with PBS and then cultured (RPMI 1640 supplemented with 5% normal human serum, 10 μg/ml gentamicin, and 10 mM Hepes) for 2 d. The inflammatory cells leaving the intact dermis were then collected and processed for total RNA.
Figure 2.
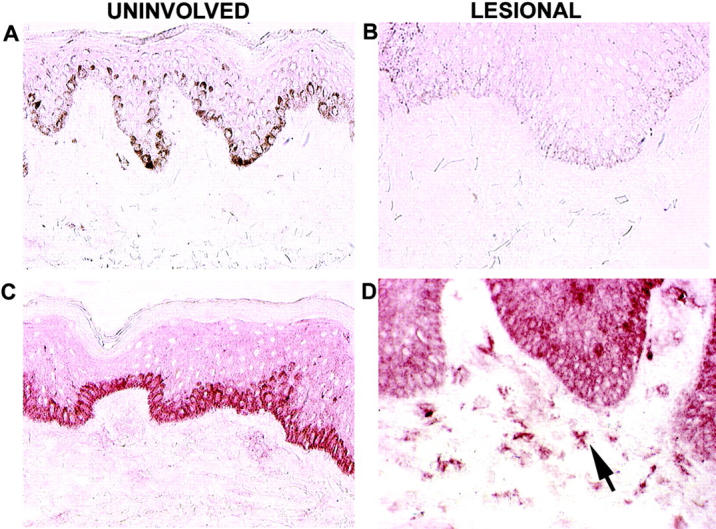
Immunohistochemical localization of p40 in psoriatic skin. The four panels are from frozen sections of human skin from psoriasis patients. A and B are IgG isotype controls in uninvolved and lesional skin, respectively. C and D are stained with mouse anti–human IL-12p40 as indicated. The arrow indicates morphology consistent with DCs. The color represents 3-amino-9-ethylcarbazole deposition at the site of antibody binding. Melanin appears in A as dark stain in the basal cell layer.
Figure 3.
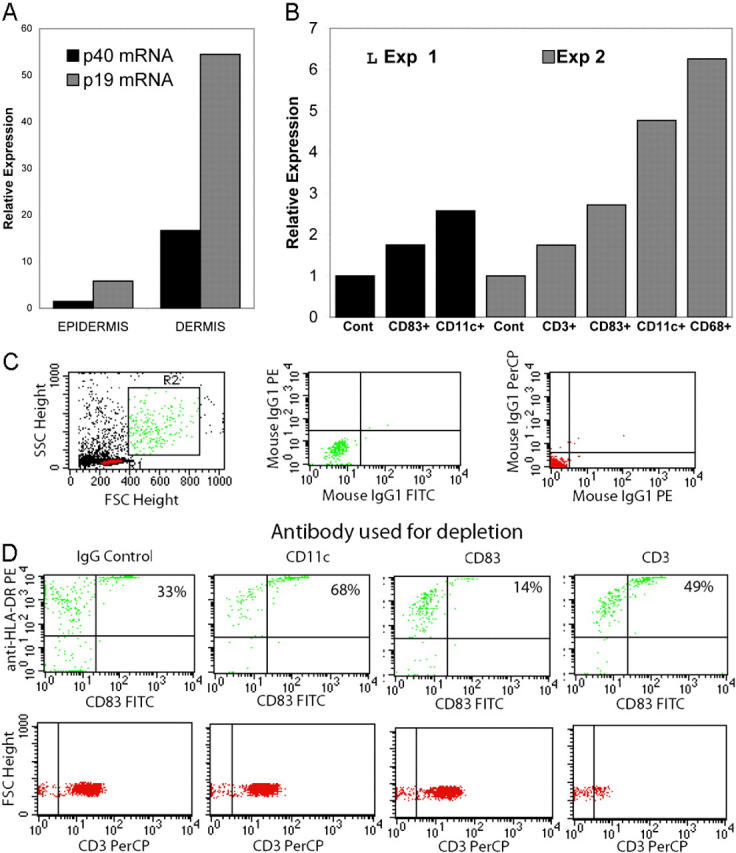
p19 and p40 gene expression in dispase split skin and dermally derived inflammatory cells. (A) Dermis from psoriasis lesions has greater p19 and p40 mRNA than epidermis. The data are from one representative experiment. A repeat run produced almost identical results. RNA was isolated from dispase split lesional skin taken from psoriatic plaques from two patients. RT-PCR for the p19 and p40 genes was performed on the epidermis and dermis and gene expression levels are shown relative to the hARP gene. In two other patients, the dermis was cultured for an additional 2 d at 37°C and the cells exiting the cultured dermis (B, C, and D) were collected for subsequent quantitative gene expression for p19 using immunomagnetic beads coated with antibodies to CD3, CD11c, or CD83. In each case, two cell populations were obtained: one adherent to the beads expressing CD83, CD11c, or CD68 (B), and one nonadherent (C and D) that was used to monitor the depletion by FACS®. For the latter, flow-through cells were analyzed for the presence of HLA-DR and CD83. (B) Total RNA was isolated and gene expression for p19 was assayed using TaqMan chemistry as described in Materials and Methods. Data are from one representative experiment. A repeat run produced almost identical results. (C, left) Side and forward scatter plots and gating (R1 for lymphocytes and R2 for DCs) for subsequent panels. (C, middle and C, right) Isotype controls. Cells depleted by isotype control (D, top, panel 1), anti-CD11c (D, top, panel 2), anti-CD83 (D, top, panel 3), and anti-CD3 (D, top, panel 4). Note that percentages represent upper right quadrant cell count (HLA-DR+ CD83+) as percent of total. Each panel has a plot of forward scatter versus CD3+ to demonstrate additional specificity of the depletion.
Dermal Cell Purification.
Cells emigrating from 48-h dermis cultures were captured by positive selection for CD1a, CD3, CD11c, CD68, and CD83 using magnetic beads (Pan Mouse IgG kit; Dynal) covalently attached to anti–mouse IgG Fc. These were coated according to the manufacturer's protocol with a mouse IgG antibody to a cell surface antigen (1 μg antibody/25 μl or 107 beads). The antibody to CD3 was from Orthoclone®, Ortho Biotech, and the others were from Becton Dickinson. Mouse IgG isotype control Ab was provided by Protein Design Labs. The psoriatic dermal cells were split into 1-ml aliquots and incubated with 500 μl of the appropriate antibody-coated magnetic bead. Cells attached to the beads were separated from nonattached cells by magnet and placed in RLT buffer with β-mercaptoethanol for p19 and p40 mRNA analysis, whereas unbound flow-through cells were verified by flow cytometry for depletion of the targeted cell type.
Online Supplemental Material.
The supplemental material describes gene transduction studies to verify the primer-probe set used for p19 amplification and detection by RT-PCR (TaqMan). Table S1 and Supplemental Results are available at http://www.jem.org/cgi/content/full/jem.20030451/DC1.
Results
Gene Expression Studies.
The expression of p40, (IL-23) p19, and (IL-12) p35 was determined in lesional and nonlesional skin of 22 psoriasis patients (Fig. 1) . The values obtained for each gene were normalized to the housekeeping gene hARP. To confirm that our primer/probe set correctly identified expression of p19 mRNA, we used recombinant adenovirus-transduced cell lines (see Table S1, which is available at http://www.jem.org/cgi/content/full/jem.20030451/DC1). Expression of p19 mRNA was increased in all 22 lesional skin biopsies (22-fold mean increase; P < 9 × 10−7) as was p40 mRNA (12-fold mean increase; P < 4.8 × 10−7). The expression of p35 mRNA was more variable, but the mean values for paired lesional and nonlesional skin samples did not differ significantly (P = 0.153).
Figure 1.
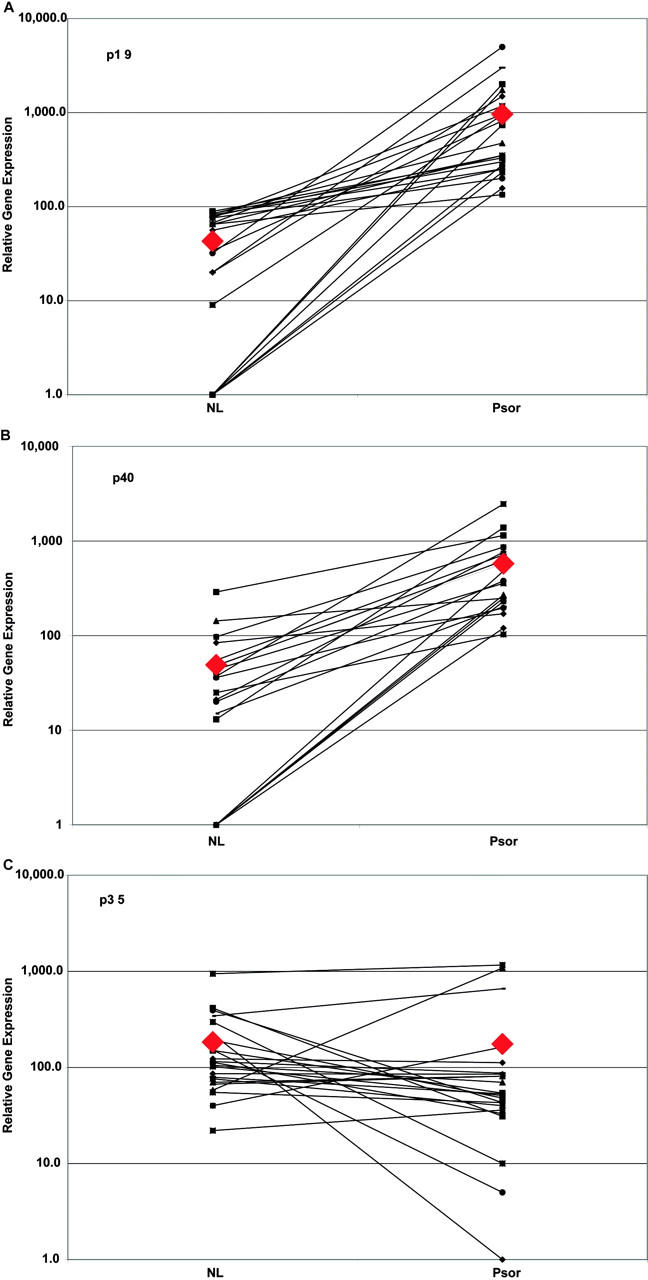
Expression of p19, p40, and p35 mRNA in paired samples of uninvolved and lesional skin from psoriasis patients. ♦, indicates the arithmetic mean for each type of specimen and is not connected by a line. A line connects the paired samples taken from each patient's nonlesional and psoriatic skin. Data is presented as the ratio of PCR yield, in picograms, of p19 (A), p40 (B), and p35 (C) normalized to the yield of hARP. Note the logarithmic scale of the abscissa.
Immunohistochemical Studies of IL-12p40 Expression.
Because an antibody to the p19 subunit is presently not available, we used a p40-reactive antibody to localize potential cells that might synthesize the IL-23 heterodimer in psoriasis lesions. Intensely positive staining cells were noted in the papillary dermis of lesional skin and were dendritic in appearance (Fig. 2 D). Little background staining was seen with an isotype control antibody in uninvolved or lesional skin (Fig. 2, A and B). The anti-p40 monoclonal antibody produced a variable degree of staining in basal keratinocytes of uninvolved skin, but very few p40-reactive cells were seen in the dermis of uninvolved skin (Fig. 2 C). Variable degrees of epidermal staining with the p40 antibody were noted, but staining of dermal cells was consistent across many different cases of psoriasis vulgaris. Overall, the staining pattern for p40 most resembled the staining of DCs in lesions, as detected previously with antibodies to CD83 or CD11c (15).
Localization of p19/p40 mRNA Expression Mainly to Dermal Cells.
Comparison of gene expression in dispase-separated epidermis and dermis from lesions showed that most of the mRNA for p19 and p40 was contained in dermal cells (Fig. 3 A). We then used immunomagnetic beads to enrich specific populations of leukocytes isolated from the dermis of psoriasis lesions (Fig. 3 B). A low level of p19 mRNA was detected in cells isolated with anti-CD3 beads, whereas higher levels were measured in cells selected with anti-CD83, anti-CD11c, and anti-CD68 beads (Fig. 3 B). To characterize the cells isolated by antibody-coated magnetic beads, we assayed the unbound (flow-through) fraction for depletion of HLA-DR+ and CD83+ or CD3+ cells (Fig. 3 D). Using two color flow cytometry and gating parameters appropriate for T cells and DCs (Fig. 3 C, R1 and R2, respectively), we found that CD11c depletion increased the relative concentration of CD83+ cells (68%), but decreased the frequency of CD14+ cells, as expected if many of the CD11c+ cells were monocytes (Fig. 3 D). Analogously, CD83 depletion decreased the relative concentration of CD83+ cells in the flow through (Fig. 3 D, 14%), but did not deplete CD14+ cells (not depicted), as expected if these cells were mature DCs. CD3 depletion resulted in only a minor increase in CD83+ cells in the flow through (Fig. 3 D, 49%). CD3+ cells (gate R1) were depleted only with CD3 antibody beads (Fig. 3 D, bottom). By immunohistochemical and FACS® staining of dermal cells from other biopsies, large increases in presumptive monocytes (CD11c+ CD14+ CD83−) and mature DCs (CD11c+ CD14− CD83+) were detected in active psoriasis lesions compared with unaffected skin of the same patients.
Strong and Sustained Expression of p19 and p40 in Mature DCs.
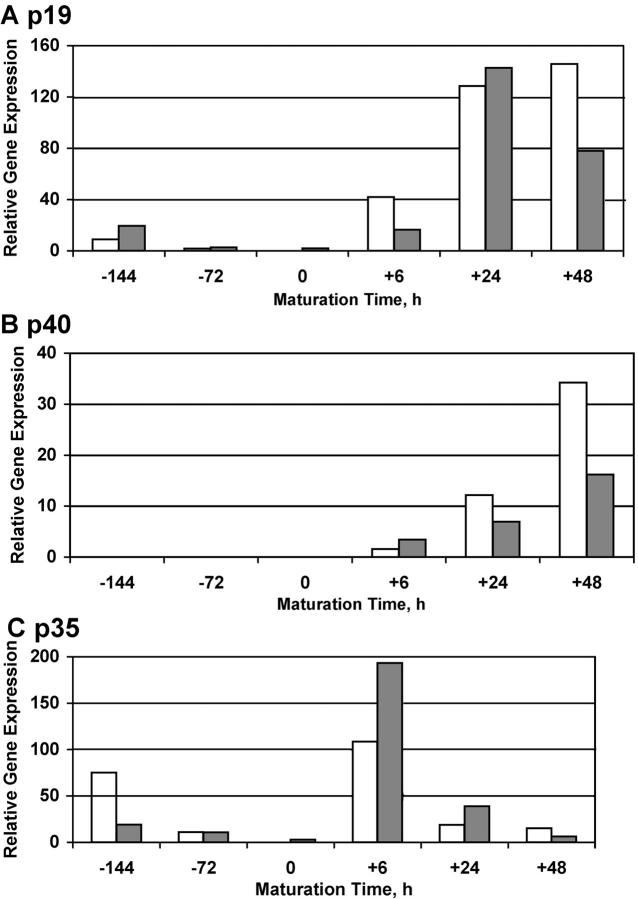
To further examine the control of IL-12 and IL-23 expression in monocytes and DCs, we used a standard procedure to generate DCs from peripheral blood monocytes using 4 d of culture in GM-CSF and IL-4 to produce immature DCs and a cytokine cocktail to mature the DCs (14). Fig. 4 displays quantitative expression of p19, p40, and p35 mRNAs in monocytes and in monocyte-derived DCs at various times after the addition of our maturation stimulus, and essentially parallels results seen with CD40L-stimulated DCs (1). Monocytes (−144 h), as well as monocytes cultured in GM-CSF and IL-4 to stimulate the differentiation of immature DCs (0 h), expressed relatively low levels of p19 mRNA and virtually undetectable levels of p40 mRNA along with gradually decreasing levels of p35 mRNA. However, p19 mRNA was increased up to 10-fold in freshly isolated monocytes by the addition of TNF-α or the maturation cocktail (IL-1β, IL-6, TNF-α, and PGE2) for 6–24 h, but p40 and p35 mRNA were not significantly induced by these stimuli (not depicted). For immature DCs, however, the addition of the maturation cocktail produced a rapid increase in mRNA for p19, p40, and p35 subunits, but with differing kinetics. Relative to immature DCs, there was at least a 50-fold increase in p19 and p40 mRNA in maturing and mature DCs. The up-regulation of these IL-23 subunits was sustained over the full 48-h maturation period and was highest in fully mature CD83+ DCs. In contrast, there was a rapid increase in p35 mRNA 6 h after giving the maturation stimulus, but the levels declined by 5–10-fold by 24 and 48 h. Other responses during DC maturation (6, 24, and 48 h) included strong up-regulation of DC-LAMP mRNA and decreased expression of DC-SIGN mRNA, and induction of CD83 protein at 48 h.
Figure 4.
Expression of p19, p40, and p35 mRNA in monocyte-derived DCs. Monocytes were placed in culture at −144 h, differentiated to immature DCs, and then stimulated beginning at 0 h with TNF-α, IL-1β, IL-6, and PGE2 for the stated amount of time. The data bars (open and solid) represent relative gene expression of p19 (A), p40 (B), and p35 (C) from two separate experiments. Note differing abscissa scales.
Discussion
Accumulating evidence supports the role of type 1 T cells and IFN-γ in the pathogenesis of psoriasis (8). Individual components of the type 1 pathway, both cellular and cytokine, are therapeutic targets in psoriasis. The development of Th1-type helper cells can be driven by IL-12 (1). We and others have previously reported that IL-12 p40 mRNA is elevated in psoriasis (12, 13), but we were perplexed by the failure to find elevated IL-12 p35 mRNA in diseased skin. We would now like to suggest that IL-23, not IL-12, is a key cytokine in psoriatic skin lesions based on the finding that p19 and p40, but not p35 mRNA, are overexpressed.
We believe that this is the first report that IL-23 p19 is up-regulated in a human disease. Recent reports have demonstrated a role for this novel cytokine in enhanced cutaneous immunity in a transgenic mouse model (16) and in inflammatory disorders such as experimental autoimmune encephalitis in a mouse model (5). With our observation that IL-23 is up-regulated in psoriatic skin, it became necessary to identify the cell of origin. The current lack of an antibody to p19 currently hampers this work. However, the reactivity of large cells in psoriasis lesions with antibodies specific for p40 suggests that IL-23 (given the failure to find increased IL-12 p35 mRNA in lesional skin) is likely to be actively synthesized in psoriasis lesions (12) and then act locally on cells that have up-regulated IL-12 receptors (13). In this study, we also found that IL-23 p19 and p40 mRNA were strongly up-regulated in monocyte-derived human DCs after encountering a maturation stimulus, and that p19 mRNA was strongly expressed in cells bearing both immature and mature DC surface markers in psoriasis lesions (CD11c+ CD14− CD83− and CD83+). The sustained expression of IL-23 subunits in cultured fully mature DCs contrasted sharply with the transient expression of p35 mRNA in maturing DCs. Of the cells known to express IL-23 p19, only macrophages and DCs concurrently express p40 and are known to express functional IL-23 protein. Monocytic and DC lines have previously been identified as IL-23 producers, whereas mature human DCs have been shown to secrete IL-23 protein (1, 17). Thus, it seems that the mRNA and protein subunits of IL-23 are coordinately up-regulated in producing cells. One of the cellular features of psoriasis skin lesions is a marked increase in mature CD83+ DCs, whereas uninvolved skin contains mostly immature Langerhans cells and dermal DCs (15, 18, 19). Based on p40 immunostaining, infiltrating mononuclear leukocytes are the likely source of IL-23 in lesions. Given that p35 mRNA is expressed only transiently in activated DCs and p35 mRNA is not elevated in lesional skin, IL-12 is likely to be expressed at lower levels than IL-23 in psoriatic lesions. Adoptive transfer experiments have clearly established leukocytes as the chief source of IL-23 in inflammatory lesions (4).
It is possible that both IL-12 and IL-23 have a role in psoriatic skin disease. However, the work in murine experimental autoimmune encephalitis indicates that IL-23 is critical for a monocyte-rich inflammatory response. Expression of IL-23 may also drive increased production of IL-17 in affected skin (20). IL-17 has been reported to increase the expression of iNOS (21), a gene that is consistently overexpressed in psoriasis lesions (13). In model systems, IL-23 acts on DCs to allow for the induction of immunity to otherwise tolerogenic peptides (22), so potentially its expression could contribute to abnormal antigenic recognition of skin-related antigens in psoriasis. Interestingly, a recent report identified skin inflammation in a skin-targeted p40 transgenic overexpressor as due to IL-23 production, but not IL-12 production, and there was a marked increase in the abundance of mature DCs in these lesions associated with increased IL-23 levels (16). In summary, IL-23 antagonists might be the most appropriate treatment for inflammatory skin diseases like psoriasis.
Acknowledgments
The authors wish to thank Joseph Krasovsky for technical assistance.
J. Krueger and E. Lee were supported by National Institutes of Health (NIH) grants AI-49572, AI-49832, 1-K23-AR49815-01, and M01-RR00102. M. Dhodapkar was supported by NIH grant CA 84512 and a Clinical Investigator Award from Damon Runyon Cancer Research Fund.
Abbreviation used in this paper: hARP, human acidic ribosomal protein gene.
The online version of this article contains supplemental material.
References
- 1.Oppmann, B., R. Lesley, B. Blom, J.C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, et al. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 13:715–725. [DOI] [PubMed] [Google Scholar]
- 2.Parham, C., M. Chirica, J. Timans, E. Vaisberg, M. Travis, J. Cheung, S. Pflanz, R. Zhang, K.P. Singh, F. Vega, et al. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168:5699–5708. [DOI] [PubMed] [Google Scholar]
- 3.Elkins, K.L., A. Cooper, S.M. Colombini, S.C. Cowley, and T.L. Kieffer. 2002. In vivo clearance of an intracellular bacterium, Francisella tularensis LVS, is dependent on the p40 subunit of interleukin-12 (IL-12) but not on IL-12 p70. Infect. Immun. 70:1936–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiekowski, M.T., M.W. Leach, E.W. Evans, L. Sullivan, S.C. Chen, G. Vassileva, J.F. Bazan, D.M. Gorman, R.A. Kastelein, S. Narula, et al. 2001. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility, and premature death. J. Immunol. 166:7563–7570. [DOI] [PubMed] [Google Scholar]
- 5.Cua, D.J., J. Sherlock, Y. Chen, C.A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748. [DOI] [PubMed] [Google Scholar]
- 6.Austin, L.M., M. Ozawa, T. Kikuchi, I.B. Walters, and J.G. Krueger. 1999. The majority of epidermal T cells in psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J. Invest. Dermatol. 113:752–759. [DOI] [PubMed] [Google Scholar]
- 7.Koo, J.Y. 1999. Current consensus and update on psoriasis therapy: a perspective from the U.S. J. Dermatol. 26:723–733. [DOI] [PubMed] [Google Scholar]
- 8.Krueger, J.G. 2002. The immunologic basis for the treatment of psoriasis with new biologic agents. J. Am. Acad. Dermatol. 46:1–23. [DOI] [PubMed] [Google Scholar]
- 9.Oestreicher, J., I. Walters, T. Kikuchi, P. Gilleaudeau, J. Surette, U. Schwertschlag, A. Dorner, J. Krueger, and W. Trepicchio. 2001. Molecular classification of psoriasis disease-associated genes through pharmacogenomic expression profiling. Pharmacogenomics J. 1:272–287. [DOI] [PubMed] [Google Scholar]
- 10.Bowcock, A.M., W. Shannon, F. Du, J. Duncan, K. Cao, K. Aftergut, J. Catier, M.A. Fernandez-Vina, and A. Menter. 2001. Insights into psoriasis and other inflammatory diseases from large-scale gene expression studies. Hum. Mol. Genet. 10:1793–1805. [DOI] [PubMed] [Google Scholar]
- 11.Zhou, X., J.G. Krueger, M.C. Kao, E. Lee, F. Du, A. Menter, W.H. Wong, and A.M. Bowcock. 2003. Novel mechanisms of T-cell and dendritic cell activation revealed by profiling of psoriasis on the 63,100-element oligonucleotide array. Physiol. Genomics. 13:69–78. [DOI] [PubMed] [Google Scholar]
- 12.Yawalkar, N., S. Karlen, R. Hunger, C.U. Brand, and L.R. Braathen. 1998. Expression of interleukin-12 is increased in psoriatic skin. J. Invest. Dermatol. 111:1053–1057. [DOI] [PubMed] [Google Scholar]
- 13.Trepicchio, W.L., M. Ozawa, I.B. Walters, T. Kikuchi, P. Gilleaudeau, J.L. Bliss, U. Schwertschlag, A.J. Dorner, and J.G. Krueger. 1999. Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions. J. Clin. Invest. 104:1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhodapkar, K.M., J. Krasovsky, B. Williamson, and M.V. Dhodapkar. 2002. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J. Exp. Med. 195:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrams, J.R., S.L. Kelley, E. Hayes, T. Kikuchi, M.J. Brown, S. Kang, M.G. Lebwohl, C.A. Guzzo, B.V. Jegasothy, P.S. Linsley, et al. 2000. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte–associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J. Exp. Med. 192:681–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kopp, T., P. Lenz, C. Bello-Fernandez, R.A. Kastelein, T.S. Kupper, and G. Stingl. 2003. IL-23 production by cosecretion of endogenous p19 and transgenic p40 in keratin 14/p40 transgenic mice: evidence for enhanced cutaneous immunity. J. Immunol. 170:5438–5444. [DOI] [PubMed] [Google Scholar]
- 17.Pirhonen, J., S. Matikainen, and I. Julkunen. 2002. Regulation of virus-induced IL-12 and IL-23 expression in human macrophages. J. Immunol. 169:5673–5678. [DOI] [PubMed] [Google Scholar]
- 18.Dieu-Nosjean, M.C., C. Massacrier, B. Homey, B. Vanbervliet, J.J. Pin, A. Vicari, S. Lebecque, C. Dezutter-Dambuyant, D. Schmitt, A. Zlotnik, et al. 2000. Macrophage inflammatory protein 3α is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J. Exp. Med. 192:705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koga, T., H. Duan, K. Urabe, and M. Furue. 2002. In situ localization of CD83-positive dendritic cells in psoriatic lesions. Dermatology. 204:100–103. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal, S., N. Ghilardi, M.H. Xie, F.J. de Sauvage, and A.L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910–1914. [DOI] [PubMed] [Google Scholar]
- 21.Miljkovic, D., I. Cvetkovic, O. Vuckovic, S. Stosic-Grujicic, M. Mostarica Stojkovic, and V. Trajkovic. 2003. The role of interleukin-17 in inducible nitric oxide synthase-mediated nitric oxide production in endothelial cells. Cell. Mol. Life Sci. 60:518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belladonna, M.L., J.C. Renauld, R. Bianchi, C. Vacca, F. Fallarino, C. Orabona, M.C. Fioretti, U. Grohmann, and P. Puccetti. 2002. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J. Immunol. 168:5448–5454. [DOI] [PubMed] [Google Scholar]