Abstract
The potassium conductance of the basolateral membrane (BLM) of proximal tubule cells is a critical regulator of transport since it is the major determinant of the negative cell membrane potential and is necessary for pump-leak coupling to the Na+,K+-ATPase pump. Despite this pivotal physiological role, the properties of this conductance have been incompletely characterized, in part due to difficulty gaining access to the BLM. We have investigated the properties of this BLM K+ conductance in dissociated, polarized Ambystoma proximal tubule cells. Nearly all seals made on Ambystoma cells contained inward rectifier K+ channels (γslope, in = 24.5 ± 0.6 pS, γchord, out = 3.7 ± 0.4 pS). The rectification is mediated in part by internal Mg2+. The open probability of the channel increases modestly with hyperpolarization. The inward conducting properties are described by a saturating binding–unbinding model. The channel conducts Tl+ and K+, but there is no significant conductance for Na+, Rb+, Cs+, Li+, NH4 +, or Cl−. The channel is inhibited by barium and the sulfonylurea agent glibenclamide, but not by tetraethylammonium. Channel rundown typically occurs in the absence of ATP, but cytosolic addition of 0.2 mM ATP (or any hydrolyzable nucleoside triphosphate) sustains channel activity indefinitely. Phosphorylation processes alone fail to sustain channel activity. Higher doses of ATP (or other nucleoside triphosphates) reversibly inhibit the channel. The K+ channel opener diazoxide opens the channel in the presence of 0.2 mM ATP, but does not alleviate the inhibition of millimolar doses of ATP. We conclude that this K+ channel is the major ATP-sensitive basolateral K+ conductance in the proximal tubule.
Keywords: ion channel, kidney, patch-clamp, sulfonylurea, epithelia
introduction
The ability of the renal proximal tubule to maintain homeostatic electrolyte and water reabsorption in the face of drastic changes in dietary solute and water intake and renal hemodynamics implies that transtubular ion transport is tightly regulated. Proximal tubule potassium channels, particularly in the basolateral membrane (BLM),1 play pivotal physiologic roles in the regulation of membrane voltage, potassium recycling, and ultimately in transepithelial solute and water reabsorption. That the basolateral membrane potential of a proximal tubule cell is dominated by the BLM K conductance is well established (Sackin and Boulpaep, 1983). The Na+,K+-ATPase pump in the BLM provides the energy that makes ion transport thermodynamically favorable, but continuous operation of the pump requires there be a K+ exit pathway. The BLM K conductance provides such a pathway and thus steady state intracellular K activity can be maintained in the face of large transcellular fluxes of salt and water (Sackin and Boulpaep, 1983). Since a major portion of the transcellular Na+ flux is reabsorbed across the BLM by the action of the Na+,K+-ATPase, a population of BLM K+ channels working in concert with the pump would allow K+ to recycle in a regulated fashion. Moreover, hyperpolarization secondary to the opening of BLM K+ channels enhances the driving force for electrogenic apical Na+ entry and basolateral Cl− efflux, resulting in net NaCl reabsorption.
The Ambystoma proximal tubule exhibits a large K+ conductance in the basolateral membrane (Siebens and Boron, 1987; Sackin and Boulpaep, 1981) that has been shown macroscopically to be sensitive to barium and pH. However, previous studies of the “macroscopic” BLM K+ conductance (Boulpaep, 1976) lack the resolution to distinguish whether there is one population of imperfectly selective K+ channels or a set of highly selective K+ channels coexisting with a population of nonselective cation channels. More recent “microscopic” single-channel patch-clamp studies of BLM K+ channels have shown diversity in both experimental design and findings (Tsuchiya et al., 1992; Hunter, 1991; Parent et al., 1988; Kawahara et al., 1987; Sackin and Palmer, 1987; Gögelein and Greger, 1987a , 1987b ), so a clear consensus has been elusive and details of the properties and regulation are lacking.
We have now characterized the properties and regulation (see Mauerer et al., 1998) of the principal K+ channel in the BLM in a preparation of dissociated yet polarized Ambystoma proximal tubule cells (Segal et al., 1996). Inwardly rectifying, ATP-sensitive K+ channels were present in >95% of recordings from the BLM, each containing from 2 to >25 KATP channels/patch. Although the regulation of this proximal tubule BLM K+ channel is similar to that of recently cloned KATP channels in the apical membrane of the distal nephron (ROMK1 and ROMK2), there are important differences, and ROMK has not been found in the proximal tubule (Chepilko et al., 1995; Zhou et al., 1994; Lee and Hebert, 1995; Boim et al., 1995; Ho et al., 1993). The studies reported in this paper and the companion paper elucidate the properties and the regulation, respectively, of the major K+ channel underlying the BLM K conductance that is coupled to transport in the proximal tubule.
materials and methods
Solutions and Drugs
The compositions of the solutions used are summarized in Table I. After titration to pH 7.5 (710A; Orion Research, Boston, MA), sucrose was added to adjust the osmolality of the solutions (3MO; Advanced Instruments Inc., Needham Heights, MA). To determine channel conductance as a function of [K+], sucrose was added to the standard pipette KCl to maintain osmolality. Chemicals used were of the highest quality and obtained from Sigma Chemical Co. (St. Louis, MO), except thallium acetate (Aldrich Chemical Co., Milwaukee, WI), diazoxide (Calbiochem Corp., La Jolla, CA), ATPγS, and ADP (Boehringer-Mannheim Biochemicals, Indianapolis, IN). Nucleotides were prepared fresh daily as 20–50-mM stocks in bath solution. Glibenclamide was dissolved in DMSO (100 mM stock).
Table I.
Solutions
| a NaCl Ringer | b Ca2+/Mg2+- free Ringer | c NaCl recording solution | d KCl recording solution | e Mg-free KCl | f KCl 200 nM Mg2+ | g Tl+ pipette solution | h 2 mM Cl− K solution | i KCl salt dilution | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | 95.9 | 98.5 | 95 | 2.5 | 2.5 | 2.5 | 5 | 2.5 | ||||||||||
| K+ | 2.5 | 2.5 | 2.5 | 95 | 95 | 95 | 95 | 14 | ||||||||||
| Cl− | 98.1 | 92.5 | 94.5 | 94.5 | 92.5 | 96.1 | 2 | 2 | 13.5 | |||||||||
| Ca2+ | 1.8 | |||||||||||||||||
| Mg2+ (added, mM) | 1 | 1 | 1 | 0.81 | 1 | 1 | 1 | |||||||||||
| Mg2+ (free, nM) | 200 | |||||||||||||||||
| Tl+ | 90 | |||||||||||||||||
| Acetate− | 90 | 90 | ||||||||||||||||
| HEPES | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||||||||
| HEPES− | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||||||||
| EGTA | 1 | 1 | 1 | 1 | 1 | |||||||||||||
| EDTA | 1 | 8 | 8 | |||||||||||||||
| H2PO4 − | 0.1 | 0.1 | ||||||||||||||||
| HPO4 2− | 0.4 | 0.4 | ||||||||||||||||
| Dextrose | 2 | 2 | ||||||||||||||||
| pH (titrand) | 7.5 (NaOH) | 7.5 (NaOH) | 7.5 (NaOH) | 7.5 (KOH) | 7.5 (KOH) | 7.5 (KOH) | 7.5 (NaOH) | 7.5 (KOH) | 7.5 (KOH) | |||||||||
| Osmolality | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 |
Cell Preparation
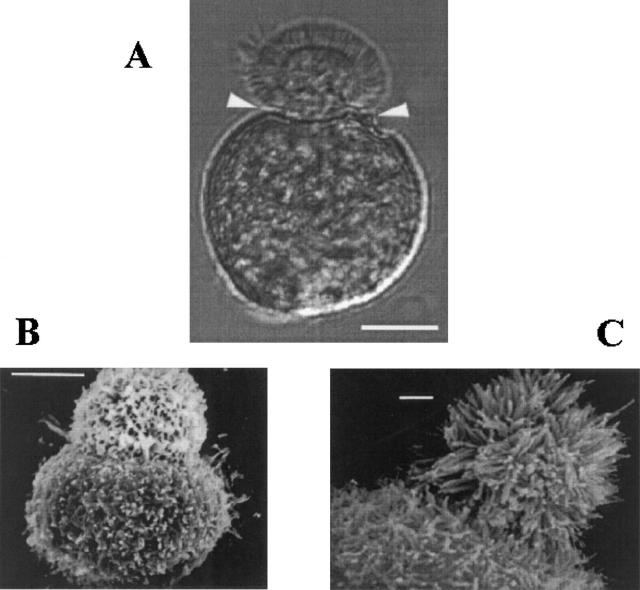
Dissociated proximal tubule cells were isolated from amphibian kidneys as previously described (Segal et al., 1996). Briefly, aquatic phase Ambystoma tigrinum kept at 4°C were killed by submersion in 0.2% tricaine methanesulfonate. The kidneys were rapidly removed and placed in iced HEPES-buffered NaCl at pH 7.5 (solution a). The adventitial tissue was removed by hand dissection, and the renal tissue was cut into 1–2 mm3 pieces and incubated in collagenase-dispase (0.2 U/ml of collagenase; Boehringer-Mannheim Biochemicals) on a gyratory shaker for 60 min at 22°C. The enzyme reaction was stopped by washing with Ca2+- and Mg2+-free NaCl (solution b ). The cells were then mechanically dispersed into suspension by repeated trituration, and a pellet was obtained by centrifugation at ∼1,600 rpm for 3 min. Finally, the cells were resuspended in 2.5 ml NaCl (solution a) in a 35-mm culture dish, and stored at 4°C until use. The dissociated proximal tubule cells can retain their epithelial polarity for up to 14 d (Segal et al., 1996). Cells were used for experiments from 2 to 12 d after dissociation. Representative cells as seen under light microscopy (Fig. 1 A) and scanning electron microscopy (Fig. 1 B) are shown (for details of methods see Segal et al., 1996).
Figure 1.
Dissociated Ambystoma proximal tubule cells retain epithelial cell polarity. (A) Light photomicrograph of a single dissociated Ambystoma proximal tubule cell shows distinct apical and basolateral membrane surfaces. The apical surface of these bilobated cells is the smaller lobe with a microvillar brush border. The robust cytoskeleton of these cells includes an actin-rich “waist-band” (arrowheads) between the two membrane domains that is important in retention of epithelial polarity. Giga-ohm seals can be made on both surfaces. Scale bar, 10 μm. (B and C) Scanning electron micrographs showing the sharp transition between the membrane domains and the detailed topology of the apical surface invested with its microvillar brush border, and the basolateral surface with its folds and projections. Scale bars: 10 μm in B, 1 μm in C.
Electrophysiology
A 5-μl aliquot of cell suspension in NaCl storage solution (solution a) was placed on a Cell-Tak™-coated glass coverslip in a recording chamber of our design (RC-5/25; Warner Instruments, Hamden, CT) mounted on an inverted microscope (Olympus IM; Olympus America, Inc., Melville, NY). The chamber has a bath volume of 500 μl, and solutions are perfused directly into an input multiplexer on the chamber at a gravity-driven flow rate of ∼10 ml/min.
Nonadherent cells were washed off the coverslip with the recording solution (either Ca2+-free NaCl, solution c, or KCl, solution d, unless otherwise noted) and the cells were visualized under Hoffman modulation optics (Modulation Optics, Greenvale, NY). Proximal tubule cells were readily recognized by their characteristic morphology (see Fig. 1; Segal et al., 1996). An individual proximal tubule cell was selected for an experiment only if it fulfilled the following criteria (Segal et al., 1996): (a) distinctly bilobated structure, (b) clearly defined brush border sharply delimited on the apical surface, (c) relatively smooth appearing basolateral membrane, and (d) absence of large vacuoles.
Patch clamp.
The standard configurations for single-channel and whole-cell tight seal patch-clamp technique (Hamill et al., 1981) were used to record channel currents from the BLM. Patch pipettes were fabricated from borosilicate glass capillaries (Warner Instruments, Hamden, CT) on a two-step puller (PP-83; Narishige Co., Ltd, Tokyo, Japan), coated with Sylgard 184™ (Dow-Corning Corp., Midland, MI) to within 200 μm of the tip, and fire-polished just before use. When filled with KCl, the open tip pipette resistance was 3–8 MΩ when placed in the initial bath solution. A hydraulic micromanipulator (Narishige) was used to guide the patch microelectrode to the BLM of the cell. High resistance giga-ohm seals (up to 50 GΩ) were obtained on the BLM in ∼75% of attempts by applying gentle suction to the pipette just after it touched the cell membrane. To achieve the whole-cell configuration, further suction was applied to rupture the cell-attached patch. Data have not been corrected for liquid-junction potentials since for most solutions they were <4 mV when measured as follows: the bath Ag-AgCl ground electrode was connected to the control KCl bath through a 3% agar bridge made of KCl pipette solution. A low resistance (<1 MΩ) pipette filled with 3 M KCl was placed in a KCl bath and the DC offset was adjusted to 0 mV in zero current clamp. The liquid-junction potentials were measured as the voltage offset resulting when the control KCl bath was replaced by the test solution. Low [Cl] solutions in which 90% of Cl− was replaced by aspartate− had a liquid-junction potential of 13.3 mV.
Voltage-clamped membrane currents were amplified (and zero current-clamp membrane potentials were measured) with an EPC-7 patch-clamp amplifier (Medical Systems, Greenvale, NY) controlled by a PDP 11/23 computer (Digital Equipment Corp., Maynard, MA). The current (filtered at 10 kHz) and voltage outputs of the EPC-7 were digitized at 44.1 kHz using a modified pulse code modulator (501ES; Sony Corp., Tokyo, Japan), and stored on videotape (SL-HF300; Sony Corp.). Whole-cell membrane currents were also digitized at 250 samples/s (Cheshire A/D; Indec, Sunnyvale, CA) and stored directly on computer disk. Signals were monitored on an oscilloscope (205; Hameg Instruments, Inc., Frankfurt, Germany) and strip-chart recorder (220; Brush, Cleveland, OH). All experiments were carried out at room temperature (20–22°C).
Data Analysis
Current data were played back and low pass filtered at 400 Hz (902LPF eight-pole Bessel filter; Frequency Devices Inc., Haverhill, MA), digitized at 1,000 samples/s, and stored on the PDP-11/ 23. In some cases, currents were filtered at 40 Hz and digitized at 100 samples/s for current binning and averaging analysis. Datafiles were transferred to a Pentium computer (Gateway 2000, North Sioux City, SD) via Kermit (Columbia University, NY) for analysis. Custom software for data acquisition and analysis was written in our laboratory using BASIC-23, AxoBASIC 1.0 (Axon Instruments, Foster City, CA), and Matlab 4.0 (The Mathworks, Natick, MA).
Channel activity (nP o) was calculated over periods of 60–500 s as follows. The closed current level (i c) was taken as the mode of the distribution around closed events. This current was subtracted from the current of a given bin, and the difference was multiplied by the number of events in that bin. The sum of these products yields the open channel area of the histogram. nP o is given by dividing the open channel area by the single-channel current, i sc. That is,
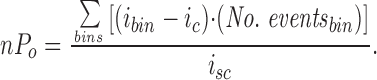 |
1 |
For kinetic analysis, currents were filtered at a corner frequency (f c) of 2 kHz and sampled at 5,000 s−1. An event (transition) was counted each time a data point crossed the 50% level of the unitary channel current. For our recording system, the patch-clamp has a 5-kHz step response, a 5-kHz tape bandwidth, and a 2-kHz eight-pole Bessel filter, yielding an effective bandwidth (−3 dB point, f ceff) of 1.74 kHz. With these settings, the “dead time” of the recording system is given by Colquhoun and Sigworth (1983), Tdead = 0.179/f ceff. The “50% delay time” of the Bessel filter is T50% = 0.506/f c. For f ceff = 1.74 kHz and f c = 2 kHz, Tdead = 102.8 μs and T50% = 253 μs. Therefore, events in time histogram bins <500 s were cut off. Since an event lasting 253 μs would be the margin of detection, all dwell lifetimes <253 μs would be missed events.
Open and closed dwell-time kinetics were fit to a probability density function expressed as a sum of exponentials,
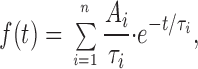 |
2 |
where n is the number of open or closed states, the Ai are the relative amplitudes, and the i are time constants. This function was transformed according to x = ln(t) and logarithmically binned (Sigworth and Sine, 1987), such that
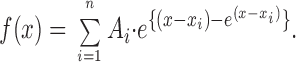 |
3 |
This function was used to fit the data using the Levenberg-Marquardt nonlinear least-squares fitting algorithm (Origin 4.0; Microcal Software, Inc., Northampton, MA) to find the appropriate set of τ's. Note that if the errors follow a Gaussian distribution, this nonlinear least-squares method is equivalent to the method of maximum likelihood (Colquhoun and Sigworth, 1983).
Dose–response relations for a drug (D) were fitted to the Hill equation as
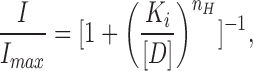 |
4 |
where I/I max is the fractional inhibition, K i is the concentration of drug giving 50% inhibition, [D] is the concentration of the drug, and n H is the Hill coefficient. When n H = 1, this equation reduces to the Langmuir adsorption isotherm.
In the text, the number of observations or experiments is reported, whereas n in the analysis denotes either the whole data set or the subset of total experiments in which precise quantitation could be reliably applied. In some figures, a running average (using a specified window width) of current versus time is displayed. Statistical values for the n elements are given as mean ± SEM. Student's t test was applied where appropriate.
results
Properties of the BLM K+ Channel
Overview.
With [K+] = 95 mM in the patch pipette, we found the K+ channel in 551 of 559 seals made on the BLM (98.6%). This K+ channel was never detected in seals made on the apical membrane (0 of 16, 0%), consistent with our previous finding that dissociated Ambystoma proximal tubule cells retain epithelial cell polarity (Segal et al., 1996). Seals on the BLM typically contained from 2 to >25 K+ channels. Despite numerous attempts to minimize patch area using small-tipped pipettes (resistance ≥ 30 MΩ), patches appearing to have one and only one channel were very infrequent (n = 4, only 0.7%).
Cell-attached patches.
When cell-attached (c/a) patches were made in NaCl bath, spontaneous inward K+ currents were usually observed (95%) at 0 mV (−Vpip) command potential. By briefly switching to zero current clamp mode, the resting membrane potential (Vm) of the cell can sometimes be estimated if Rseal >> Rpatch. Using this method under these conditions, Vm averaged −37.2 ± 2.1 mV (n = 16), in good agreement with Vm = −40 mV as measured by conventional impalement (Segal et al., 1996). The Vm of the dissociated cells is ∼15–20 mV less than that for a cell in the intact tubule (Sackin and Boulpaep, 1983), suggesting an anion conductance exists at either the apical or basolateral membrane. Indeed, we have characterized a cAMP-activated Cl− channel in the BLM of these cells, which often appears in the same membrane patch as the K+ channel (not shown). Alternatively, the isolated cells may have acquired a nonspecific leak pathway that shunts the normally high K diffusion potential. Since the K+ and Cl− activity of the pipette solution (aP K = 0.80*[95] = 76 mM and aP Cl = 0.78*[92] = 71.8 mM) is greater than the intracellular K+ and Cl− activity (ai K = 0.80*[68] = 54.4 mM and ai Cl = 0.78*[20.5] = 16 mM (Sackin and Boulpaep, 1983), yielding a reversal potential (Erev) of −28.7 mV. Thus, at −Vpip = 0 mV, the inward current must be carried by K+ moving down its electrochemical gradient from the pipette into the cell.
Representative c/a current records at various command potentials are shown in Fig. 2 A, and the current–voltage (I-V) characteristic is shown in Fig. 2 B. The inward slope conductance is 22.2 ± 1.4 pS (taken from −40 to −80 mV, n = 8), and the outward slope conductance (from +20 to +80 mV) is 3.5 ± 0.1 pS (n = 5), and channel activity appears to increase with hyperpolarization (Fig. 2 A). Although the c/a I-V characteristic displays inward rectification, suggesting that the channel is an inward rectifier; under these conditions, the I-V relation would be expected to “inwardly rectify” due to Goldman-Hodgkin-Katz rectification.
Figure 2.
The BLM K+ channel appears to be an inward rectifier. (A) Representative current records at various command potentials (−Vpip) from a cell-attached basolateral membrane patch containing at least two K+ channels. Note the increase in channel activity with hyperpolarization. The patch pipette contains 95 mM K+ (solution d) and the bath is NaCl (solution c). The dashed line represents the all channels closed (leak) current at each potential. Each open channel level is denoted by a dotted line. (B) Current–voltage relation for the BLM K+ channel for the conditions described in A. The limiting inward slope conductance is 22.2 ± 1.4 pS (n = 8) and the outward chord conductance 3.5 ± 0.1 pS (between 120 and 180 mV, n = 5). Symbols represent mean (•) ± SEM (bars).
Excised patches.
When BLM membrane patches were excised in the inside-out (i/o) configuration into the standard bath solutions (solution c or d), channel activity typically began to decline and then disappear. Addition of 0.2 mM ATP to the bath before or just after patch excision prevented rundown and maintained channel activity indefinitely.
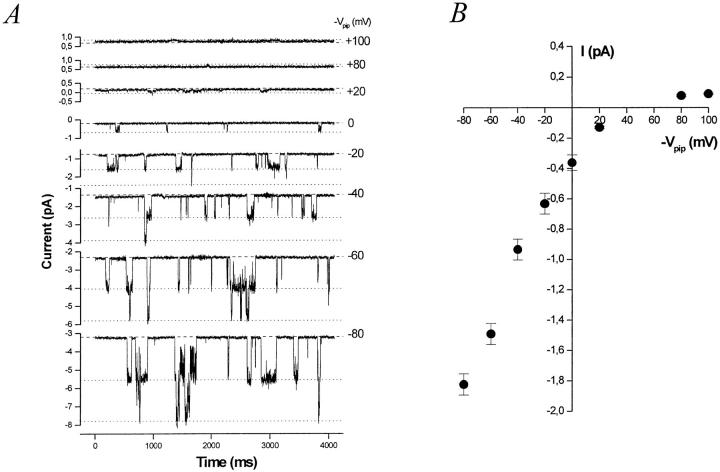
Measurements of single-channel current with [K+] = 95 mM on both sides of the membrane patch (plus 0.2 mM ATP on the cytosolic side) demonstrate that the BLM K+ channel is a true inward rectifier (Fig. 3, A and B). The I-V relation in symmetrical [K+] inwardly rectifies and reverses very close to EK = 0 mV. The channel has an inward slope conductance of γslope, in = 24.5 ± 0.6 pS (n = 8, measured between −60 and −100 mV), and an inward chord conductance of γchord, in = 20.5 ± 0.4 pS (n = 8, measured between Erev = 0 and −100 mV). The outward chord conductance measured at +80 mV is γchord, out = 3.7 ± 0.4 pS (n = 2). The outward slope conductance between +20 and +80 mV is clearly smaller.
Figure 3.
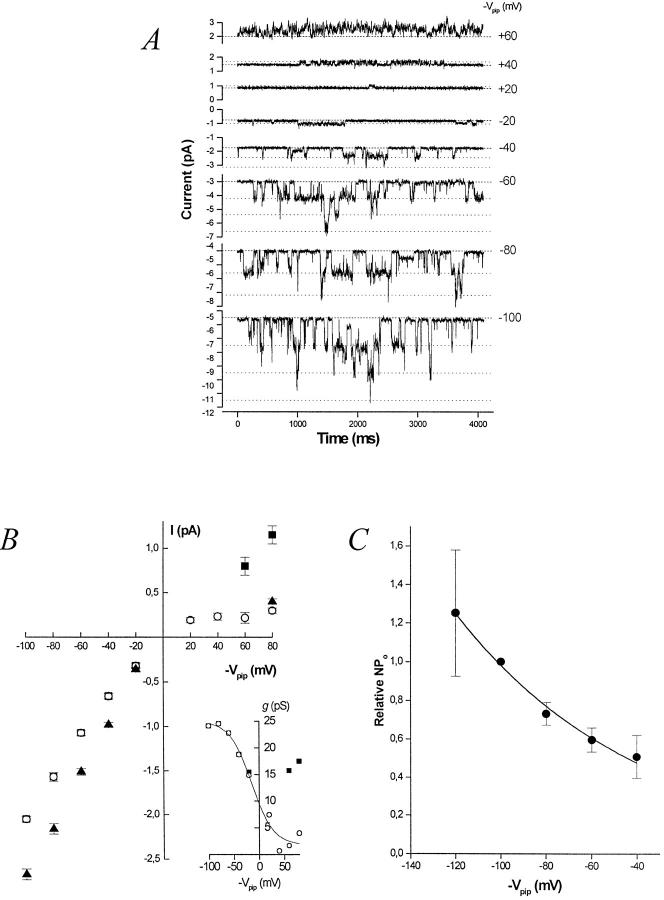
The BLM K+ channel is a true inward rectifier. Representative current records at various command potentials (−Vpip) from an inside-out basolateral membrane patch in symmetrical [K] containing at least three K+ channels. The patch pipette and bath each contain 95 mM K+ (solution d) with 0.2 mM ATP added to the bath. The dashed line represents the all channels closed (leak) current at each potential. Each open channel level is denoted by a dotted line. (B) Effect of [Mg2+]i on the I-V relation of the BLM K+ channel. The inward rectification evident in 1 mM [Mg2+]i (○) is relieved when [Mg2+]i is lowered to 200 nM (▪). (inset) Slope conductance–voltage (g-V) relation for the BLM K+ channel in 1 mM [Mg2+]i (○) and 200 nM [Mg2+]i (▪). Symbols represent mean ± SEM. The K+ channel prefers Tl+ over K+. The I-V relation from inside-out patches with Tl-acetate (solution g) in the pipette and K-acetate (solution h) in the bath is also shown (▴). The limiting inward slope conductance is 29.0 ± 1.0 pS (n = 4) for Tl+ compared with 24.5 ± 0.6 pS (n = 8) for K+. (C) Channel activity (nP o) increases with hyperpolarization. Data from four inside-out membrane patches in symmetrical [K] are plotted. Channel activity at command potentials of −120, −100, −80, −60, and −40 mV was normalized to that at −100 mV for comparison. Solid line is a single exponential fit with a voltage constant of ∼83 mV. Symbols represent mean (•) ± SEM (bars).
Voltage dependence of nPo.
Since BLM membrane patches almost always contained more than one K+ channel, nP o (channel activity) was used to assess the relative voltage dependence of P o. This assumes that the number of active channel proteins in an excised patch remains constant as voltage varies. (We take n as the maximum number of simultaneously open channels observed, which places a minimum on the actual number of channel proteins in the patch.) As was the case for cell-attached patches, channel activity with KCl on both sides of the excised membrane patch increases with increasing hyperpolarization (Fig. 3 C). Although the absolute values of nP o differed significantly among patches, relative nP o increased e-fold per ∼83-mV hyperpolarization between −40 and −120 mV, reflecting a 27% increase of nP o for every 20 mV of hyperpolarization.
Role of magnesium.
It has been shown that [Mg2+]i mediates at least part of the inward rectification in other inwardly rectifying K+ channels (Matsuda et al., 1987; Horie et al., 1987; Ficker et al., 1994) by blocking outward currents in a voltage-dependent manner. When Mg2+ i was removed from the “cytosolic” side of i/o patches (solution e plus 0.2 mM Na2ATP), we observed flickering, and then rundown of the channel. This is in sharp contrast to the behavior of ROMK1 channels, in which channel rundown is slowed in a Mg2+-free bath (McNicholas et al., 1994). Since rundown occurs in the absence of free Mg2+ i and the presence of 0.2 mM Na2ATP (n = 4), it appears that at least the complex of Mg-ATP is required to prevent rundown. Interestingly, it has been shown that both Mg-ATP and free Mg2+ i are required to sustain channel activity for an ATP-insensitive inward rectifier K+ channel (Fakler et al., 1994). However, since higher levels of ATP block the BLM K+ channel (see below), it is not possible for us to dissociate the role of free Mg2+ i from that of the Mg-ATP complex. Millimolar concentrations of ATP (or indeed, any nucleotide) block the BLM K+ channel, and this effect is independent of both free Mg2+ i and Mg-ATP.
In a solution containing 368 nM Mg-ATP and 200 nM free [Mg2+]i (solution f ) (Fabiato and Fabiato, 1979), channel rundown did not occur. Although Fakler et al. (1994) showed that >10 μM free [Mg2+]i is required to prevent rundown from occurring in the Kir2.1 channel, just 200 nM free [Mg2+]i is sufficient for the BLM K+ channel. Under this condition, the outward unitary conductance of the latter increases, but the inward conductance is essentially unaffected. The enhanced outward current is ATP sensitive, as 5 mM ATP blocked 89.3 ± 6.3% (n = 3) of the current. The outward chord conductance with [Mg2+]i = 200 nM increased from 4.25 ± 0.59 pS to 14.4 ± 1.2 pS (n = 4, measured at +80 mV); inward currents were not affected by the change in [Mg2+]i (Fig. 3 B). Inward rectification was reestablished by returning bath [Mg2+]i to 1 mM.
Assuming a single-binding site, the Mg2+ block may be described using a one-site model according to Woodhull (1973):
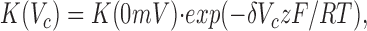 |
5 |
where V c is the command potential, z is the valence of 2, and K(V c) and K(0 mV) are the concentrations of Mg2+ causing half-maximal block at V c and 0 mV, respectively. The factor δ may be the electrical distance of the binding site from the outside of the channel pore. Alternatively, δ can be considered together with another term to yield either the equivalent valence (z) of, or the effective potential (V c) sensed by, the Mg2+ (Hille, 1992).
Current–voltage relations from inside-out patches in symmetrical K+ for several [Mg2+]i were constructed, from which the current was normalized to that observed in a Mg2+-free bath, and the relative current was plotted against command potential for each [Mg2+]i (not shown). These data were then fitted (nonlinear least squares fitting routine) by the following function that incorporates Eq. 5:
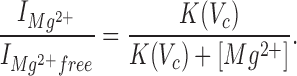 |
6 |
The fit according to Eq. 6 yields the following results: δ = 0.57, K(0 mV) = 7.7 mM, K(60 mV) = 0.72 mM.
Concentration and voltage dependence of isc.
To further investigate the biophysical properties of this channel, we asked the question of how varying extracellular [K+] would affect the current carried by the channel. To isolate the change in [K+] from any change in driving force across the patch, the same [KCl] was used in the pipette and bath, thus clamping Erev to 0 mV. This approach allowed us to measure the change in absolute conductance while maintaining a constant relative permeability (i.e., we varied the Goldman-Hodgkin-Katz current equation while holding the result of the Goldman-Hodgkin-Katz voltage equation constant). Under these conditions, the command potential is the only driving force for net K+ movement across the membrane patch. Pipette and bath [KCl] ranged from 5 to 205 mM while osmolality was kept at 400 mosm/kg using sucrose as necessary. Since isc for [KCl] = 95 mM was the same in both standard KCl solution (solution d, 200 mosm/kg) or 400 mosm/kg KCl solution, the tonicity change itself does not significantly alter the conducting properties of the channel.
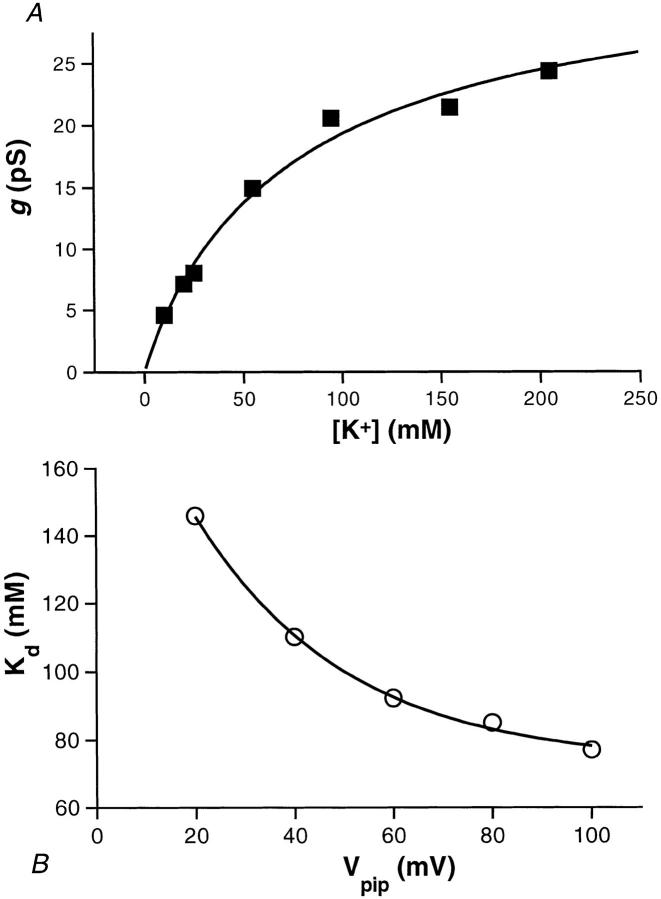
Channel events (inward current) were analyzed at command potentials of −20, −40, −60, −80, and −100 mV for [K+] = 5, 10, 20, 25, 55, 95, 155, and 205 mM, and the I-V relationship was determined for each of these KCl concentrations. Channel events for [K+] = 5 mM could not be adequately resolved and were not included in the further analysis. For each voltage, the single channel current increases with [K+] and approaches a limiting current (i max,sc). Similarly, the single channel chord conductance (γchord) also increases with [K+] and saturates (as shown for −100 mV in Fig. 4 A). Fitting the γchord versus [K+] data at −100 mV with the Hill equation:
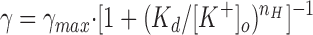 |
7 |
Figure 4.
The operating surface of the BLM K+ channel. (A)
The single channel conductance (γ) at a command potential of
−100 mV plotted as a function of [K+] and fit to the Hill equation
according to 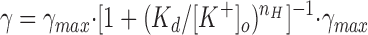 is the maximal value of the conductance, K
d is the apparent dissociation constant, and n
H is the Hill coefficient. For the chord conductance at
−100 mV (▪) and its least-squares fit (solid line), γmax,slope = 34.3
pS, K
d = 77 mM, and n
H = 0.94. (B) The plot of K
d versus Vpip
shows that the apparent binding–unbinding rate of K+ to the
channel is voltage dependent. The data (○) were fit with a single
exponential (solid line) as follows: K
d = 73 + 141 · exp(−Vpip/30).
is the maximal value of the conductance, K
d is the apparent dissociation constant, and n
H is the Hill coefficient. For the chord conductance at
−100 mV (▪) and its least-squares fit (solid line), γmax,slope = 34.3
pS, K
d = 77 mM, and n
H = 0.94. (B) The plot of K
d versus Vpip
shows that the apparent binding–unbinding rate of K+ to the
channel is voltage dependent. The data (○) were fit with a single
exponential (solid line) as follows: K
d = 73 + 141 · exp(−Vpip/30).
yielded a K d of 77 mM, a Hill coefficient of 0.95, and a maximum value for γchord (γmax) of 36.6 pS (Fig. 4 A). Plots of γchord versus [K+] at different voltages yielded similar results for γmax. The average value for γmax determined at −60, −80, and −100 mV was 34.3 pS. γmax,sc was the same for all command potentials fitted. Indeed, the intersection of the channel's operating surface with the I-V plane as K shows a linear conductance. This mean value for γmax was applied to obtain K d values and Hill coefficients for all the voltages (Table II).
Table II.
Kd Values and Hill Coefficients for the BLM K+ Channel
| Command potential | K d | Hill coefficient | ||
|---|---|---|---|---|
| mV | mM | nH | ||
| −20 | 146 | 0.94 | ||
| −40 | 110 | 1.02 | ||
| −60 | 92 | 0.96 | ||
| −80 | 85 | 1.00 | ||
| −100 | 77 | 0.95 |
Comparison of the dissociation constants K d for the different command potentials shows that K d decreases with hyperpolarization (Fig. 4 B and Table II). The relationship between K d (mM) and voltage (mV) can be described by a single exponential decay
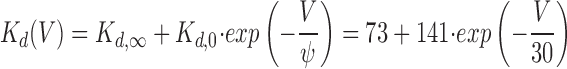 |
8 |
where K d, is the asymptotic value for K d as Vpip → ∞•, K d,0 is the difference of K d at the reversal potential (0 mV) and K d,∞•. Finally, ψ is the voltage change required to effect an e-fold change in (K d −K d,∞•).
Cation selectivity.
The cation to anion preference of the total BLM conductance was determined by salt dilution experiments using i/o membrane patches. Starting in symmetrical 95 mM K+ and 94.5 mM Cl− (EK = ECl = 0 mV) and holding at −Vpip = 0 mV, the bath was changed to a 14 mM K+ and 13.5 mM Cl− solution (solution i, EK = +48.6 mV, ECl = −49.4 mV) plus sucrose to maintain isoosmolality. This maneuver resulted in large inward currents (n = 3), reflecting the cation (K+) moving down its chemical gradient. The reversal potential for this membrane current (including leak) was at least +40 mV (n = 3). While holding at this E rev, outward current developed when the 10% KCl bath was replaced with 100% KCl, due to K+ moving along its electrical gradient. Thus, the BLM conductance is cation selective.
Using the Goldman-Hodgkin-Katz voltage equation (Hodgkin and Katz, 1949; Goldman, 1943) for K+ and Cl−, the permeability ratio pK:pCl can be estimated (see materials and methods):
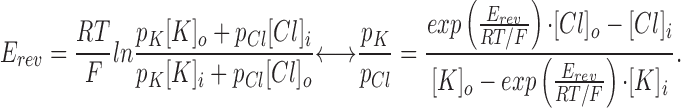 |
9 |
where R = 8.315 J K−1 mol−1, F = 9.648 · 104 C mol−1, T = 293 K, E rev ≥ +40 mV, [Cl]o = 94.5 mM, [K]o = 95 mM, [Cl]i = 13.5 mM, and [K]i = 14 mM, yielding pK: pCl of at least 17:1. Note that even a small change in E rev to +44 mV would double the selectivity ratio. It is emphasized that this value represents the minimum cation to anion preference of the K+ channel since this is the selectivity ratio of the whole basolateral membrane patch including chloride and leak conductances.
Selectivity among cations.
Two approaches were used to determine the selectivity of this inwardly rectifying K+ conductance on the BLM. (a) Using c/a and i/o patches, the K+ in the patch pipette was replaced with the chloride salt of Na+ (n = 48), Rb+ (n = 4), Li+ (n = 4), Cs+ (n = 2), or NH1 4 (n = 7). Each solution was adjusted to pH 7.5 with the respective hydroxide salt. In all cases, c/a and i/o patches failed to show inward channel currents. These results strongly suggest that the BLM K+ channel is highly selective for K+ and excludes these cations, since channel activity is seen in >98% of seals made on the BLM when K+ is in the pipette. (b) Outside-out patches were made to exclude the remote possibility that the c/a and i/o patches used above did not contain any channels. The pipette was filled with KCl, and 0.2 mM Mg-ATP was included to prevent channel rundown. After recording in a KCl bath, the test cation was introduced into the bath (as the chloride salt) and the voltage protocol was repeated. KCl bath exchanges were interposed between test cations.
The cation selectivity as determined from outside-out patches is exemplified in Fig. 5. For Na+ and Li+, the current at −40 mV is negligible, but a modest inward current is carried by Rb+, Cs+, and NH1 4. Exchanging the test cation with KCl returned the ensemble currents to the control level in all cases. That the outward current carried by K+ remains essentially unchanged during each bath cation substitution indicates that the test cations do not act as channel blockers from the outside. The ensemble current obtained at Vpip = −40 mV shows that the selectivity of the channel for these cations is K+ >> Rb+ ≈ Cs+ ≈ NH4 + > Na+ ≈ Li+.
Figure 5.
The BLM K+ conductance is highly selective for K+. The cationic selectivity of the BLM is demonstrated in this outside-out patch with KCl (solution d) + 0.2 mM ATP in the pipette and Cl-salt of the test cation in the bath. The running average of current at −40 mV shows that cationic selectivity is K+ >> Rb+ ≈ Cs+ ≈ NH4 + > Na+ ≈ Li+. The dashed line is the zero-current line, and the dotted line is the all channels closed (leak) current at a command potential of −40 mV. The voltage protocol is indicated below the current data.
Thallium.
Many types of K+ channels have been shown not only to conduct Tl+, but often better than they conduct K+ (Hille, 1992). To assess the Tl+ conductance of the BLM K+ channel, the patch pipette was filled with 90 mM Tl-acetate (solution g ; the Cl salt of Tl+ was not used due to its low aqueous permeability) and the bath was filled with K-acetate (solution h). Under these conditions, inward Tl+ currents were observed in both c/a and i/o patches. The kinetic behavior of these channel events was notably different from those seen when the channel conducts K+. When conducting Tl+, the channel openings displayed more bursting, with each opening interrupted by fast flickery closures. The probability that this is actually a different channel is low since (a) the frequency of finding the K+ channel exceeds 98%, (b) other cation-selective channels were rarely observed, (c) the disparate kinetics were only seen when Tl+ was in the pipette, and (d) Tl+ currents were sensitive to glibenclamide (see below).
The I-V relationship for this biionic condition is shown in Fig. 3 B. The limiting inward slope conductance for Tl+ was 29.0 ± 1.0 pS (measured between −60 and −100 mV, n = 4). Thus the channel conductance is slightly higher for Tl+ than it is for K+ (g*inTl: g*inK = 1.2:1). Since small inward currents were observed while holding at 0 mV in two i/o experiments with Tl+ in the pipette and K+ in the bath, the reversal potential is positive. This implies that the permeability (zero-current conductance) for Tl+ is also greater than that for K+. Therefore, compared with K+, Tl+ has a higher conductance and is more permeant but probably interacts with the pore, causing a fast channel block.
In conclusion, these findings indicate that the permeability sequence of this K+ channel is Tl+ > K+ >> Cs+ ≈ Rb+ ≈ NH4 + > Li+ ≈ Na+ > Cl−.
Kinetics.
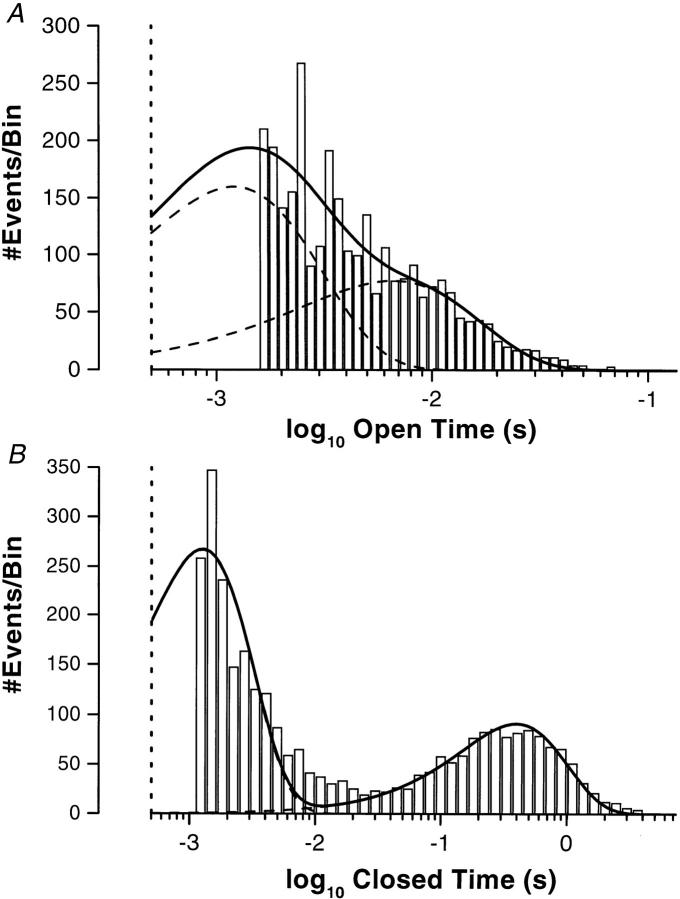
Due to the high density of this K+ channel in the BLM, a patch apparently containing only one channel is extremely rare. In over 550 seals, only four membrane patches appeared to contain only one channel (0.7%). Since the open probability (P o) of the channel is only 0.05 ± 0.01 (n = 4), long recordings were required to accumulate enough transitions for meaningful analysis of the long closed state. Kinetic analyses from such patches show that under resting state conditions at −Vpip = −60 mV, the BLM K+ channel has two apparent open states and two apparent closed states. Parameters from one c/a patch and one i/o patch show that the open dwell lifetimes are (ms): τo1 = 0.78 (c/a), 1.21 (i/o), and τo2 = 4.7 (c/a), 6.6 (i/o). The closed dwell lifetimes are (ms): τc1 = 1.27 (c/a), 0.72 (i/o), and τc2 = 397 (c/a), 502 (i/o). Fig. 6 shows the open and closed time histograms for a c/a patch.
Figure 6.
Kinetics of the BLM K+ channel. (A) Open-time histogram for the BLM K+ channel in a cell-attached patch at −60 mV with the time intervals logarithmically binned (Sigworth and Sine, 1987). The data were fitted with two probability density functions (dashed lines) to give the overall fit (solid line), yielding time constants of τo1 = 0.78 ms (78%) and τo2 = 4.7 ms (22%). Based on the bandwidth of the recording system, the data and the fit were cutoff at 500 μs (vertical dotted line). (B) Closed-time histogram for the BLM K+ channel in a cell-attached patch at −60 mV with the time intervals logarithmically binned. The data were fitted with two probability density functions (dashed lines) to give the overall fit (solid line), yielding time constants of τc1 = 1.27 ms (74%) and τc2 = 397 ms (26%). Based on the bandwidth of the recording system, the data and the fit were cutoff at 500 μs (dotted vertical line).
Inhibitor Profile
The BLM K+ channel is inhibited by barium and glibenclamide. The channel is insensitive to tetraethylammonium (up to 10 mM) applied either to the extracellular or cytoplasmic side.
Barium.
We have previously shown by recording single-cell membrane potential that the whole cell conductance is dominated by a barium-sensitive K+ conductance (Segal et al., 1996). Perforated patch whole-cell recordings show that the barium-sensitive whole cell conductance inwardly rectifies. When 2 mM Ba2+ was included in the KCl pipette solution (unpaired experiments), channel openings were rare and a flickery state was noted. In contrast to the voltage dependence of channel activity without Ba2+ in the pipette (see Fig. 3 C), steady depolarization now has the effect of increasing nP o, and subsequent hyperpolarization reduced activity. This is presumably due to the voltage dependence of the Ba2+ block of the channel (data not shown).
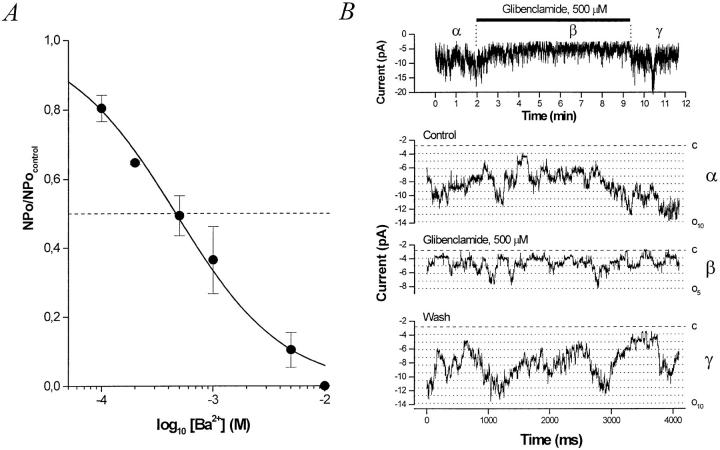
To quantify the Ba2+ block and use each patch as its own control, outside-out patches were made (n = 3). In outside-out membrane patches at −40 mV, Ba2+ inhibits the channel with a K i = 460 μM (Fig. 7 A).
Figure 7.
Inhibitors of the BLM K+ channel. (A) Dose– response curve for inhibition of the BLM K+ channel by external Ba2+. Relative nP o (nP o/nP o,control) determined from outside-out patches at a command potential of −40 mV with KCl in the pipette and bath (solution d) is plotted versus [Ba2+]. The data at −40 mV were fitted with the Hill equation (solid curve), yielding a K i = 460 μM and n H = 0.90. The inhibition by Ba2+ is fully reversible. (B) Glibenclamide inhibits the BLM KATP channel. (top) A running average (window width 16 ms) of current versus time. Glibenclamide (500 μM) is added to the bath where indicated. (bottom) Sample traces from a representative experiment at −60 mV with KCl in the pipette and bath (solution d). Exposure of this inside-out patch to 500 μM glibenclamide reversibly decreases nP o by ∼50%. The dashed line denotes the all channels closed (leak) current level and the dotted lines indicate each open channel level.
Glibenclamide.
This sulfonylurea is known to inhibit ATP-sensitive K+ channels in a number of epithelial tissues by binding to the sulfonylurea receptor (SUR). SUR1 has recently been cloned (Aguilar-Bryan et al., 1995) and is thought to associate with the KATP channel, thereby conferring sulfonylurea sensitivity. However, SUR1 may not be present in the kidney (Inagaki et al., 1995), which may explain in part the much higher dose of glibenclamide required to inhibit renal KATP channels (Hebert and Ho, 1994). In this context, the BLM K+ channel is glibenclamide sensitive, albeit at “renal doses.” We treated 22 patches with glibenclamide; 16 excised inside-out patches were exposed to 500 μM, while 6 cell-attached patches were exposed to low (0.01–10 μM) concentrations. In 7 of 16 inside-out patches, 500 μM glibenclamide inhibited activity by 42.3 ± 5.6% (Fig. 7 B). Remarkably, the inhibition was much more potent in the cell-attached patches: 10 μM exerted an 83 ± 2% inhibition in three patches, and 100 nM exerted a 70 ± 3% inhibition in three other patches (data not shown).
ATP Sensitivity
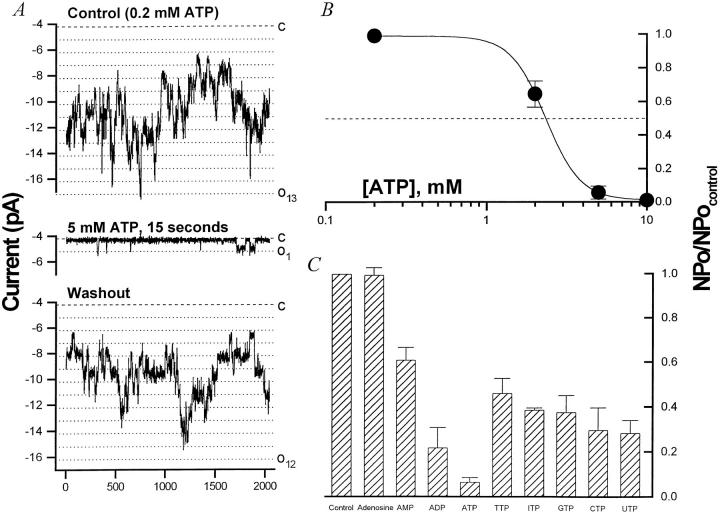
Similar to other KATP channels, low doses of ATP are required to prevent Ambystoma BLM K+ channel rundown, whereas millimolar doses inhibit channel activity. At a dose of 5 mM ATP, >90% of channel activity is inhibited. This effect is reversible as nP o returns to baseline when the bath [ATP] is returned to 0.2 mM (Fig. 8 A). The dose–response curve for ATP has a K i ∼ 2.4 mM (Fig. 8 B). The Hill coefficient of ∼4 may suggest that the channel has a tetrameric structure, with each subunit possessing an ATP binding site. Thus, in the Ambystoma proximal tubule, the BLM K+ channel that appears to be the major K+ conductance of the cell is ATP sensitive.
Figure 8.
Nucleotides reversibly inhibit the BLM K+ channel. (A) ATPi reversibly inhibits the BLM K+ channel in inside-out patches. In the experiment depicted, up to 13 channel open levels are seen under control conditions (top) at −60 mV with KCl (solution d) in the pipette and NaCl (solution c) plus 0.2 mM ATP in the bath. (middle) Addition of 5 mM ATP to the cytoplasmic side almost completely blocks channel activity (98% decrease) with only rare openings to one open level. (bottom) The inhibition is readily reversed upon returning to 0.2 mM ATPi. (B) Dose–response curve for inhibition by ATPi. The inhibitory effect of ATPi was determined in inside-out patches under the conditions described in A. Relative nP o (nP o/nP o,control) is plotted versus [ATP]i. The data (•) was fitted with the Hill equation (solid line) yielding a K i ∼ 2.4 mM and n H = 3.95. Adenosine nucleotides and nucleoside triphosphates (each at 5 mM) inhibit the BLM K+ channel. The inhibitory effect of ATP, ADP, AMP, and adenosine was determined under the conditions described in A. Values plotted are average (nP o,test/nP o,control) SEM (n = 3–9). The rank order of inhibition is ATP (93.3%) > ADP (65.6%) > AMP (38.7%). Adenosine has no inhibitory effect on nP o of the BLM K+ channel. Although all the nucleoside triphosphates inhibit the channel, ATP exerts a significantly stronger block than the other NTPs tested (P < 0.02). There is no significant difference among the other NTPs.
Among the nucleoside diphosphates, ADP is less potent than ATP (ADP inhibits by 65.6 ± 8.1%, n = 4, P < 0.01), but more potent than CDP, GDP, IDP, TDP, or UDP (data not shown). This suggests that the putative nucleotide binding site(s) recognize NDPs as well as NTPs, and that nucleotide hydrolysis is probably not occurring at this site. Indeed, even nucleoside monophosphates have a moderate inhibitory effect, although nucleosides themselves are without effect. The relative potency of the adenosine nucleosides (at 5 mM) in inhibiting the BLM KATP channel is ATP (93.3 ± 1.9%) > ADP (65.6 ± 8.1%) > AMP (38.7 ± 3.7%) > adenosine (1 ± 2%) (Fig. 8 C, n = 3–9).
Other nucleotides.
The effect of nucleotides was tested in excised i/o patches. All the NTPs tested reversibly inhibited BLM K+ channel activity at a dose of 5 mM (ATP 93.3 ± 1.9%, n = 9; CTP 70.3 ± 10.0%, n = 5; GTP 62.3 ± 7.5%, n = 3; ITP 61.1 ± 0.8%, n = 2; TTP 53.7 ± 6.7%, n = 2; UTP 71.7 ± 5.7%, n = 2) (Fig. 8 C). These results suggest that each compound probably interacts with common cytoplasmic nucleotide binding site(s). Note that ATP is significantly more potent than the other nucleoside triphosphates (P < 0.02), but there is no significant difference among the nonadenosine nucleotides.
Rundown of the BLM K+ Channel
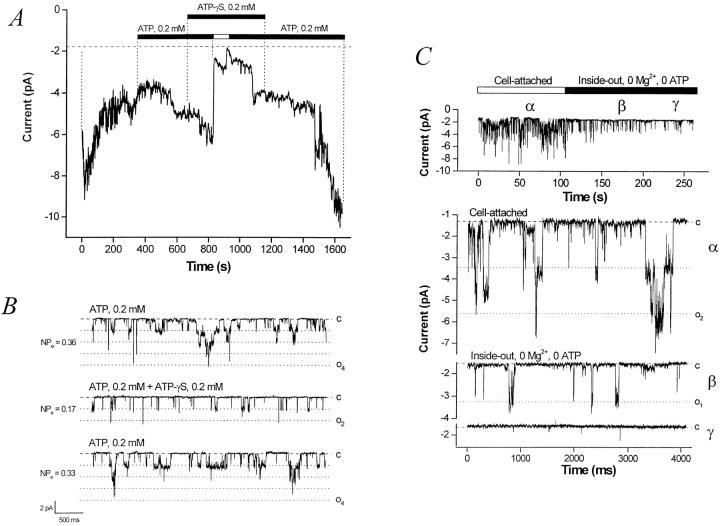
One characteristic of KATP channels is “rundown,” a gradual loss of activity when the membrane patch is deprived of cytosolic ATP (Findlay and Dunne, 1986). Typically, both Mg2+ and ATP are required to prevent rundown in KATP channels (Ashcroft and Ashcroft, 1990). Likewise, the BLM K+ channel runs down in the absence of either Mg2+ or ATP (or both). Lower concentrations of ATP (100–200 μM) will prevent or “rescue” channel rundown. The experiment shown in Fig. 9 A summarizes the characteristics of BLM K+ channel rundown. Channel activity typically begins to decrease (rundown) upon excision of the membrane patch into a nucleotide-free bath. If this process is allowed to continue, channel activity will cease, usually irreversibly. When 0.2 mM of ATP is added back, channel activity can be restored. When ATP is removed, all channels rapidly close. In the continued presence of ATP-γS, readdition of ATP is again able to rescue rundown, and activity returns to baseline upon washout of the ATP-γS. Frequently (but not invariably), ATP-γS has an inhibitory effect on single channel activity when added in the presence of ATP, which is reversible as long as the exposure is not prolonged (n = 6), as shown in Fig. 9 B. When ATP-γS is added in the absence of ATP, channel activity runs down very quickly, usually irreversibly (n = 4, data not shown).
Figure 9.
ATP but not ATP-γS prevents and rescues channel rundown. Hydrolyzable nucleoside triphosphates prevent and rescue BLM K+ channel rundown. A running average (current versus time, window width 768 ms) of a representative experiment is shown. The pipette is KCl (solution d), the bath is NaCl (solution c), the command potential is −60 mV. Upon excision in a nucleotide-free bath, channel activity decreases (channel rundown). After the addition of 0.2 mM ATP to the bath, channel activity slowly recovers. The addition of 0.2 mM ATP-γS (a poorly hydrolyzable ATP analogue) in the continued presence of 0.2 mM ATP has no effect. However, when ATP is removed, ATP-γS is not able to support channel activity, which rapidly declines and runs down. Readdition of ATP leads to full recovery of channel activity. Single-channel traces showing that ATP-γS has an inhibitory effect on KATP channel activity in excised inside-out BLM patches. When compared with control conditions (top), the addition of ATP-γS (middle) reduces nP o. This inhibition is reversible as long as the exposure to ATP-γS is not prolonged (bottom). (C) Removal of Mg2+ does not prevent channel rundown in an ATP-free bath. The top panel shows that rundown of the BLM KATP channel upon excision into an ATP-free bath proceeds despite removal of bath Mg2+. Representative traces from the regions marked by α, β, and γ are shown at bottom.
Thus, ATP-γS cannot substitute for ATP in sustaining channel activity, suggesting that phosphorylation itself is not sufficient to prevent rundown. Although phosphorylation may be necessary, it appears that the nucleoside triphosphate must be hydrolyzable to maintain channel activity. This hypothesis is supported by the finding that CTP, GTP, ITP, TTP, and UTP could all prevent or rescue rundown, but the corresponding NDPs could not. Rescue does not appear to require the cAMP-dependent protein kinase, since channel activity can be restored even in the presence of a high concentration of protein kinase inhibitor (PKI, 1 μg/ml, P-0300; Sigma Chemical Co.).
Since it has been reported that removal of free Mg2+ nearly abolishes rundown of KATP in cultured CRI-G1 insulin-secreting cells (Kozlowski and Ashford, 1990) and partially inhibits rundown of ATP-regulated ROMK1 channels excised in an ATP-free bath (McNicholas et al., 1994), we assessed BLM KATP channel activity under these conditions. The representative experiment shown in Fig. 9 C shows that rundown of the BLM KATP channel still occurs in the absence of ATP despite excision of the patch into a Mg2+-free bath.
Diazoxide.
The synthetic KATP channel opener diazoxide was applied to the cytoplasmic side of i/o patches. It has been shown that this benzothiadiazine can open KATP channels in the presence of Mg-ATP, but it may have an inhibitory effect in the absence of Mg-ATP (Kozlowski et al., 1989). Initial excision of the patch into an ATP-free bath leads to channel rundown as discussed above, and 200 μM diazoxide alone does not rescue rundown. However, addition of 0.2 mM Mg-ATP in the continued presence of, or after exposure to, diazoxide increases channel activity well in excess of that before rundown (n = 3, Fig. 10). The inhibitory effect of 5 mM ATP is not diminished in the presence of, or by previous exposure to, diazoxide (n = 5, data not shown).
Figure 10.
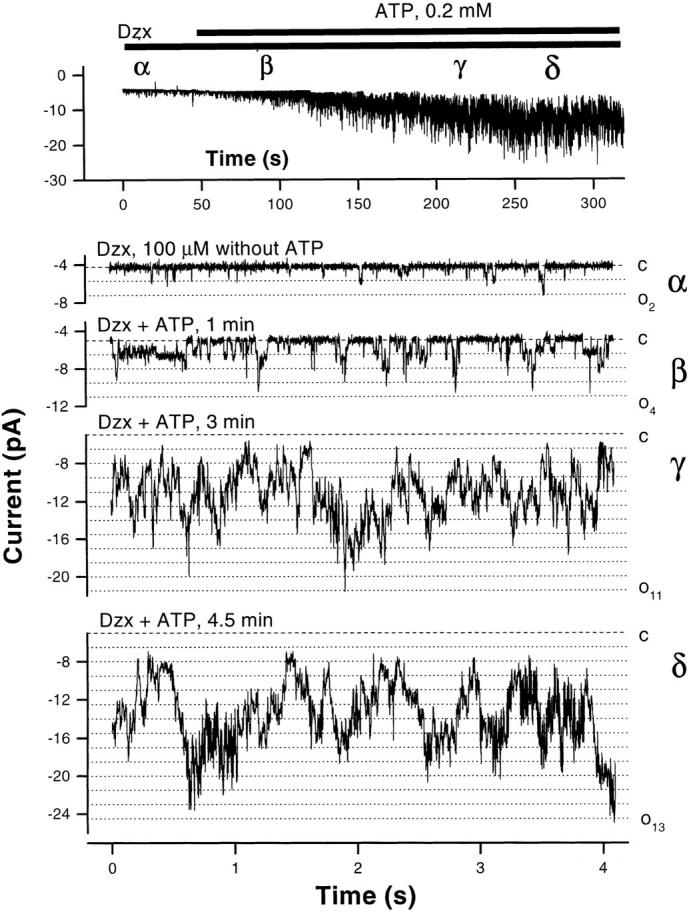
The K channel opener diazoxide activates the BLM K+ channel. Diazoxide activates the BLM K+ channel in the presence of ATP. In the experiment depicted, the pipette contains KCl (solution d), the bath contains NaCl (solution c), and the command potential is −80 mV. (top) A running average (window width 16 ms) of current versus time. (bottom) Sample current traces from the same experiment. With diazoxide alone, the channel opens infrequently (nP o = 0.15) and no more than two channels are open at a time. Diazoxide combined with 0.2 mM ATP, however, promptly increases channel activity and within 5 min, up to 13 simultaneously open channels are evident (nP o = 5.27). The all channels closed level (dashed line) and open channel levels (dotted lines) are indicated.
Since low levels of Mg-ATP are required for diazoxide to open the BLM K+ channel, diazoxide probably does not interact with the rundown site. As hypothesized by others (Edwards and Weston, 1993, 1995), diazoxide may be acting at or near the nucleotide binding site that mediates inhibition. On the other hand, the interaction at this second site is more complex than a simple competition between the K channel opener and the nucleotide, since diazoxide does not relieve the inhibition by millimolar levels of ATP.
discussion
The proximal tubule (a leaky epithelium) can be considered to function within the general scheme of the epithelial transport model first proposed by Koefoed-Johnsen and Ussing (1958) (KJU), in which the apical membrane is primarily Na+ selective and the BLM is primarily K+ selective. Although this model was first applied to tight epithelia such as frog skin (Koefoed Johnsen and Ussing, 1958) and urinary bladder (Davis and Finn, 1982), its essence holds for leaky epithelia such as small intestine (Gunter-Smith et al., 1982) and the proximal tubule (Matsumura et al., 1984). In the KJU model, maintenance of unidirectional Na transport requires that K moves in a closed circuit (recycling) across the BLM. That is, barring intracellular accumulation of K (or K+ secretion), the K+ pumped into the cell by the pump must be matched by an outward K+ current across the BLM.
Steady state vectorial transport in the proximal tubule thus requires continuous activity of the basolateral Na+,K+-ATPase pump, which consumes ATP and obligates intracellular accumulation of K+. A conductance for K+ is necessary both to allow this K+ to recycle and to maintain Vbl. Experiments performed by Matsumura et al. (1984) on perfused Necturus proximal tubules first demonstrated that the BLM GK varies as a function of pump activity, and they suggested that the regulation of BLM GK was linked to cellular metabolism, as had been previously proposed for red cells (Romero, 1978) and suspensions of rabbit cortical tubules (Balaban et al., 1980).
This hypothesis was bolstered when the first single-channel records of an ATP-sensitive K+ channel (K i = 0.1 mM) from cardiac muscle were published (Noma, 1983). Similar ATP-sensitive K+ (KATP) channels were subsequently found in pancreatic β-islet cells (Cook and Hales, 1984), skeletal muscle (Spruce et al., 1985), and smooth muscle (Standen et al., 1989). The K i for ATP is 10–100 μM for all these Type I KATP channels (Ashcroft and Ashcroft, 1990), and they are inhibited by sulfonylurea agents (Edwards and Weston, 1993). A KATP channel with these Type I properties has not been identified in epithelial tissues. However, a related KATP channel was recently demonstrated in nonperfused (Tsuchiya et al., 1992) and perfused (Hurst et al., 1993; Beck et al., 1993) rabbit proximal tubules. In preliminary experiments, we have also found this channel in the BLM of nonperfused rabbit proximal tubule, and in the present study we provide the first detailed description of the analogous BLM KATP channel in an amphibian using a novel preparation of dissociated proximal tubule cells that retain epithelial polarity (see Fig. 1 and Segal et al., 1996).
Investigators have used several techniques to patch the BLM, including mechanically stripping off the basement membrane (Sackin and Palmer, 1987; Kawahara et al., 1987) and tearing tubules to access the lateral membrane (Gögelein and Greger, 1987b ). However, enzymatic treatment of nonperfused tubules (Tsuchiya et al., 1992; Parent et al., 1988), perfused tubules (Hurst et al., 1993; Beck et al., 1993), and single cells (Hunter, 1991) has been employed most commonly.
We have previously shown that the dissociated Ambystoma proximal tubule cells (see Fig. 1) retain epithelial cell polarity (Segal et al., 1996). Indeed, the K+ channel described in the present study was present in >98% of seals made on the BLM, but was never detected in the apical membrane. With high [K] on both sides of the membrane patch, this BLM K+ channel exhibits inward rectification with γslope, in = 24.5 ± 0.6 pS and γchord, out = 3.7 ± 0.4 pS. This non-ohmic behavior in both cell-attached and -free membrane patches is due at least in part to a voltage-dependent open channel block by cytosolic Mg2+ (Matsuda et al., 1987). Removal of Mg2+ from the cytosolic side of i/o patches leads to an increase in outward conductance without affecting inward conductance. In c/a patches made on frog BLM (NaCl pipette in a KCl bath), Hunter (1991) estimated that the blocking site was 51.3% across the membrane from the cytosolic side (i.e., 0.513). This compares well to the value of 0.57 obtained for the Ambystoma BLM K+ channel in the present study.
Inward rectification of current is not necessarily due to a decrease in the intrinsic outward conductance of the channel pore. Indeed, Matsuda et al. (1987) first described how ohmic single channel units could behave as rectifiers. They showed that inward rectification of a cardiac myocyte K+ channel was mediated by two effects: (a) Mg2+-independent voltage gating in which ohmic channels are open at hyperpolarized voltages and essentially inactivate upon depolarization, and (b) a Mg2+-induced voltage-dependent open channel (fast) block. Our data for the BLM K+ channel are in keeping with these observations since (a) the conductance is ohmic in the absence of cytosolic Mg2+, and (b) Mg2+ appears to cause a flickery open channel block of the outward current in the steady state (e.g., see Fig. 3 A). Naturally occurring polyamines (e.g., spermine) have also been shown to mediate inward rectification of some K+ channels (Ficker et al., 1994). However, spermine (up to 5 mM) does not affect the Ambystoma BLM K+ channel.
Properties of K+ conduction through the pore.
Constant-field theory predicts that net K+ flux across the channel should increase linearly with rising [K+]. Most channels do not exhibit this behavior due to “competition” for binding sites within the pore, and saturation occurs at higher concentrations of the permeant ion (Hille, 1992). In our study, conductance versus [K+] was fit with the Hill equation (Table II). Hill coefficients of ∼1 suggest that one K+ ion interacts with the channel at a time, allowing permeation to be modeled as follows:
 |
At high [K+] concentrations, saturation occurs when the rate of ion entry approaches the maximum rate of unbinding. For the BLM K+ channel, the maximum inward conductance (γmax = 34.3 pS) is voltage-independent, suggesting that at high concentrations of K+, the electrical driving force is the rate-limiting factor for ion flux. However, the binding-unbinding rate is voltage dependent, suggesting that the loading of the binding site for K+ is dependent on the membrane potential (Hille, 1992). Kawahara et al. (1987) also noted saturation of the Necturus BLM K+ channel conductance with external [K+] in c/a patches.
Voltage dependence.
Hyperpolarization increases nP o. Such voltage sensitivity renders the channel susceptible to changes in membrane voltage, a potential regulator of the BLM K+ conductance. On the BLM in the Necturus proximal tubule, hyperpolarization increased K+ channel activity (Sackin and Palmer, 1987; Kawahara et al., 1987). In frog kidney, one study showed that nP o increased with hyperpolarization (Kawahara, 1990), whereas in another study, no voltage dependence of nP o was observed (Hunter, 1991). In preliminary experiments using rabbit proximal tubule, we again found that hyperpolarization increases nP o, although Parent et al. (1988) reported the opposite. Note that for the cloned renal K+ channels, ROMK1 (Ho et al., 1993) and ROMK2 (Chepilko et al., 1995), which have not been detected in proximal tubule, nP o decreases with hyperpolarization.
Selectivity and blockade.
The channel is highly selective for K+ over a number of cations (Na+, Rb+, Li+, NH4 +, and Cs+) as well as over Cl−. When K+ in the patch pipette was replaced by these cations, c/a and i/o patches failed to show inward channel currents. Paired experiments in outside-out patches show that gK:gNa exceeds 30:1, and that the cation selectivity of the BLM K+ channel is K+ >> Rb+ ≈ Cs+ ≈ NH4 + > Na+ ≈ Li+ (see Fig. 5). Detailed selectivity data are lacking in other studies on BLM K+ channels in the proximal tubule, but more distal K+ channels, including ROMK, conduct Rb+. With Rb+ in the patch pipette, gRb/gK = 0.36 for ROMK2 (Chepilko et al., 1995). Under similar conditions, we did not observe inward Rb+ currents through the BLM K+ channel.
Like other types of K+ channels (Hille, 1992), the BLM K+ channel can conduct thallium. Indeed, the single channel conductance is higher for Tl+ than it is for K+ (g*inTl/g*inK = 1.2). Since the reversal potential under biionic conditions (Tl+ pipette, K+ bath) is slightly positive, Tl+ is also more permeant than K+. This differs from ROMK2, in which Tl+ is also more permeant than K+ but the conductance for Tl+ is less than that for K+ (g*inTl/g*inK = 0.7) (Chepilko et al., 1995). Thus, for the BLM K+ channel, Tl+ has a higher conductance and is more permeant than K+.
Similar to other renal K+ channels, this BLM K+ channel is insensitive to tetraethylammonium. However, it is well known that the proximal tubule K+ conductance can be inhibited by millimolar concentrations of Ba2+ (Sackin and Boulpaep, 1981) and the whole-cell conductance of the dissociated Ambystoma cells is barium sensitive (Segal et al., 1996). Outside-out patches from the BLM show that K+ channel activity is inhibited by barium with a K i of 460 μM at a command potential of −40 mV (see Fig. 7). Complete inhibition occurs at [Ba2+]o = 10 mM.
Sensitivity to Nucleotides, Sulfonylureas, and Activation by Diazoxide
Nucleotides.
The Ambystoma BLM K+ channel is sensitive to ATP, albeit at millimolar levels. Since the first demonstration of an ATP-sensitive K (KATP) channel in cardiac muscle (Noma, 1983) and pancreatic cells (Cook and Hales, 1984), potassium channels inhibited by nucleotides have been described in a wide variety of tissues (Ashcroft and Ashcroft, 1990). In pancreatic β cells, KATP channels function in the regulation of insulin secretion. Ashcroft and Ashcroft (1990) have classified such inwardly rectifying KATP channels as Type I based on their exquisite sensitivity to ATP (K i ∼ 10–100 μM) and sulfonylureas.
There is no doubt that KATP channels function primarily to couple cell metabolism to membrane potential (Ashcroft and Ashcroft, 1990), and perhaps to epithelial transport (Mauerer et al., 1998). Curiously, epithelial KATP channels appear to lack the exquisite sensitivity of Type I KATP channels, possibly for reasons discussed below. The proximal tubule BLM K+ channel described in this study is reversibly inhibited by ATP with a K i of 2.4 mM at pH 7.5. This is a reasonable operating point given that intracellular ATP levels in proximal tubule are 4–5 mM, and ATP levels have been shown to fall to ∼2 mM when transport is stimulated (Beck et al., 1991; Tsuchiya et al., 1992). The role of intracellular ATP in coupling channel activity to pump turnover is discussed in the companion paper.
It is likely that the intracellular ATP-binding site contains a motif that recognizes a spectrum of nucleotides since the channel is also reversibly inhibited by GTP, ITP, UTP, CTP, and TTP (see Fig. 8 C). This putative binding site recognizes NTPs, NDPs, and NMPs, but not the nucleosides themselves. Adenosine nucleotides exert significantly stronger inhibition than their nonadenosine counterparts, and the triphosphate of a given nucleoside is a better inhibitor than the diphosphate and the monophosphate. Since the binding of NDPs and even NMPs is effective, channel inhibition probably does not require hydrolysis. In contrast to the KATP channel on the apical membrane of principal cells (Wang and Giebisch, 1991), the inhibitory effect of the nucleotides is probably not critically dependent on Mg2+ since nucleotide sodium salts (free Mg2+ ≅ 25 μM) are as effective as the magnesium salts (free Mg2+ ≅ 1.3 mM).
Sulfonylureas.
In cell-free patches, the Ambystoma and rabbit BLM K+ channels are (variably) inhibited by submillimolar levels of glibenclamide, whereas native cell Type I KATP channels are sensitive to nanomolar concentrations of glibenclamide (Ashcroft and Ashcroft, 1990). Indeed, no renal and perhaps no epithelial K+ channel has the sensitivity to sulfonylureas (or ATP) that Type I KATP channels possess. This may suggest that either Type I KATP channels are only found in specific tissues, or that associated molecules (e.g., the sulfonylurea receptor) conferring sulfonylurea sensitivity are cell specific. In this regard, it is of interest that all but one of the cloned KATP channels fail to exhibit the degree of sulfonylurea sensitivity seen in the native β cell KATP channels. The exception is the KATP channel composed of Kir6.2 and the sulfonylurea receptor itself (Inagaki et al., 1995).
In the kidney, cloned ROMK channels are ATP regulated in the sense that they rundown in the absence of ATP, but also have a much lower (if any) sensitivity to ATP and sulfonylureas (Ho et al., 1993; Zhou et al., 1994). At least on Northern analysis, the kidney also appears to lack SUR1 (Inagaki et al., 1995), which may partially account for the decrease in glibenclamide sensitivity. Of note, the renal K+ channel ROMK2 appears to be sensitive to glibenclamide when coexpressed with the cystic fibrosis transmembrane conductance regulator, but becomes much less sensitive after PKA phosphorylation of the patch (McNicholas et al., 1996). Since phosphorylation by PKA activates the BLM KATP channel (Mauerer et al., 1998), it may be that high current patches are already highly phosphorylated, and thus relatively insensitive to glibenclamide. That glibenclamide inhibition of the BLM KATP channel appears significantly more potent in cell-attached patches with smaller currents is consistent with this idea.
Diazoxide.
Diazoxide is a benzothiadiazine K channel opener (KCO) that activates several types of KATP channels. In many tissues, it is thought that activation by KCOs requires Mg-ATP (Edwards and Weston, 1993; Ashcroft and Ashcroft, 1990), as illustrated for the BLM K+ channel in Fig. 10. Diazoxide also does not alter the requirement for Mg-ATP to prevent BLM K+ channel rundown (see below). Indeed, diazoxide can be inhibitory in the absence of Mg-ATP, perhaps by accelerating channel rundown (Kozlowski et al., 1989). Therefore, it is not likely that diazoxide exerts its effect on the rundown site of the BLM K+ channel.
For both the frog muscle KATP channel and the Ambystoma BLM K+ channel, KCOs lose their effectiveness at high (5–10 mM) intracellular [ATP]. This reciprocal effect on channel activity suggests a competitive interaction between KCOs and nucleotides. The activation by KCOs in the frog skeletal KATP channel is not Mg2+ dependent and, like the BLM K+ channel, does not occur in the absence of ATP. Further studies may thus show that diazoxide opens the BLM K+ channel by a mechanism similar to that recently proposed for KCO activation of the skeletal muscle KATP channel (Forestier et al., 1996).
Rundown of the BLM K+ Channel
A hallmark of KATP channels is that they exhibit the phenomenon of rundown, a gradual loss of activity when the membrane patch is deprived of cytosolic ATP. As first noted by Findlay and Dunne (1986), rundown is “a paradoxical situation in that K+ channels that are inhibited by intracellular ATP require intracellular ATP to retain the ability to open.” In a recent study on frog proximal tubule cells (Robson and Hunter, 1997), washout of intracellular ATP reduced the whole-cell barium-sensitive conductance by ∼60% over 10 min. Inclusion of 2 mM ATP in the patch pipette not only prevented this rundown, but the barium-sensitive conductance increased by 54% over 10 min.
Accordingly, the BLM K+ channel runs down in the absence of ATP, and low concentrations of ATP (100– 200 μM) are required to prevent or rescue channel rundown in cell-free patches. Once rundown has begun, application of ATP will restore channel activity, but the ATP-restorable current decreases as the time between rundown and ATP application lengthens. The BLM K+ channel specifically requires Mg-ATP to support activity as rundown occurs if either ATP, Mg2+, or both are removed (see Table III). Typically, both Mg2+ and ATP are required to prevent rundown in KATP channels (Ashcroft and Ashcroft, 1990). Mg2+ is required as a cofactor, as channel rundown occurs in a Mg2+-free solution despite the presence of Na-ATP. Free Mg2+ concentrations as low as 200 nM are sufficient to prevent BLM K+ channel rundown in the presence of ATP. The possibility that free Mg2+ itself (rather than the Mg-ATP complex) plays a distinct role in the rundown of the BLM K+ channel cannot be excluded, but is difficult to test since rundown occurs if either Mg2+ or ATP is removed. Interestingly, free Mg2+ itself (≥10 μM) can prevent rundown of Kir2.1, an ATP-insensitive inward rectifier (Fakler et al., 1994).
Table III.
Rundown for ROMK1 Compared with the BLM K+ Channel
| Nucleotide | Free Mg2+ | ROMK1 rundown* | BLM K+ rundown | |||
|---|---|---|---|---|---|---|
| 0.2 mM ATP | 1 mM Mg2+ | No | No | |||
| 0.2 mM ATP-γS | 1 mM Mg2+ | ? | Yes | |||
| ATP-free | 1 mM Mg2+ | Yes | Yes | |||
| 0.2 mM ATP | Mg2+-free | Yes | Yes | |||
| ATP-free | Mg2+-free | No | Yes |
As expressed in Xenopus oocytes; data from McNicholas et al. (1994).
Paradoxically, removal of free Mg2+ nearly abolishes rundown of KATP in cultured CRI-G1 insulin-secreting cells (Kozlowski and Ashford, 1990) and partially inhibits rundown of ATP-regulated ROMK1 channels excised in an ATP-free bath (McNicholas et al., 1994). The latter effect is presumed to occur via the inhibition of a Mg2+-dependent phosphatase (McNicholas et al., 1994). Thus, removal of Mg2+ inhibits rundown in ROMK1, but actually produces rundown in the BLM K+ channel (see Fig. 9 C). We conclude that the Mg2+-dependent phosphatase thought to be involved in the rundown of ROMK1 (McNicholas et al., 1994) does not mediate rundown of the BLM K+ channel.
ATP can serve as a substrate in hydrolysis reactions mediated by an ATPase and in phosphorylation reactions mediated by a kinase. We asked whether phosphorylation itself (without hydrolysis) can prevent BLM K+ channel rundown, as has been proposed for other channels, including ROMK1 (McNicholas et al., 1994). To address this issue, we used ATP-γS, a poorly hydrolyzable ATP analogue that is an effective substrate for most kinases (e.g., PKA) in (thio)phosphorylation reactions (Eckstein, 1985). Similar to results reported for the cell KATP channel (Ohno-Shosaku et al., 1987), our findings (see Fig. 9 and Table III) indicate that ATP-γS cannot substitute for ATP in sustaining channel activity, whereas all hydrolyzable nucleoside triphosphates we tested do prevent or rescue rundown. The corresponding nucleoside diphosphates were also ineffective.
In summary, rundown of the BLM K+ channel in excised patches seems to be prevented by a high affinity nucleotide binding site, which hydrolyzes nucleoside triphosphates in the presence of Mg2+. Rundown is prevented by ATP even in the presence of high concentrations of a protein kinase inhibitor, strongly suggesting that typical protein phosphorylation processes alone are insufficient to prevent/rescue channel rundown. Fakler et al. (1994) obtained similar results in Kir2.1 channels.
Comparison with Other Studies
Gögelein and Greger (1987b) found a very weak inward rectifier K+ channel on the lateral membrane of rabbit proximal straight tubule. In symmetrical KCl with 1 mM Mg2+ in the bath, this channel had a limiting gin of ∼45 pS and a limiting gout of ∼40 pS. In contrast to the results of the present study, their channel's open probability did not increase with hyperpolarization, and nP o was unaffected by Ca2+ (<1 nM–1 μM) applied to inside-out patches (Mauerer et al., 1998). They did not observe channel rundown in excised patches, the channel was tetraethylammonium sensitive, and the effect of ATP was not tested. Thus, it is not likely that their channel was the BLM K+ channel we find in Ambystoma proximal tubule.
Kawahara et al. (1987) found a 31-pS K+ channel in 42% of cell-attached patches made on the BLM of Necturus proximal tubule. They also found saturation of inward channel conductance at ∼50 pS (compared with ∼35 pS for Ambystoma) and an apparent K m of 65.5 mM (compared with 77 mM in Ambystoma). Whereas we observed inward rectification for all [K], their I-V plots were restricted to inward currents. Similar to our findings in Ambystoma, the K+ channel in Necturus showed high selectivity (PK:PNa ≈ 10:1 in Necturus versus ≈ 30:1 in Ambystoma), channel activity increased with hyperpolarization, and external Na+ did not influence channel behavior. They also found that hyperpolarization did not affect channel open time; rather, the increase in nP o was due to shortening a closed state lifetime. Significantly, Kawahara et al. (1987) were unable to maintain channel activity in excised patches, stating that “patches became unstable and noisy after excision.” It is likely that this was channel rundown since this description is reminiscent of the channel “choking” and rundown we observe in an ATP-free bath.
Similarly, Sackin and Palmer (1987) observed two BLM K+ channels in Necturus, a short open-time channel that they studied in detail, and a long open-time channel that disappeared after excision, perhaps because of rundown. The short open-time channel is different from the BLM K+ channel of Ambystoma since it was nonrectifying and did not rundown when excised in an ATP-free bath. The long open-time channel was thought to be the same as that studied in cell-attached patches by Kawahara et al. (1987), and probably corresponds to the BLM K+ channel in Ambystoma except the channel in Necturus had a mean open time of ∼60 ms, much longer than that seen in our study.
Parent et al. (1988) made cell-attached patches on rabbit BLM and found an inward-rectifier K+ channel that had different properties than both the Ambystoma BLM K+ channels in the present study. Their voltage dependence was opposite to ours, as they concluded that hyperpolarization induced a long closed state.
Hunter (1991) made cell-attached patches on the BLM of single frog proximal tubule cells and found only one kind of K+ channel, an inward rectifier with an inward gslope = 32.4 pS, and an outward gchord = 6.2 pS at +80 mV, similar to the one we studied on the BLM of Ambystoma proximal tubule. Depolarization reduces the macroscopic BLM K+ conductance and single-channel conductance in both preparations. A Boltzmann fit of the g–V curves (rederived using Fig. 5 of Hunter, 1991, and the inset of Fig. 3 B in the present study) shows a V1/2 = +31.7 mV and a width of 14.5 mV for the BLM K+ channel in c/a patches of frog, compared with a V1/2 = −14.9 mV and a width of 21.3 mV for the BLM K+ channel in i/o patches of Ambystoma. Thus, the sensitivity of conductance versus voltage is such that there is an e-fold change in g per 14.5 mV in frog (c/a), and per 21.3 mV in Ambystoma (i/o). The discrepancy in V1/2 may be due to different experimental conditions and levels of cytosolic Mg2+. Hunter (1991) also reported that the addition of glucose and/ or alanine did not affect BLM K+ channel activity in the frog cells. Although addition of alanine depolarized Vm > 30 mV, he was unable to explain the repolarization of the BLM under his experimental conditions. In contrast, the Ambystoma BLM K+ channel under discussion is activated by these substrates, which can explain the repolarization of the BLM (Mauerer et al., 1998). In a recent follow-up study, Robson and Hunter (1997) proposed that, based on differential sensitivity to barium and quinidine, the frog cells have two separate K+ conductances. The inwardly rectifying conductance that was inhibited by both agents appears similar to the Ambystoma BLM KATP channel, although direct sensitivity to ATP or glibenclamide was not tested in their study.
Tsuchiya et al. (1992) found an ATP-sensitive K+ channel in five patches made on the BLM of nonperfused rabbit S1 and S2 segments. With 145 mM K+ in the patch pipette and a bath containing 140 mM Na+ and 5 mM K+, they reported an inward gslope = 56 pS, although an I-V plot was not given. In c/a patches at a command potential of 0 mV, they found a baseline nP o of 0.72, much higher than that found in the present study and the two studies in which perfused tubules were patched (see below) (Hurst et al., 1993; Beck et al., 1993). Although 1 mM ATP applied to the cytoplasmic side of an inside-out patch reversibly reduced nP o by 77% (from 0.70 to 0.16), the sensitivity of the basolateral conductance was reduced in a perfused tubule when 1 mM ATP was added to the bath.
Beck et al. (1993) were the first to patch clamp the BLM of (collagenase-treated) perfused rabbit proximal tubules, and found a K+ channel whose activity correlated to transport activity. This channel showed inward rectification in c/a patches (with gin = 61 pS and gout = 17 pS) and P o was voltage independent. In a follow-up study (Hurst et al., 1993), the same group showed that 2 mM ATP (i/o) inhibited channel activity by ∼80% and 100 μM diazoxide (c/a) effectively opened this BLM K+ channel.
The ROMK family of K+ channels is, to date, the only cloned ATP-regulated renal K+ channel (Ho et al., 1993). The ROMK1 channel cloned from rat kidney is an inwardly rectifying K+ channel with gin = 39 pS and a high open probability (0.8–0.9) at voltages more depolarized than −60 mV, and is not activated by hyperpolarization. ROMK1 exhibits channel rundown when membrane patches are excised in ATP-free bath, although ROMK1 is not sensitive to ATP or glibenclamide (Ho et al., 1993). In situ hybridization studies show that transcripts (mRNA) for ROMK are absent from the proximal tubule (Lee and Hebert, 1995). Combined with the differences in mechanism of channel rundown (see above and Table III), it is highly unlikely that the BLM K+ channel is ROMK1, although it may be within the same family of inwardly rectifying K+ channels with two transmembrane domains (Ho et al., 1993; Kubo et al., 1993).
Contribution of the BLM K+ Channel to Total BLM Conductance
The component of BLM conductance (GBLM) due to the BLM K+ channel (GK) can be estimated as:
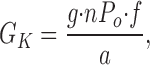 |
10 |
where g is the “physiologic” single channel conductance, n is the average number of channels in a patch, P o is the mean open time of one channel, f is the incidence of finding a channel in the patch, and a is the area of the membrane patch.
In the present study, the conductance (g) for outward currents was ∼4 pS, the average nP o extrapolated to 0 mV was 0.20, and channel activity was found in 98.6% of patches. Given that the average inner diameter of our patch pipette was 1.5 μm, the minimal membrane area of a patch is 1.77 μm2. Using these estimates in Ambystoma, the value for GK is (4 pS*0.20*0.986)/ 1.77 μm2 = 0.446 pS/μm2 or 44.6 S/cm2 (RK = 22.4 kΩ · cm2). Considering the estimate of GBLM = 51.3 μS/cm2 (RBLM = 19.5 kΩ · cm2) in Ambystoma proximal tubule (Maunsbach and Boulpaep, 1984), GK/GBLM = 44.6/ 51.3 = 0.87. Thus, under the conditions used in our experiments, GK may account for 87% of GBLM. However, to the extent that the area of the membrane patch is larger than our estimate, GK will be smaller.
The basolateral membrane area of dissociated Ambystoma proximal tubule cells exhibits less infoldings than these cells in situ (Segal et al., 1996). Assuming a whole-cell membrane capacitance of ∼120 pF (Segal et al., 1996) and a specific capacitance of 1 pF per 100 μm2, we can estimate that the surface of a single isolated cell is ∼12,000 μm2. Using a BLM surface area of 10,000 μm2 (100 × 10−6 cm2) and a GBLM = 51.3 μS/ cm2, the total BLM conductance is 5.13 nS. Using the transport number GK/GBLM of 0.87 from Table IV, GK = 4.46 nS. Despite possible changes in membrane area (e.g., due to membrane recycling) and/or channel density that could occur in the dissociated cells over time, this is a reasonable estimate since perforated-patch whole-cell studies with KCl pipette and NaCl bath (EK = −84 mV) show current that reverses at Vm = −40 mV with ∼240 pA of outward current at 0 mV command potential, and a whole-cell conductance of 6 nS. Thus, the total BLM conductance is 5.13 nS, of which 4.46 nS is K conductance, and the total apical conductance is 0.87 nS (the difference of 6–5.13).
Table IV.
Contribution of the K+ Conductance to the BLM Conductance of Proximal Tubule
| Species | g | NPo | f | A | GK | GBLM | GK/GBLM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pS | 10−8cm2 | μS/cm2 | μS/cm2 | |||||||||||
| Necturus | 31.2* | 0.14* | 1.0* ‡ | 1.3* | 33.6* | 33–59§ | 0.57–1.00 | |||||||
| 0.42* | 14.1 | 0.24–0.43 | ||||||||||||
| Rana | 5.6‖ | 0.22‖ | 0.62‖ | 1.57‖ | 48‖ | 33–59§ | 0.81–1.45 | |||||||
| 51.3¶ | 0.94 | |||||||||||||
| Rabbit | 10** | 0.06** | 1.0†† | 1.0** | 100** | 714§§ | 0.14 | |||||||
| Ambystoma | 4‖‖ | 0.20‖‖ | 0.986‖‖ | 1.77‖‖ | 44.6‖‖ | 51.3¶ | 0.87 |
From Kawahara et al., 1987; cell-attached at 0 mV;
assumed in the calculation in Kawahara et al. (1987), despite finding f = 0.42;
from Maunsbach and Boulpaep (1984), for Necturus;
from Hunter (1991), cell attached at 0 mV;
from Maunsbach and Boulpaep (1984), for Ambystoma;
from Parent et al. (1988), cell-attached at −50 mV;
presumed, since data not given in Parent et al. (1988);
from Lapointe et al. (1984);
present study.
Similar comparisons between GK derived from single-channel data and GBLM based on cable analysis are shown in Table IV for Necturus, Rana, and rabbit proximal tubule, respectively. With the exception of the rabbit, the transport numbers show that the BLM conductance of all other species is dominated by K+ and, specifically for Ambystoma, KATP channels. From this information, one can estimate the K+ channel density on the BLM of dissociated Ambystoma proximal tubule cells. If P o was unity, the number of channels would be given by 4.46 nS/4 pS = 1,115 channels. However, since P o = 0.05, the number of K+ channels on the BLM is ∼22,300 per cell; a channel density of 2.23 channels/μm2. Using the estimate of membrane patch area of 1.77 μm2, one would expect on average approximately four channels per patch, in good agreement with our experience.
Physiological Role of the BLM KATP Channel
The task of the proximal tubule under physiologic conditions is to effect transport; i.e., to maintain ionic flux in the direction of reabsorption. The electrochemical difference across the BLM of proximal tubule, given by Vbl − EK, is an invariably positive driving force promoting K+ efflux (Boulpaep, 1979). The presence of other conductances in the BLM with reversal potentials less negative than EK provides a nonzero difference between EK and Vbl that maintains K efflux. The BLM Na+,K+-ATPase pump loads the cell with two K+ ions to effect the transcellular transport of three Na+ ions. An efflux pathway for K+ is necessary to recycle K+ across the BLM, thus permitting continuous operation of the pump. Pump activity and the opening of these K+ channels hyperpolarize the BLM, favoring apical Na+ entry. An interesting contrast is that the negative shift in membrane potential consequent to the opening of KATP channels tends to depress cell function in excitable cells, but tends to promote transport in epithelia. The magnitude of BLM K+ efflux must match the K+ current of the pump. The regulatory effects of hyperpolarization and intracellular ATP levels on the BLM KATP channels thus subserve this role.
This system of a variable BLM K+ conductance coupled to changes in pump rate in a single cell has further implications when extrapolated to the level of an epithelium with a paracellular shunt pathway. Such an epithelial model should include all current pathways (see Fig. 11, adapted from Fig. 5 of Sackin and Boulpaep, 1983); namely, (a) the active Na+ and K+ pump currents, (b) the basolateral K+ leak current, (c) the other basolateral leak currents, principally carried by Cl−, and (d) the shunt current carried by Cl− and, to a lesser extent, by Na+. In this equivalent electrical cell/ circuit, Kirchoff's current law at node A gives
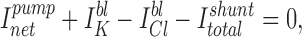 |
11 |
Figure 11.
Equivalent electrical cell/circuit. Adjacent proximal tubule cells are outlined as dashed lines, and the equivalent circuit discussed in the text is diagrammed on the left. The basolateral membrane potential is denoted by voltage source Vbl. The current produced by the Na,K-ATPase pump, Inet pump is given by INa pump + IK pump. Basolateral ion channel currents are shown as their Thévenin equivalents with their conductances, GK bl and GCl bl, represented by the equivalent resistor, in series with their Nernst potentials, EK and ECl, respectively. The paracellular current, Itotal shunt is represented by INa shunt + ICl shunt. RA is the resistance of the apical membrane. Kirchoff's current law at Node A is given by Eq. 11.
where 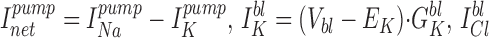 is
the transcellular component of Cl− absorption, and
is
the transcellular component of Cl− absorption, and
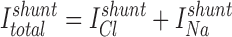 (i.e., the sum of the paracellular
component of Cl− absorption and any Na+ backleak).
If the outward current through BLM K channels could
be exactly matched to
(i.e., the sum of the paracellular
component of Cl− absorption and any Na+ backleak).
If the outward current through BLM K channels could
be exactly matched to 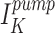 , then Eq. 11 becomes
, then Eq. 11 becomes
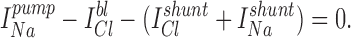 |
12 |
When Eq. 12 is satisfied, all actively transported Na+
is accompanied by Cl− absorption (sum of transcellular
and paracellular), except for the backleak, 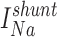 . Under these optimal conditions, the proximal tubule accomplishes NaCl reabsorption at maximum efficiency.
. Under these optimal conditions, the proximal tubule accomplishes NaCl reabsorption at maximum efficiency.
Note that if 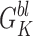 did not increase with pump rate,
did not increase with pump rate, 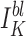 would rise only slightly due to the small increase in
driving force. Under these conditions,
would rise only slightly due to the small increase in
driving force. Under these conditions, 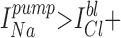
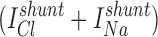 and the efficiency of NaCl reabsorption
deteriorates. That is, the incremental increase in NaCl
reabsorption would be less than that of the pump.
Therefore, the tight linkage between the Na+,K+-ATPase pump and the K+ leak through the BLM KATP channels is especially important in leaky epithelia that absorb large, fluctuating quantities of NaCl such as the
proximal tubule. The regulation of the BLM KATP channels and how they are coupled to the pump during
transport is the subject of the companion paper
(Mauerer et al., 1998).
and the efficiency of NaCl reabsorption
deteriorates. That is, the incremental increase in NaCl
reabsorption would be less than that of the pump.
Therefore, the tight linkage between the Na+,K+-ATPase pump and the K+ leak through the BLM KATP channels is especially important in leaky epithelia that absorb large, fluctuating quantities of NaCl such as the
proximal tubule. The regulation of the BLM KATP channels and how they are coupled to the pump during
transport is the subject of the companion paper
(Mauerer et al., 1998).
Acknowledgments
This work was supported by grant DK-17433 from the National Institutes of Health (NIH). Dr. A. Segal is a recipient of a Physician-Scientist Award from the NIH (DK-02103).
Footnotes
This work is dedicated to the memory of Dr. Roman Mauerer (father of Ulrich Mauerer), who passed away during the preparation of the manuscript. The authors thank Ms. Christine Macol for excellent technical assistance.
Abbreviations used in this paper: BLM, basolateral membrane; c/a, cell-attached (patch); I-V, current–voltage; i/o, inside-out (patch); KCO, K channel opener; nP o, channel activity represented as the product of the minimum number of channels (n) times the open probability (P o) of the channel; SUR, sulfonylurea receptor.
references
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, IV, Boyd AE, III, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson DA. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Ashcroft SJ, Ashcroft FM. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- Balaban RS, Mandel LJ, Soltoff SP, Storey JM. Coupling of active ion transport and aerobic respiratory rate in isolated renal tubules. Proc Natl Acad Sci USA. 1980;77:447–451. doi: 10.1073/pnas.77.1.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JS, Hurst AM, Lapointe JY, Laprade R. Regulation of basolateral K channels in proximal tubule studied during continuous microperfusion. Am J Physiol. 1993;264:F496–F501. doi: 10.1152/ajprenal.1993.264.3.F496. [DOI] [PubMed] [Google Scholar]
- Beck JS, Breton S, Mairbäurl H, Laprade R, Giebisch G. Relationship between sodium transport and intracellular ATP in isolated perfused rabbit proximal convoluted tubule. Am J Physiol. 1991;261:F634–F639. doi: 10.1152/ajprenal.1991.261.4.F634. [DOI] [PubMed] [Google Scholar]
- Boim MA, Ho K, Shuck ME, Bienkowski MJ, Block JH, Slightom JL, Yang Y, Brenner BM, Hebert SC. ROMK inwardly rectifying ATP-sensitive K+channel. II. Cloning and distribution of alternative forms. Am J Physiol. 1995;268:F1132–F1140. doi: 10.1152/ajprenal.1995.268.6.F1132. [DOI] [PubMed] [Google Scholar]
- Boulpaep EL. Electrical phenomena in the nephron. Kidney Int. 1976;9:88–102. doi: 10.1038/ki.1976.14. [DOI] [PubMed] [Google Scholar]
- Boulpaep, E.L. 1979. Electrophysiology of the kidney. In Transport Across Biological Membranes. G. Giebisch, D.C. Tosteson, and H.H. Ussing, editors. Springer-Verlag, Berlin, Germany. 97–144.
- Chepilko S, Zhou H, Sackin H, Palmer LG. Permeation and gating properties of a cloned renal K+channel. Am J Physiol. 1995;268:C389–C401. doi: 10.1152/ajpcell.1995.268.2.C389. [DOI] [PubMed] [Google Scholar]
- Colquhoun, D., and F.J. Sigworth. 1983. Fitting and statistical analysis of single-channel records. In Single-Channel Recording. B. Sakmann and E. Neher, editors. Plenum Publishing Corp., New York. 191–263.
- Cook DL, Hales CN. Intracellular ATP activity directly blocks K+channels in pancreatic B-cells. Nature. 1984;311:271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Davis CW, Finn AL. Sodium transport inhibition by amiloride reduces basolateral membrane potassium conductance in tight epithelia. Science. 1982;216:525–527. doi: 10.1126/science.7071599. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Edwards, G., and A.H. Weston. 1995. Pharmacology of the potassium channel openers. Cardiovasc. Drugs Ther. 9(Suppl. 2):185– 193. [DOI] [PubMed]
- Edwards G, Weston AH. The pharmacology of ATP-sensitive potassium channels. Annu Rev Pharmacol Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol Paris. 1979;75:463–505. [PubMed] [Google Scholar]
- Fakler B, Brandle U, Glowatzki E, Zenner HP, Ruppersberg JP. Kir2.1 inward rectifier K+channels are regulated independently by protein kinases and ATP hydrolysis. Neuron. 1994;13:1413–1420. doi: 10.1016/0896-6273(94)90426-x. [DOI] [PubMed] [Google Scholar]
- Ficker E, Taglialatela M, Wible BA, Henley CM, Brown AM. Spermine and spermidine as gating molecules for inward rectifier K+channels. Science. 1994;266:1068–1072. doi: 10.1126/science.7973666. [DOI] [PubMed] [Google Scholar]
- Findlay I, Dunne MJ. ATP maintains ATP-inhibited K+channels in an operational state. Pflügers Arch. 1986;407:238–240. doi: 10.1007/BF00580683. [DOI] [PubMed] [Google Scholar]
- Forestier C, Pierrard J, Vivaudou M. Mechanism of action of K channel openers on skeletal muscle KATP channels. J Gen Physiol. 1996;107:489–502. doi: 10.1085/jgp.107.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gögelein H, Greger R. Potassium-selective channels in the basolateral and luminal membranes of the late proximal tubule of the rabbit kidney. Pflügers Arch. 1987a;410:288–295. doi: 10.1007/BF00580279. [DOI] [PubMed] [Google Scholar]
- Gögelein H, Greger R. Properties of single K+channels in the basolateral membrane of rabbit proximal straight tubules. Pflügers Arch. 1987b;410:288–295. doi: 10.1007/BF00580279. [DOI] [PubMed] [Google Scholar]
- Goldman DE. Potential, impedance, and rectification in membranes. J Gen Physiol. 1943;27:37–60. doi: 10.1085/jgp.27.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunter-Smith PJ, Grasset E, Schultz SG. Sodium-coupled amino acid and sugar transport by Necturus small intestine: an equivalent circuit analysis of a rheogenic cotransport system. J Membr Biol. 1982;66:25–39. doi: 10.1007/BF01868479. [DOI] [PubMed] [Google Scholar]
- Hamill O, Marty E, Neher E, Sakmann B, Sigworth F. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hebert SC, Ho K. Structure and functional properties of an inwardly rectifying ATP-regulated K+channel from rat kidney. Renal Physiol Biochem. 1994;17:143–147. doi: 10.1159/000173804. [DOI] [PubMed] [Google Scholar]
- Hille, B. 1992. Ionic Channels of Excitable Membranes. 2nd edition. Sinauer Associates, Inc., Sunderland, MA. 607 pp.
- Ho K, Nichols CG, Lederer WJ, Lytton J, Vassilev PM, Kanazirska MV, Hebert SC. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Katz B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J Physiol (Camb) 1949;108:37–77. doi: 10.1113/jphysiol.1949.sp004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie M, Irisawa H, Noma A. Voltage-dependent magnesium block of adenosine-triphosphate-sensitive potassium channel in guinea-pig ventricular cells. J Physiol (Camb) 1987;387:251–272. doi: 10.1113/jphysiol.1987.sp016572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter M. Potassium-selective channels in the basolateral membrane of single proximal tubule cells of frog kidney. Pflügers Arch. 1991;418:26–34. doi: 10.1007/BF00370448. [DOI] [PubMed] [Google Scholar]
- Hurst AM, Beck JS, Laprade R, Lapointe JY. Na+ pump inhibition downregulates an ATP-sensitive K+channel in rabbit proximal convoluted tubule. Am J Physiol. 1993;264:F760–F764. doi: 10.1152/ajprenal.1993.264.4.F760. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, IV, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Kawahara K. A stretch-activated K+channel in the basolateral membrane of Xenopus kidney proximal tubule cells. Pflügers Arch. 1990;415:624–629. doi: 10.1007/BF02583516. [DOI] [PubMed] [Google Scholar]
- Kawahara K, Hunter M, Giebisch G. Potassium channels in Necturusproximal tubule. Am J Physiol. 1987;253:F488–F494. doi: 10.1152/ajprenal.1987.253.3.F488. [DOI] [PubMed] [Google Scholar]
- Koefoed Johnsen, V., and H. Ussing. The nature of the frog skin potential. Acta Physiol Scand. 1958;42:298–308. doi: 10.1111/j.1748-1716.1958.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Kozlowski RZ, Hales CN, Ashford ML. Dual effects of diazoxide on ATP-K+currents recorded from an insulin-secreting cell line. Br J Pharmacol. 1989;97:1039–1050. doi: 10.1111/j.1476-5381.1989.tb12560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski RZ, Ashford ML. ATP-sensitive K+-channel run-down is Mg2+dependent. Proc R Soc Lond B Biol Sci. 1990;240:397–410. doi: 10.1098/rspb.1990.0044. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Lapointe JY, Laprade R, Cardinal J. Transepithelial and cell membrane electrical resistances of the rabbit proximal convoluted tubule. Am J Physiol. 1984;247:F637–F649. doi: 10.1152/ajprenal.1984.247.4.F637. [DOI] [PubMed] [Google Scholar]
- Lee WS, Hebert SC. ROMK inwardly rectifying ATP-sensitive K+channel. I. Expression in rat distal nephron segments. Am J Physiol. 1995;268:F1124–F1131. doi: 10.1152/ajprenal.1995.268.6.F1124. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Saigusa A, Irisawa H. Ohmic conductance through the inwardly rectifying K channel and blocking by internal Mg. Nature. 1987;325:156–159. doi: 10.1038/325156a0. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Cohen B, Guggino WB, Giebisch G. Regulation of the basolateral potassium conductance of the Necturusproximal tubule. J Membr Biol. 1984;79:153–161. doi: 10.1007/BF01872119. [DOI] [PubMed] [Google Scholar]
- Mauerer UR, Boulpaep EL, Segal AS. Regulation of an inwardly rectifying adenosine triphosphate–sensitive K+channel in the basolateral membrane of renal proximal tubule. J Gen Physiol. 1998;111:161–180. doi: 10.1085/jgp.111.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maunsbach AB, Boulpaep EL. Quantitative ultrastructure and functional correlates in proximal tubule of Ambystoma and Necturus. . Am J Physiol. 1984;246:F710–F724. doi: 10.1152/ajprenal.1984.246.5.F710. [DOI] [PubMed] [Google Scholar]
- McNicholas CM, Guggino WB, Schwiebert EM, Hebert SC, Giebisch G, Egan ME. Sensitivity of a renal K+channel (ROMK2) to the inhibitory sulfonylurea compound glibenclamide is enhanced by coexpression with the ATP-binding cassette transporter cystic fibrosis transmembrane regulator. Proc Natl Acad Sci USA. 1996;93:8083–8088. doi: 10.1073/pnas.93.15.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas CM, Wang W, Ho K, Hebert SC, Giebisch G. Regulation of ROMK1 K+channel activity involves phosphorylation processes. Proc Natl Acad Sci USA. 1994;91:8077–8081. doi: 10.1073/pnas.91.17.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Zunkler BJ, Trube G. Dual effects of ATP on K+currents of mouse pancreatic beta-cells. Pflügers Arch. 1987;408:133–138. doi: 10.1007/BF00581342. [DOI] [PubMed] [Google Scholar]
- Parent L, Cardinal J, Sauve R. Single-channel analysis of a K channel at basolateral membrane of rabbit proximal convoluted tubule. Am J Physiol. 1988;254:F105–F113. doi: 10.1152/ajprenal.1988.254.1.F105. [DOI] [PubMed] [Google Scholar]
- Robson L, Hunter M. Two K+-selective conductances in single proximal tubule cells isolated from frog kidney are regulated by ATP. J Physiol (Camb) 1997;500:605–616. doi: 10.1113/jphysiol.1997.sp022046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero PJ. Is the Ca2+-sensitive K+ channel under metabolic control in human red cells? . Biochim Biophys Acta. 1978;507:178–181. doi: 10.1016/0005-2736(78)90385-1. [DOI] [PubMed] [Google Scholar]
- Sackin H, Boulpaep EL. Isolated perfused salamander proximal tubule. II. Monovalent ion replacement and rheogenic transport. Am J Physiol. 1981;241:F540–555. doi: 10.1152/ajprenal.1981.241.5.F540. [DOI] [PubMed] [Google Scholar]
- Sackin H, Boulpaep EL. Rheogenic transport in the renal proximal tubule. J Gen Physiol. 1983;82:819–851. doi: 10.1085/jgp.82.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackin H, Palmer LG. Basolateral potassium channels in renal proximal tubule. Am J Physiol. 1987;253:F476–F487. doi: 10.1152/ajprenal.1987.253.3.F476. [DOI] [PubMed] [Google Scholar]
- Segal AS, Boulpaep EL, Maunsbach AB. A novel preparation of dissociated renal proximal tubule cells that maintain epithelial polarity in suspension. Am J Physiol. 1996;270:C1843–C1863. doi: 10.1152/ajpcell.1996.270.6.C1843. [DOI] [PubMed] [Google Scholar]
- Siebens AW, Boron WF. Effect of electroneutral luminal and basolateral lactate transport on intracellular pH in salamander proximal tubules. J Gen Physiol. 1987;90:799–831. doi: 10.1085/jgp.90.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth FJ, Sine SM. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce AE, Standen NB, Stanfield PR. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985;316:736–738. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Wang W, Giebisch G, Welling PA. ATP is a coupling modulator of parallel Na,K-ATPase-K-channel activity in the renal proximal tubule. Proc Natl Acad Sci USA. 1992;89:6418–6422. doi: 10.1073/pnas.89.14.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Giebisch G. Dual effects of adenosine triphosphate on the apical small conductance K+channel of the rat cortical collecting duct. J Gen Physiol. 1991;98:35–61. doi: 10.1085/jgp.98.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull AM. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973;61:687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Tate SS, Palmer LG. Primary structure and functional properties of an epithelial K channel. Am J Physiol. 1994;266:C809–C824. doi: 10.1152/ajpcell.1994.266.3.C809. [DOI] [PubMed] [Google Scholar]