Abstract
We have investigated the effect of extracellular proteases on the amiloride-sensitive Na+ current (INa) in Xenopus oocytes expressing the three subunits α, β, and γ of the rat or Xenopus epithelial Na+ channel (ENaC). Low concentrations of trypsin (2 μg/ml) induced a large increase of INa within a few minutes, an effect that was fully prevented by soybean trypsin inhibitor, but not by amiloride. A similar effect was observed with chymotrypsin, but not with kallikrein. The trypsin-induced increase of INa was observed with Xenopus and rat ENaC, and was very large (∼20-fold) with the channel obtained by coexpression of the α subunit of Xenopus ENaC with the β and γ subunits of rat ENaC. The effect of trypsin was selective for ENaC, as shown by the absence of effect on the current due to expression of the K+ channel ROMK2. The effect of trypsin was not prevented by intracellular injection of EGTA nor by pretreatment with GTP-γS, suggesting that this effect was not mediated by G proteins. Measurement of the channel protein expression at the oocyte surface by antibody binding to a FLAG epitope showed that the effect of trypsin was not accompanied by an increase in the channel protein density, indicating that proteolysis modified the activity of the channel present at the oocyte surface rather than the cell surface expression. At the single channel level, in the cell-attached mode, more active channels were observed in the patch when trypsin was present in the pipette, while no change in channel activity could be detected when trypsin was added to the bath solution around the patch pipette. We conclude that extracellular proteases are able to increase the open probability of the epithelial sodium channel by an effect that does not occur through activation of a G protein-coupled receptor, but rather through proteolysis of a protein that is either a constitutive part of the channel itself or closely associated with it.
Keywords: trypsin, chymotrypsin, amiloride, G protein, epithelial Na+ channel
introduction
The passage of sodium through the epithelial Na+ channel (ENaC)1 is the rate limiting step in the sodium reabsorption by the epithelial cells of the distal nephron and colon and in airways (Garty and Palmer, 1997). It thereby plays a key role in the regulation of the sodium balance, extracellular fluid volume, and blood pressure by the kidney, and in the controlled fluid reabsorption in the airways. The activity of ENaC has to be tightly regulated with regard to the whole organism sodium balance, but also with regards to the epithelial cell transport capacity so that the homeostasis of the intracellular milieu is preserved. This regulation is under the control of several hormones and intracellular factors by mechanisms that are not yet completely understood (Garty and Palmer, 1997).
Concerning luminal factors, Garty and Edelman (1983) observed that trypsin, at a concentration of 1 mg/ml, induced an irreversible inhibition of the sodium transport and this effect could be prevented by amiloride. They concluded that a component of the Na+ channel protein could be cleaved by a protease at a site protectable by amiloride bound to its receptor. Lewis and Alles (1986) and Lewis and Clausen (1991) studied the effects of proteases such as kallikrein, which is normally present in mammalian urine, on the Na+ channel of the rabbit urinary bladder. To briefly summarize their findings, they observed that these proteases altered the highly selective and amiloride-sensitive Na+ channel into a nonselective cation channel, and further proteolysis rendered this channel unstable in the membrane so that the proteolyzed channel could eventually be recovered from the fluid bathing the apical medium (Zweifach and Lewis, 1988). These authors speculated that luminal proteolytic activity could be a physiological regulator of the epithelial Na+ channel (Lewis and Alles, 1986).
Orce et al. (1980) made an interesting observation suggesting, in contrast, that an extracellular protease was able to activate the electrogenic Na+ transport: they reported that the trypsin inhibitor aprotinin induced a decrease of the amiloride-sensitive short circuit current in the toad bladder. In support of an activating effect of a protease on ENaC, Vallet et al. (1997) have recently identified a novel endogenous serine protease in A6 cells that is able to activate the amiloride-sensitive Na+ current when coexpressed with ENaC in oocytes. In addition, they also show that trypsin can activate the amiloride-sensitive Na+ current in the A6 cell. To try to understand the mechanism of these effects of proteases, we have studied the effect of serine proteases on the Na+ current carried by rat (Canessa et al., 1993, 1994) or Xenopus (Puoti et al., 1995) ENaC expressed in Xenopus oocytes. Trypsin is known to induce a transient activation of a calcium-activated chloride current in Xenopus oocytes, an effect described by Durieux et al. (1994) that is mediated through a G protein-coupled trypsin receptor naturally present in the oocyte membrane and an IP3-induced release of calcium from intracellular stores. In addition to these known effects, we observed that low concentrations of trypsin or chymo-trypsin induced an activation of the amiloride-sensitive current carried by ENaC expressed in oocytes. A number of experiments performed to evaluate the role of G proteins or potential intracellular second messengers in this regulation yielded negative results. The data from macroscopic current and single channel experiments rather suggest that the effect of trypsin occurs through proteolysis of the channel protein itself or of a closely associated protein.
methods
Expression of Rat and Xenopus ENaC in Xenopus Oocytes
In vitro-transcribed cRNA for the α, β, and γ subunits of Xenopus ENaC (XENaC) and rat ENaC (rENaC) were injected into stage V–VI Xenopus oocytes (0.3–1 ng of cRNA of each subunit in a total volume of 50 nl) as described previously (Canessa et al., 1993, 1994; Puoti et al., 1995). Electrophysiological experiments were performed 1–2 d after cRNA injection. In other experiments, 1 ng of human cystic fibrosis transmembrane conductance regulator (CFTR) cRNA (clone generously provided by R. Boucher, University of North Carolina, Chapel Hill, NC), 1 ng of human β2 adrenergic receptor cRNA (clone generously provided by S. Cotecchia, Institute of Pharmacology, Lausanne, Switzerland), or 0.1 ng rat ROMK2 cRNA (cDNA generously provided by L.G. Palmer, Cornell University Medical College, New York) were in vitro transcribed and injected into Xenopus oocytes.
Ion Current Measurement in Whole Ooctyes
Amiloride-sensitive and cAMP-stimulated Cl− currents were measured as previously described (Canessa et al., 1993) using the two-electrode voltage clamp technique by means of a Dagan TEV voltage clamp apparatus (Dagan Corp., Minneapolis, MN), at room temperature (22–25°C) and at a holding potential of −100 mV in a solution containing (mM) 100 Na gluconate, 0.4 CaCl2, 10 Na HEPES, pH 7.4, 5 BaCl2, and 10 tetraethylammonium chloride. The current signal was filtered at 20 Hz using the internal filter of the Dagan apparatus and continuously recorded on a paper chart. Low chloride concentration and K+ channel blockers were used to reduce the background membrane conductance.
ROMK2-induced K+ currents were measured as the inward current induced by addition of 5 mM K+ at −100 mV under the conditions described by Zhou et al. (1994).
Single Channel Recordings
Before patch-clamp experiments, the oocytes were placed for 3–5 min at room temperature in a hypertonic medium (475 mosM) with the following composition (mM): 200 K aspartate, 20 KCl, 1 MgCl2, 10 EGTA, 10 Na HEPES, pH 7.4. The vitelline membrane could then be manually removed from the cell using fine forceps (Methfessel et al., 1986). The oocytes were then immediately transferred to the recording chamber.
Both the cell-attached and excised outside-out patch clamp experiments were carried out according to the methods described by Hamill et al. (1981). Patch pipettes were made of borosilicate glass (Corning Glass Works, Corning, NY), pulled in two stages with a PP-83 vertical puller (Narishige, Japan) and fire polished. They had a resistance of 10–20 MΩ. Single channel currents were recorded with a patch clamp amplifier (LM EPC 7; List Electronics, Darmstadt, Germany), displayed on an oscilloscope (Tektronix, Heerenveen, The Netherlands) and stored on a digital tape recorder (Biologic, France). By convention, for outside-out patches the intracellular potential corresponds to the pipette potential (Vpip), and negative (downward) single channel currents correspond to Li+ flux from the extracellular to the intracellular side of the membrane; for cell-attached patches the membrane potential should be close to the actual potential across the membrane patch because the oocyte membrane potential is close to 0 mV under our experimental conditions.
Current signals were filtered at 200 Hz with an eight pole Bessel filter (Frequency Devices Inc., Haverhill, MA) and digitized at 1 kHz using a Labmaster analog–digital interface and Fetchex Software (Axon Instruments, Foster City, CA). The n.Po product (n = number of channel, Po = open probability) was calculated as n.Po = IENaC/i, where IENaC is the current due to ENaC (= total current minus current with no channel open) averaged over a 1-min recording, divided by the unitary current measured as the peak to peak interval in the amplitude histogram.
Chemicals and Enzymes
Amiloride, guanosine 5′-O-(3-thiotriphosphate) (GTP-γS), soybean trypsin inhibitor, and trypsin (DPCC-treated trypsin from bovine pancreas, a form of trypsin with low level of chymotrypsin contamination) were obtained from Sigma Chemical Co. (St. Louis, MO). Porcine kallikrein was obtained from Calbiochem Corp. (La Jolla, CA) and chymotrypsin (TLCK-treated α-chymo-trypsin, a form of chymotrypsin with a low level of trypsin contamination), was purchased from Fluka (Buchs, Switzerland).
results
Effect of Trypsin on ENaC Expressed in Xenopus Oocytes
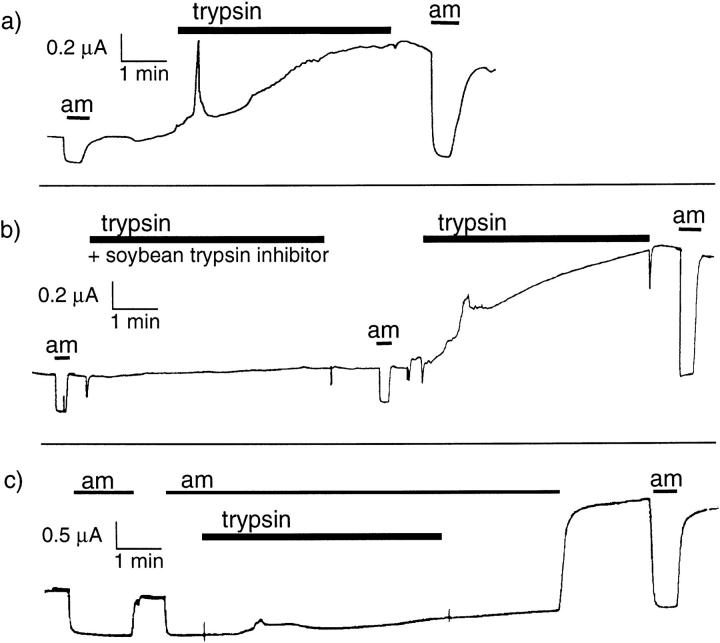
As shown in Fig. 1 a, 2 μg/ml trypsin had a biphasic effect: it induced first a peak of inward current lasting 10–60 s; the amplitude and the duration of this current was highly variable, sometimes undetectable and sometimes amounting to 2–3 μA. This effect of trypsin has been described by Durieux et al. (1994). This current could also be observed in native noninjected oocytes or oocytes expressing ENaC in the presence of 10 μM amiloride and was therefore not related to ENaC. In oocytes expressing αβγENaC, trypsin induced in addition a slower increase of a steady inward current, with a time course of 1–5 min to reach its maximal amplitude (Fig. 1 a). This current was amiloride sensitive.
Figure 1.
Effect of trypsin on the amiloride-sensitive current in oocytes expressing αβγENaC. (a) Original current recording in an oocyte expressing αβγrENaC. The current sensitive to 10 μM amiloride was measured at −100 mV before and after a 4-min exposure to 2 μg/ml trypsin. A transient spike of current, attributed to Ca-activated Cl channels occurs in the 10–20 s after the beginning of trypsin treatment, and there is an approximately threefold increase in the amiloride-sensitive current. (b) Original current recording in an oocyte expressing αβγXENaC. The current sensitive to 10 μM amiloride was measured at −100 mV before and after 4-min exposures to 2 μg/ml trypsin, first in the presence of 10 μg/ml soybean trypsin inhibitor, and then without inhibitor. Trypsin has no detectable effect on either INa or a chloride current in the presence of the inhibitor. (c) Original current recording in an oocyte expressing αβγrENaC. The current sensitive to 10 μM amiloride was measured at −100 mV before and after a 4-min exposure to 2 μg/ml trypsin in the presence of 10 μM amiloride. A transient increase in current, corresponding to the Ca-activated Cl− current can be observed ∼1 min after the beginning of exposure to trypsin. A more than twofold increase is seen when amiloride is removed after trypsin treatment.
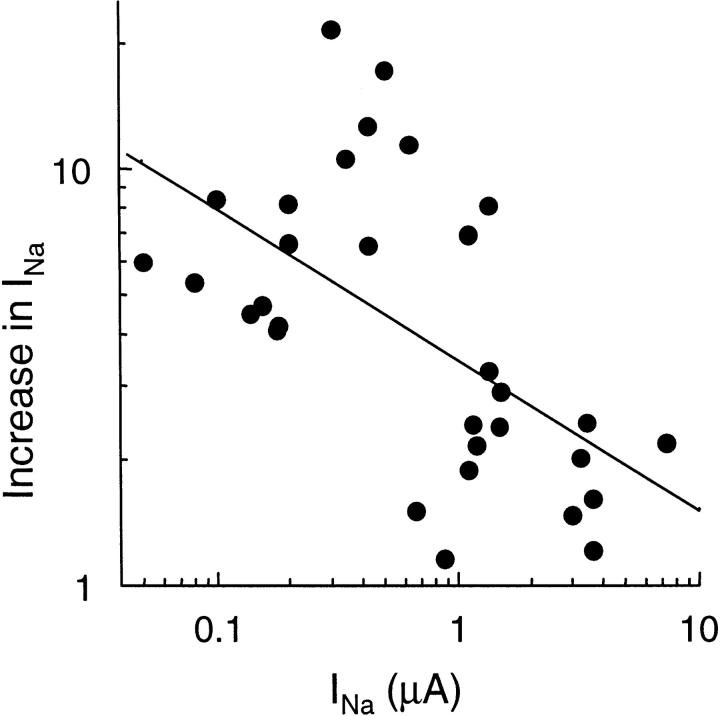
The amplitude of the increase of the amiloride-sensitive sodium current (INa) was quite variable, ranging from a hardly detectable increase to a sixfold increase in oocytes expressing rat αβγENaC, and a 2- to more than 10-fold increase in oocytes expressing Xenopus αβγENaC. Heteromeric channels composed of the α subunit of the Xenopus ENaC and the β and γ subunits of rat ENaC expressed a small INa, but in these oocytes trypsin induced a very large increase of INa, often >20-fold, following the same time course. The mean values of the baseline INa and of the increase of INa after a 3–5-min exposure to 2 μg/ml trypsin in Xenopus ENaC, (αXβXγX), in rat ENaC (αrβrγr), and in heteromeric rat/Xenopus channels, αXβrγr and αrβXγX, are given in Table I. The response to trypsin was inversely correlated with the initial INa value as shown in Fig. 2, suggesting that trypsin effect was larger in oocytes either expressing a low number of channels or channels with a low open probability.
Table I.
Effect of Trypsin on the Amiloride-sensitive Na+ Current (INa)
| Control INa | Trypsin INa | INa increase* | n | |||||
|---|---|---|---|---|---|---|---|---|
| μA | μA | |||||||
| αXβXγX | 1.28 ± 0.10 | 4.1 ± 0.2 | 5.7 ± 0.4 | 31 | ||||
| αrβrγr | 0.81 ± 0.11 | 2.3 ± 0.3 | 2.9 ± 0.3 | 16 | ||||
| αXβrγr | 0.13 ± 0.04 | 2.0 ± 0.6 | 21 ± 5 | 13 | ||||
| αrβXγX | 3.6 ± 0.7 | 6.5 ± 1.0 | 2.0 ± 0.3 | 11 |
Mean ± SEM INa (= currents sensitive to 10 μM amiloride at −100 mV) before (control) and after (trypsin) a 3–5-min exposure to 2 μg/ml trypsin.
Increase indicates the mean values of the change in INa; i.e., the ratio of INa after trypsin exposure to INa before trypsin exposure (n = number of observations). Experiments were performed with oocytes expressing Xenopus ENaC (αXβXγX), rat ENaC (αrβrγr), or heteromeric Xenopus/rat channels (αXβrγr and αrβXγX).
Figure 2.
Trypsin-induced increase in amiloride-sensitive Na current (INa): relationship to the initial INa value. The increase in INa after a 3–5-min exposure to 2 μg/ml trypsin is reported as a function of the initial value of INa in 31 oocytes expressing αβγ Xenopus ENaC. Note that both scales are logarithmic. The straight line is the regression line of log(increase in INa) versus log(INa). The corresponding r = 0.5794 (n = 31), indicating a statistically significant inverse relationship (P < 0.001) between these two variables.
To examine the question of whether the effect of trypsin was due to its catalytic activity or to some other mechanism such as binding to a receptor, we measured the effect of trypsin (2 μg/ml) first in the presence and then absence of fivefold excess of soybean trypsin inhibitor (10 μg/ml) in oocytes expressing αβγrENaC. As shown in the example of Fig. 1 b, the presence of trypsin inhibitor completely prevented the effect of trypsin, while trypsin alone was subsequently able to induce a large increase of INa. In eight measurements, a 3-min exposure to trypsin + trypsin inhibitor resulted in a slight decrease of INa to 87 ± 13% of its initial value (no significant difference), while subsequent exposure to trypsin alone induced an increase of INa to 277 ± 36% of the initial control value (P < 0.001, paired t test).
The inhibitory effect of trypsin described earlier in toad or rabbit bladder (Garty and Edelman, 1983; Lewis and Clausen, 1991) could be prevented by amiloride. We tested the effect of amiloride on the stimulatory effect of trypsin using the following protocol to ensure that no channels were exposed to trypsin in the absence of amiloride (see example in Fig. 1 c). After recording the Na+ current in the control solution, amiloride (10 μM) was first added to block the channel. 30 s later, trypsin (2 μg/ml) was added in the presence of amiloride for 3 min, and then removed while amiloride was still present. Finally, amiloride was removed, allowing measurement of the Na+ current. As shown in Fig. 1 c, the stimulatory effect on INa was not prevented by amiloride. In a series of comparative measurements, the increase of the amiloride-sensitive current when trypsin was added for 3 min in the absence of amiloride (8.6 ± 1.6, n = 11) and when trypsin was added in the presence of 10 μM amiloride (9.8 ± 2.1, n = 9) was similar.
To evaluate the selectivity of the action of trypsin, we also studied the effect of trypsin on the inward K+ current induced in oocyte by expression of ROMK2, a renal K+ channel that is most probably expressed in the apical membrane of the cortical collecting tubule principal cells, the same membrane in which ENaC is expressed (Zhou et al., 1994). In 11 oocytes expressing ROMK2, the inward K+ current amounted to 365 ± 69 nA and was not increased by trypsin (331 ± 66 nA, after a 3-min exposure to 2 μg/ml trypsin).
Trypsin Concentration–Effect Relationship and Effects of Other Proteolytic Enzymes
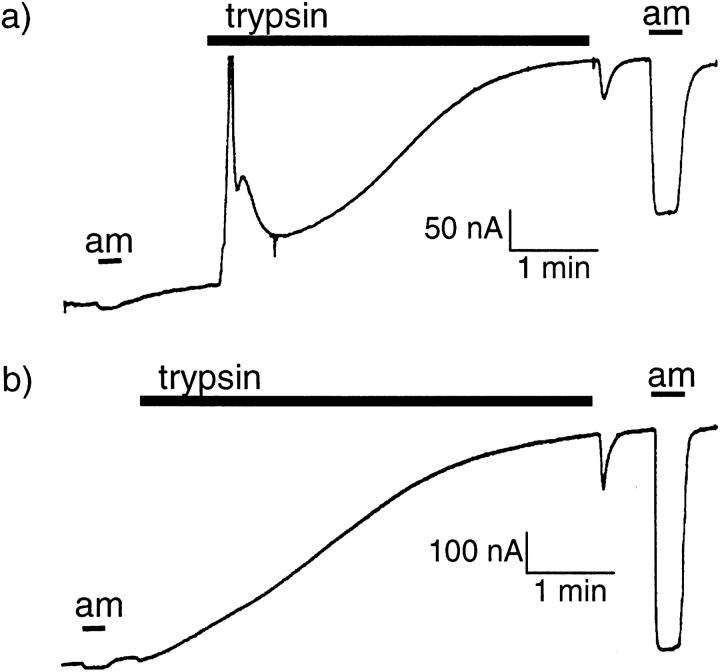
Because of the large variability of the response to trypsin and the large and variable Cl− current induced early after exposure to trypsin, we were not able to establish a relationship between the concentration of trypsin and the initial rate of Na+ current increase. However, to demonstrate that a maximal effect could be obtained by a 3-min exposure to a 2-μg/ml trypsin concentration, we showed that a subsequent 3-min exposure to 50 μg/ml trypsin could not further increase INa (see Fig. 3 a). In addition, in an attempt to observe the inhibitory effect described earlier, we measured INa in oocytes expressing ENaC before and after a 3-min exposure to 10 μg/ml trypsin, a maneuver that induced the usual increase in INa, and then we incubated these oocytes for 30 min in a solution containing 1 mg/ ml trypsin and measured INa again in the same oocytes. This experiment was performed in 18 oocytes expressing Xenopus ENaC and 14 oocytes expressing rat ENaC. In both cases, we failed to observe a decrease of the Na+ current (Fig. 3 b).
Figure 3.
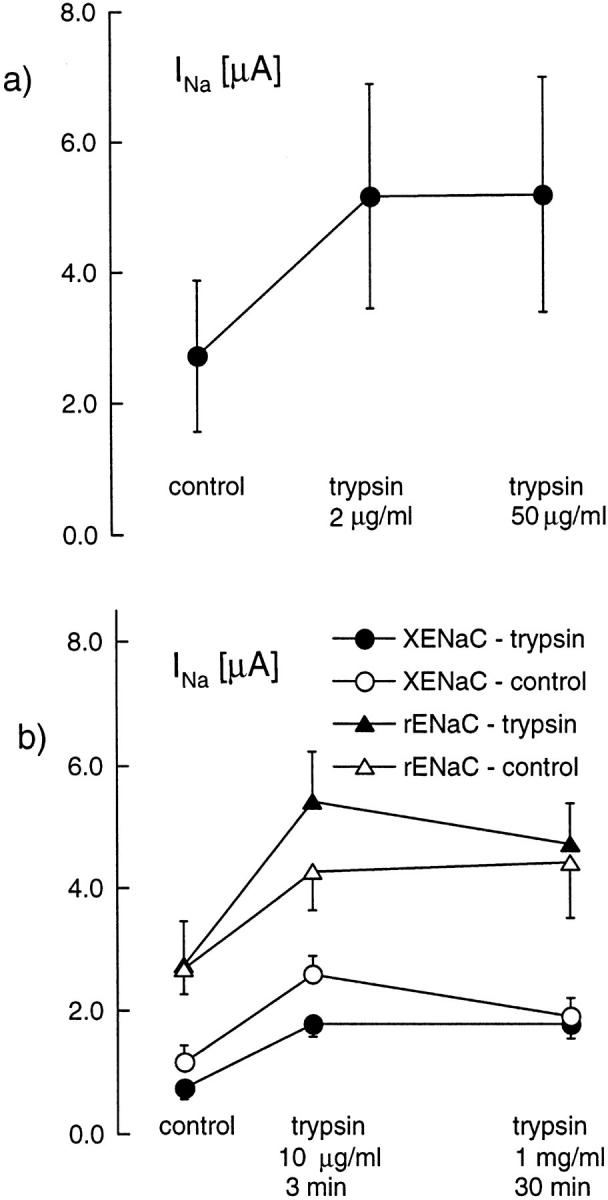
Response to high concentration of trypsin. The amiloride-sensitive current (INa) was measured before exposure to trypsin, first after a 3-min exposure to a 2-μg/ml concentration of trypsin, and then after a 3-min exposure to 50 μg/ml trypsin. Although the low concentration induced a significant increase of INa (P < 005, n = 8, paired student's t test), there was no significant further increase nor decrease of INa after the high concentration of trypsin. The amiloride-sensitive current (INa) was measured before exposure to trypsin, first after a 3-min exposure to a 2 μg/ml concentration of trypsin, and then after a 30-min incubation in a 1 mg/ml-containing bath solution (experimental group, filled symbols) or in the same solution without trypsin (control group, open symbols). Results are shown for oocytes expressing Xenopus ENaC (circles; experimental, n = 18; control, n = 8) and rat ENaC (open squares; experimental, n = 14; control, n = 12). In all groups, the first treatment with trypsin induced a significant increase of INa (P < 0.01 in each case). However, the long exposure to the high concentration of trypsin did not induce any significant further increase or decrease of INa. In the XENaC control group only, there was a small decrease (P < 0.02) of INa after the 30-min incubation in the control medium.
The effect of chymotrypsin on INa was roughly similar to the effect of trypsin. With oocytes expressing Xenopus αβγENaC, a 3-min treatment with 2 or 10 μg/ml induced a mean increase of 1.50 ± 0.08-fold (n = 14) and 2.65 ± 0.36-fold (n = 13), respectively. With rat ENaC, 2 μg/ml chymotrypsin induced a 2.69 ± 0.33-fold increase in INa (n = 13). Remarkably, chymotrypsin never induced the early transient peak of current that was usually observed with trypsin and attributed to a calcium-activated chloride channel.
We also tested the effect of kallikrein on oocytes expressing Xenopus ENaC. Kallikrein, at a concentration of 10 μg/ml and for a 5-min exposure, produced no detectable effect. In fact, INa decrease from 5.9 ± 1.1 to 4.8 ± 1.0 μA, a mean 17% decrease, which corresponds to the usual slow run down of the ENaC current expressed in oocytes (Awayda et al., 1996). Subsequent exposure to 2 μg/ml trypsin induced an increase of INa to 10.2 ± 2.1 μA, showing that the oocytes were indeed able to react to trypsin as usual.
Effect on the Properties of ENaC
To test for the possibility of an alteration of the channel ionic selectivity after trypsin treatment, we measured the amiloride-sensitive current carried by Na+, Li+, or K+ before and after activation by trypsin. No significant amiloride-sensitive inward current at −100 mV could be detected when K+ was the only extracellular monovalent cation before (0.02 ± 0.02 μA, n = 6) or after a 5-min trypsin (2 μg/ml) treatment (0.03 ± 0.03 μA, n = 6). The amiloride-sensitive Na+ channel has been shown to be more conductive for Li+ than for Na+ both in native tissue (Palmer and Frindt, 1988) and as the result of ENaC expression in Xenopus oocyte (Canessa et al., 1994). We measured the amiloride-sensitive current in oocytes expressing αβγXENaC before and after a 3-min exposure to trypsin. In 15 measurements, the mean Na current was 3.4 ± 1.1 μA before and increased to 6.9 ± 1.4 μA after trypsin treatment. The ratio of the amiloride-sensitive current in 100 mM Na+ vs. 100 mM Li+ was 1.51 ± 0.05 before trypsin, and decreased slightly to 1.44 ± 0.05 after trypsin (P < 0.005, paired t test). We also measured the inhibitory constant (KI) of amiloride before and after a 3-min trypsin treatment in oocytes expressing αβγXENaC in the presence of 100 mM Na+, at −100 mV, according to the procedure used earlier (Puoti et al., 1995), using concentrations of 0.1, 1.0, 10, and 50 μM amiloride. In a group of 13 oocytes, INa increased about six times after trypsin treatment (from 1.09 ± 0.56 to 6.49 ± 1.20 μA, P < 0.001), while the amiloride KI did not change significantly (0.55 ± 0.03 μM before vs. 0.49 ± 0.03 μM after trypsin).
Role of Intracellular Calcium
As shown by Durieux et al. (1994), the first component of the response to trypsin, the chloride current, is dependent on a rise of intracellular calcium. To evaluate the role of intracellular calcium on the INa response to trypsin, we injected Xenopus oocytes expressing αXENaC and βγrENaC with 50 nl of 100 mM EGTA or water (control). As shown in the examples of Fig. 4 and in accordance with Durieux et al. (1994), we found that this treatment abolished the transient chloride current, but it did not prevent the increase of the amiloride-sensitive current. In oocytes expressing αβγXENaC, trypsin induced a 5.6 ± 1.2-fold increase (n = 9) in INa 10–20 min after EGTA injection, a value similar to the mean 6.3 ± 1.2-fold increase observed in control oocytes injected with water (n = 7) or noninjected oocytes (n = 4). In 10 oocytes expressing the αXβrγr ENaC and injected with EGTA, trypsin induced a mean 14.5 ± 5.4-fold increase, which was not significantly different from the effect observed without EGTA injection.
Figure 4.
Effect of intracellular EGTA injection. Original current recordings obtained in oocytes expressing αXENaC and βγrENaC. The current sensitive to 10 μM amiloride was measured at −100 mV before and after a 4-min exposure to 2 μg/ml trypsin. In these oocytes, the amiloride-sensitive sodium current (INa) is hardly detectable before trypsin treatment. (a) The recording obtained in a control oocyte injected with 50 nl of water before the electrophysiological measurement; a large transient current is observed almost immediately after trypsin treatment and a >20-fold increase in INa was induced. (b) The recording obtained with an oocyte injected with 50 nl of 200 mM EGTA; no transient Ca-activated Cl− current could be observed, but the activation INa was similar to that observed in the absence of intracellular EGTA.
Relation with Regulation of ENaC through G Protein
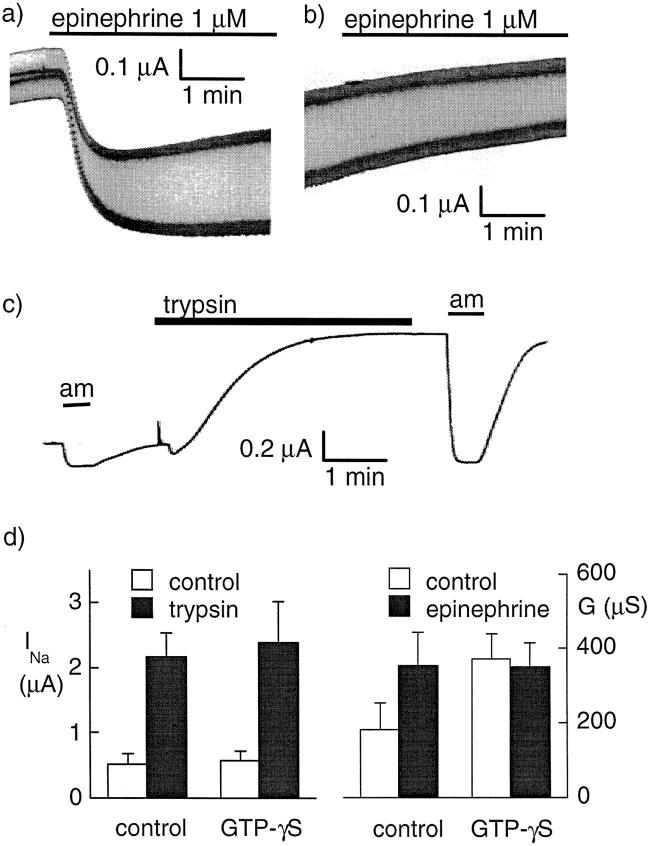
To evaluate the potential role of G protein in the response to trypsin, we followed the amiloride-sensitive current before and after trypsin treatment in oocytes injected with GTP-γS, a nonspecific activator of all G proteins. As a positive control, we coexpressed human CFTR and the human β2 adrenergic receptor and tested for activation of a chloride current by 1 μM epinephrine. Epinephrine induced a fast increase of the conductance in oocytes expressing CFTR and the β2 adrenergic receptor (Fig. 5 a). When oocytes were injected with GTP-γS (0.09 nmol = 50 nl of a 1.8-mM GTP-γS solution) 10–30 min before the electrophysiological measurement, the initial conductance of the oocytes expressing CFTR was larger and the response to epinephrine was abolished (Fig. 5 b), indicating that GTP-γS injection was efficient to activate G proteins. In contrast, when we studied oocytes expressing Xenopus ENaC, the baseline amiloride-sensitive current was similar in the GTP-γS–injected and water-injected groups, and trypsin induced a large increase of the amiloride-sensitive current (Fig. 5 c) similar to that observed in the control group. The mean values of INa before and after trypsin and of the oocyte conductance (G) before and after epinephrine stimulation are shown in Fig. 5 d.
Figure 5.
Effect of G protein stimulation with GTP-γS. (a) Original current recording showing the effect of 1 μM epinephrine on the conductance of an oocyte expressing the β-adrenergic receptor and CFTR. The holding potential was alternating between −40 and −60 mV with a 1 Hz frequency. (b) The same protocol was applied to an oocyte injected 13 min before with 50 nl of a 1.8-mM GTP-γS solution. (c) Effect of trypsin (2 μg/ml for 3.5 min) on the amiloride-sensitive Na+ current (INa) at −100 mV after intracellular injection with GTP-γS (50 nl, 1.8 mM). (d) The effect of a 3-min trypsin treatment (left) on INa (before trypsin, white bars; after trypsin, black bars) in oocytes expressing αβγXENaC. Nine oocytes were injected with GTP-γS, and seven control oocytes were noninjected. The effect of trypsin was not affected by previous GTP-γS intracellular injection. (right) The whole oocyte conductance in oocytes expressing CFTR and the β2-adrenergic receptor before (open bars) and after (filled bars) stimulation with 1 μM epinephrine. In control oocytes (n = 11) (i.e., without previous GTP-γS injection), epinephrine induced an increase of the whole oocyte conductance (P < 0.005, paired t test). Intracellular GTP-γS injection (n = 13) increased the oocyte conductance (P < 0.05, unpaired t test) and completely prevented the effect of epinephrine.
Effect of Trypsin and Channel Density
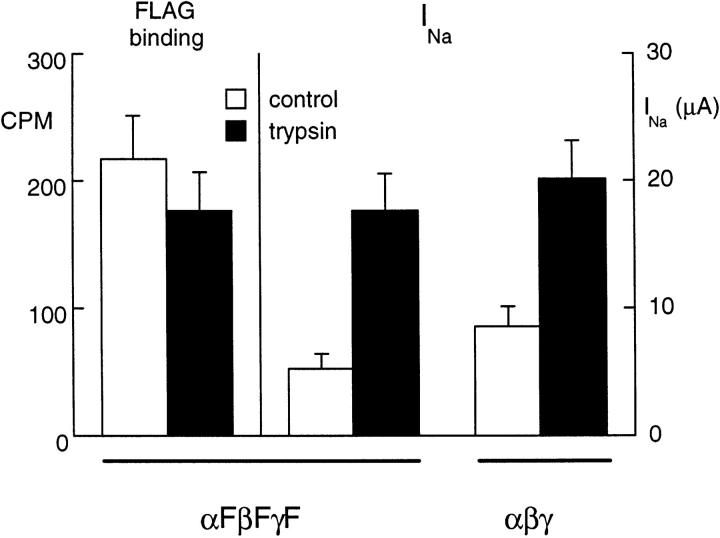
To evaluate the effect of trypsin on the number of channels at the oocyte surface, we used channel mutants in which a FLAG epitope had been added to the extracellular domain of each subunit, and estimated the channel density by binding of iodinated anti–FLAG antibody as described earlier (Firsov et al., 1996). Anti– FLAG antibody binding was measured after a 10-min exposure to trypsin (5 μg/ml) and in control oocytes not exposed to trypsin. At the same time, the amiloride-sensitive current was measured in two parallel groups of oocytes exposed to the same treatment. The results of specific anti–FLAG binding and the amiloride-sensitive current with or without trypsin treatment (shown in Fig. 6) indicate that trypsin does not induce a modification of the channel density at the cell surface. Therefore, the increase in INa is not due to translocation of channels from an intracellular pool to the surface, and so the increase in current must be due either to an increase in single channel conductance or to a modification of the open probability.
Figure 6.
Effect of trypsin on channel density. The channel density estimated by anti–FLAG antibody in oocytes injected with αF, βF, and γF ENaC cRNA (F indicates insertion of a FLAG epitope) was not modified by a 10-min exposure to trypsin (control group, n = 43; trypsin group, n = 44), indicating no effect of trypsin on the surface channel density, while the amiloride-sensitive Na current (INa) increased more than threefold in a parallel group of oocytes (P < 0.01, n = 24). For comparison, the effect of trypsin was tested in a group of oocytes injected with wild-type (without FLAG epitope) αβγ rat ENaC in which there was no specific anti– FLAG antibody binding. Trypsin had a similar effect on INa in oocytes expressing the channel with FLAG epitope (middle columns) and in the wild-type channel (right columns).
Effect of Trypsin at the Single Channel Level
The effects of trypsin on the single channel current were examined in Xenopus oocytes expressing ENaC using the cell-attached mode and excised patches in the outside-out configuration. Cell-attached patches were obtained using pipettes filled with a 100-mM LiCl solution with or without 5 μg/ml trypsin. Under these conditions, typical Xenopus ENaC single channel currents (8 pS inward current) were observed in 7 of 16 successful patches (44%) in the absence of trypsin and in 8 of 11 successful patches (73%) in the presence of trypsin. This difference is marginally significant (P < 0.05 by χ2 test). Analysis of open probability did not show obvious differences between the two groups, a fact that is not surprising considering the very large spontaneous variation in open probability observed with ENaC (Palmer and Frindt, 1988, 1996). To confirm the activation of the channel by trypsin, we took advantage of the observation, described above, that the heteromer composed of the α subunit of Xenopus and the β and γ subunits of rat ENaC (αXβrγr) showed a much larger increase after trypsin treatment than either the rat or Xenopus αβγENaC. Channels were observed in 8 of 12 successful patches (67%) when the pipette contained trypsin, but only in 1 of 16 successful patches (6%) without trypsin (P < 0.001). There was no difference in single channel conductance between any of these groups. The increase in active channel density observed in these experiments is consistent with those found in macroscopic current (see Table I).
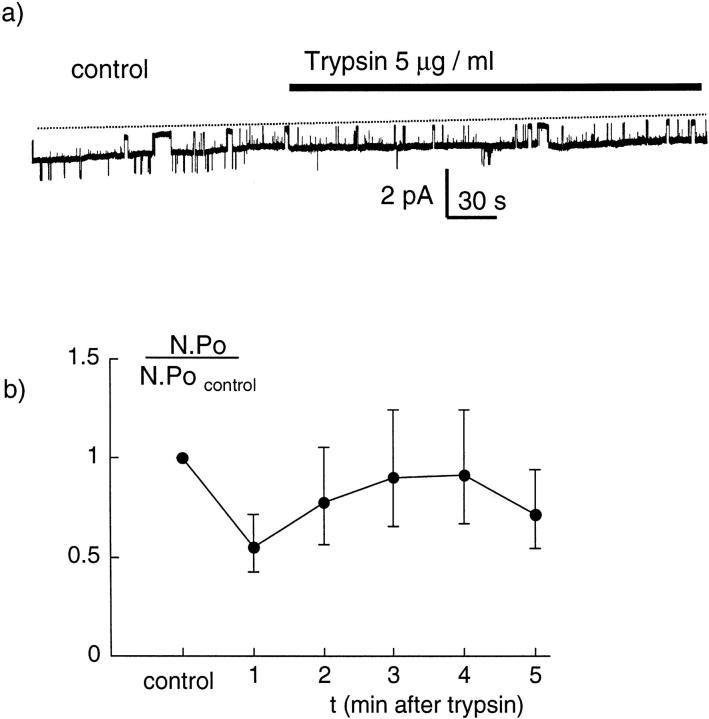
Another series of experiments was carried out to test the possibility that trypsin could activate the sodium channel indirectly, for instance through the diffusion of a soluble intracellular second messenger. Patches in the cell-attached configuration were obtained with LiCl solution–containing pipettes. The channel activity was recorded for 2–5 min under control conditions, and then 5 μg/ml trypsin was added to the bath solution surrounding the pipette and the oocyte. A representative trace is shown in Fig. 7 a. Although neither the absolute number of channels nor the open probability could be determined accurately, there was no obvious increase in channel activity after perfusion with trypsin. The mean values of the n.Po before and during exposure to trypsin are given in Fig. 7 b. Together with the results obtained with trypsin in the pipette, these results suggest that the effect of trypsin is local (i.e., by action of trypsin on the channel itself or on a closely associated protein) and is not mediated by a soluble intra-cellular second messenger.
Figure 7.
Effect of oocyte superfusion with trypsin on the Na+ channel activity. (a) Original channel recording showing the activity of Na+ channels in a cell-attached patch. At the time indicated by the bar, a solution containing 5 μg/ml trypsin was perfused around the oocyte. Trypsin did not induce any evident change in the channel activity. The pipette was filled with a solution containing 100 mM LiCl and the pipette potential was +100 mV. A downward deflection indicates current flowing from the pipette into the cell. The closed-channel level is indicated by the dotted line. (b) Mean Na+ channel activity expressed as n.Po in the absence and presence of the trypsin in the bath. Because of the large range and the highly asymmetrical distribution of the n.Po values, the mean n.Po was calculated as exp(mean(log(n.Po))). The control value is the mean n.Po over a 3-min period before trypsin and during each minute after superfusion with trypsin. The number of measurements is 16 for the control and 1-min values, and between 15 and 11 for the 2–5-min values.
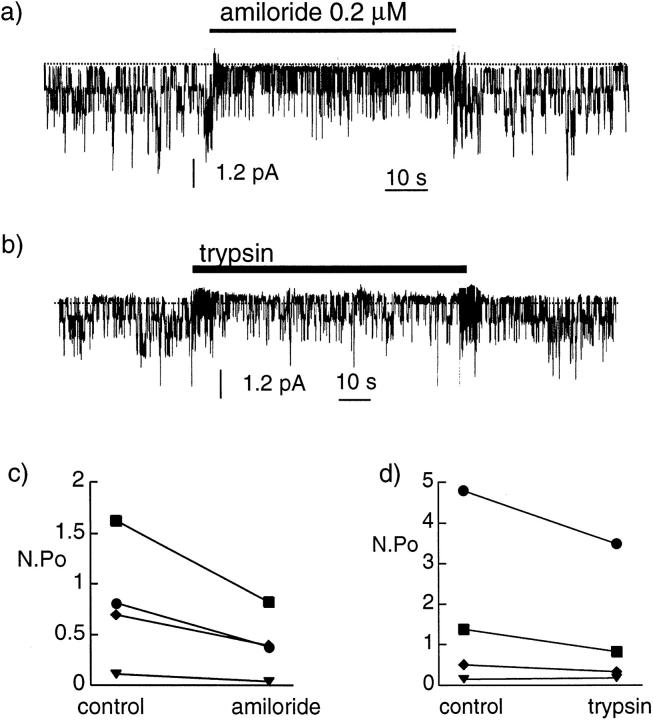
We have also tested the effect of extracellular trypsin in the excised patch, outside-out configuration, with patches obtained on oocytes expressing Xenopus αβγENaC. For these experiments, the pipette solution had the following composition (mM): 20 KCl, 80 K gluconate, 2 EGTA, 10 Na HEPES, pH 7.4, and the bath solution was a 100-mM LiCl solution, buffered to pH 7.4 with 10 mM Na HEPES. Under these circumstances, we could observe inward single channel currents, carried by lithium, at a holding potential Vpip of −100 mV. Most often after patch excision, there was either no channel activity or a rapidly running down channel activity, but in a few cases a stable channel activity was observed. In four such cases, the identity of the channel could be first verified by the effect of low concentration of external amiloride: 0.2 μM amiloride induced a reversible decrease of n.Po to 45 ± 6% of its initial value (Fig. 8), a decrease compatible with the KI 0.6 μM observed for Xenopus ENaC in macroscopic current experiments (Puoti et al., 1995). Trypsin (5 μg/ml) added to the extracellular side of the membrane did not induce any increase of the activity of ENaC (n.Po control 1.7 ± 1.0 vs. n.Po trypsin 1.2 ± 0.8, n = 4).
Figure 8.
Effect of extracellular trypsin on Na+ channel activity in outside-out excised patches. (a) In this original recording, amiloride (200 nM) added to the bath (extracellular side) solution, reduced the activity of a single Na+ channel, an effect that was quickly reversible. (b) With the same patch, trypsin (5 μg/ml) added to the external solution did not produce any obvious increase either in the single channel conductance or in the channel activity. The pipette was filled with a K+ gluconate solution and the bath solution contained 100 mM LiCl solution. The pipette potential was −100 mV. A downward deflection indicates a current flowing into the pipette and the closed-channel level is indicated by the dotted line. (c and d) The n.Po values in four patches in which recordings were obtained before, during, and after exposure to amiloride (c) and before and during exposure to trypsin (d) are shown.
discussion
In contrast to several earlier reports indicating an inactivating effect of proteases on the epithelial sodium channel (Garty and Edelman, 1983; Lewis and Alles, 1986; Lewis and Clausen, 1991), we have observed that trypsin and chymotrypsin induce a significant activation of the sodium or lithium current carried by ENaC expressed in Xenopus oocytes. The stimulatory effect of proteases was obtained with much lower concentrations of trypsin than those used in the experiments reported by Garty and Edelman (1983) or Lewis et al. (1991). Even using a large concentration of trypsin for a long time, we failed to observe a decrease of the amiloride-sensitive Na+ current, suggesting a direct proteolytic degradation of the channel. The different experimental model, native tight epithelia versus Xenopus oocytes artificially expressing ENaC, might be the reason for this discrepancy. In the oocyte, the expressed channel might be in a different conformation or be missing a protein component distinct from the α, β, and γ subunits, a component that might be necessary for the sensitivity to the inhibitory effect of trypsin. Another difference is the species: we used rat and Xenopus ENaC, while the results published by Garty and Edelman (1983) and Lewis et al. (1991) were obtained with Bufo marinus and rabbit tissues; minor sequence differences might determine the presence or absence of a proteolytic site. This hypothesis is supported by the observation of Jungwirth et al. (1991), who also failed to observe stimulatory effect of trypsin or kallikrein on the transepithelial potential of rat distal convoluted tubules.
The observation of a stimulatory effect of trypsin of the Na+ current mediated by ENaC goes in the same direction as the observation of Orce et al. (1980) showing that inhibition by aprotinin of a proteolytic activity in the apical medium from toad urinary bladder led to a reduced transepithelial sodium transport. The demonstration of the presence of a “kallikreinlike” protease activity in the apical medium of A6 cells grown on permeable support (Jovov et al., 1990) could well provide the physiological equivalent in a native epithelia of what we have observed with trypsin and Na+ channels expressed in Xenopus oocytes. This hypothesis has received strong support from the recent identification of an endogenous serine protease naturally expressed in A6 cells that is able to activate INa when coexpressed with ENaC in oocytes and the observation that trypsin also activates the electrogenic transepithelial Na+ transport in A6 cells provided that the endogenous protease had first been inhibited (Vallet et al., 1997). The experiments presented here give some first indications about the mechanism by which a protease active from the extracellular side of the membrane might activate the Na+ channel.
The Effect of Trypsin Does Not Occur through G Protein-mediated Increase in cAMP or Intracellular Calcium
Trypsin is known to produce a transitory activation of calcium-activated chloride current, an effect probably mediated by the activation of a G protein-coupled trypsin receptor naturally present at the surface of the native oocyte, followed by activation of phospholipase C, and IP3-mediates release calcium from endogenous calcium stores (Durieux et al., 1994). Several observations indicate that the activation of the sodium current is not due to the same mechanism. First, the chloride current can be prevented by intracellular calcium buffering, while the increase of the amiloride-sensitive current is not affected by this maneuver. Second, no detectable transient chloride currents were observed after chymotrypsin treatment, suggesting that this enzyme was not able to activate the endogenous oocyte trypsin receptor while it produced a large increase in the Na+ current with both Xenopus and rat ENaC. We therefore conclude that the ENaC response to proteases is not related to the presence of an oocyte-endogenous trypsin receptor, but is mediated by a different mechanism than that responsible for the calcium-activated chloride current response.
In amphibian and mammalian tight epithelia, the amiloride-sensitive Na+ transport is stimulated by cAMP (Schafer and Hawk, 1992; Verrey, 1994) and this is the main mechanism by which vasopressin can increase the transepithelial Na+ transport. It was tempting to make the hypothesis that trypsin stimulation of INa in Xenopus oocytes occurred through the same mechanism, especially considering the similar time course of the responses (compare for instance our data with the results obtained by Verrey, 1994, on A6 cells), but several observations indicate that this is not the case. First, INa, due to ENaC expression in Xenopus oocytes, does not appear to be stimulated by an increase of cAMP production (Awayda et al., 1996). Second, we could not modify the response to trypsin by a pretreatment with GTP-γS, which is a strong, although indirect, indication that no G proteins are involved in the response of ENaC to trypsin.
Finally, the observation of the effect of trypsin on single Na+ channel activity, in the “cell-attached” configuration, when trypsin was applied inside, but not outside, the pipette, indicated that the effect of trypsin is a “local” phenomenon; i.e., a mechanism that involves protein(s) that can be present together with the channel within the ∼1 μm2 of membrane area included in a patch and probably does not include the diffusion of a soluble second messenger.
All these data taken together indicate clearly that the effect of trypsin on the sodium current carried by ENaC is not mediated by the “classical” pathway of receptor binding, G protein activation, second messenger production, protein kinase activation, or channel phosphorylation.
Hypothetical Mechanism of Action of Trypsin on ENaC
The similar effects of two different serine proteases on the Na+ channel activity suggest that this effect is due to a proteolytic event. The abolishment of the effect of trypsin by soybean trypsin inhibitor is also compatible with this hypothesis. What might be the substrate of this proteolytic action?
The earlier published observations of an inhibitory effect of trypsin and other proteases on the epithelial Na+ channel had demonstrated important modifications of the channel properties: change in selectivity (Lewis and Alles, 1986; Lewis and Clausen, 1991) or inability to further respond to aldosterone (Garty and Edelman, 1983). In addition, the effect of proteases could be prevented by the presence of an amiloride molecule in its binding site (Garty and Edelman, 1983; Schlichter et al., 1986). All these observations clearly supported the hypothesis that the channel itself (i.e., one of its protein components) was the substrate of the protease. Unfortunately, the situation is less clear concerning the stimulatory effect of trypsin. Although our results are all compatible with a direct effect of trypsin on one or several of the ENaC subunits, we could not find any direct evidence supporting this hypothesis. The effect of trypsin on the Na+/Li+ selectivity ratio is compatible with a modification of the selectivity filter of the channel, to which the “pre-M2” segment of each subunit seems to contribute (Schild et al., 1997). However, the observed change, although statistically significant, is of such a small amplitude that we could not convince ourselves that this observation is a reliable proof of the direct action of the protease on the channel protein. The absence of modification of the Na+/ K+ selectivity and of the affinity to amiloride does not support a direct effect. Sodium channel activation by trypsin could not be blocked or even slowed down by a concentration of amiloride sufficient to occupy >95% of the channels. However, our patch clamp data clearly indicate a local effect. Therefore, the substrate of trypsin, if it is not the channel protein itself, is probably a closely associated protein.
Although the effect of trypsin could be observed when applied inside a patch pipette in the cell-attached mode, no effect of trypsin could be detected in excised outside-out patches. These observations should be interpreted with care because, due to the “run down” phenomenon, only a small fraction of Na+ channels remain active in excised patches, and this fraction may not be representative of the whole channel population; they nevertheless suggest that, although the proteolytic action of trypsin is most probably extracellular, the activation process includes some intracellular component that is disturbed by the patch excision procedure. This situation is somewhat similar to that observed with the effect of vasopressin on the Na+ channel density that could well be observed when the hormone was applied before a patch was obtained, but not when vasopressin was added after the formation of a seal (Marunaka and Eaton, 1991). However, in contrast with the well supported hypothesis of channel insertion for the response to vasopressin (Garty and Palmer, 1997), our data obtained using FLAG insertion mutants and anti– FLAG antibody binding do not support a significant change of surface channel density to explain the large increase in Na+ current density. Since the single channel conductance was not modified, the effect of trypsin must be due to an increase of the proportion of open channels in the total population of channels present at the cell surface. Our data do not allow us to determine whether this increase results from a change in the open probability of already active channels or from recruitment out of a pool of completely silent channels. The large increase that is often observed after trypsin treatment indicates that the overall open probability of all the channels present at the oocyte surface must be very low. As the mean open probability of active channels observed in cell-attached patches on oocytes expressing ENaC is often of the order of 0.5 (Canessa et al., 1994), our results suggest that there may be a significant fraction of “silent” channels that are not seen in patch clamp experiments, but are present at the oocyte surface and can be activated by proteolytic treatment. A similar conclusion was reached by Firsov et al. (1996) using quantitative measurements of cell surface channel protein expression. The inverse relationship between the initial INa and the effect of trypsin (see Fig. 2) could be explained if there was a variable degree of “spontaneous” activation, possibly by an oocyte-endogenous protease. In oocytes with a low degree of spontaneous activation, trypsin would reveal a large portion of silent channels while, in oocytes with a high level of spontaneous activation, trypsin could only activate a small portion of silent channels.
In summary, trypsin or chymotrypsin can induce a large activation of the sodium channel obtained by expression of the α, β, and γ subunits of the rat or Xenopus ENaC in Xenopus oocytes. This effect is due to an increase of the open probability of channels already present at the oocyte surface, through a proteolytic action of the enzyme on the channel protein itself or a closely associated component.
Acknowledgments
We are grateful to L. Schild, B. Rossier, and S. Cotecchia for critical reading of the manuscript and for helpful suggestions.
This work was supported by the Human Frontier Science Program, grant RG-0464.
Footnotes
Abbreviations used in this paper: CFTR, cystic fibrosis transmembrane conductance regulator; ENaC, epithelial Na+ channel; INa, amiloride-sensitive Na+ current.
references
- Awayda MS, Ismailov II, Berdiev BK, Fuller CM, Benos DJ. Protein kinase regulation of a cloned epithelial Na+ channel. J Gen Physiol. 1996;108:49–65. doi: 10.1085/jgp.108.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Horisberger J-D, Rossier BC. Functional cloning of the epithelial sodium channel: relation with genes involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thorens B, Gautshi Y, Horisberger J-D, Rossier BC. The amiloride-sensitive epithelial sodium channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Durieux ME, Salafranca MN, Lynch KR. Trypsin induces Ca2+-activated Cl− currents in X. laevisoocytes. FEBS Lett. 1994;337:235–238. doi: 10.1016/0014-5793(94)80198-3. [DOI] [PubMed] [Google Scholar]
- Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome—a quantitative approach. Proc Natl Acad Sci USA. 1996;93:15370–15375. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H, Edelman IS. Amiloride-sensitive trypsinization of apical sodium channels. Analysis of hormonal regulation of sodium transport in toad bladder. J Gen Physiol. 1983;81:785–803. doi: 10.1085/jgp.81.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels— function, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp technique for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Jovov B, Wills NK, Donaldson PJ, Lewis SA. Vectorial secretion of a kallikrein-like enzyme by cultured renal cells. I. General properties. Am J Physiol. 1990;259:C869–C882. doi: 10.1152/ajpcell.1990.259.6.C869. [DOI] [PubMed] [Google Scholar]
- Jungwirth A, Lewis S, Lang F. Kallikrein does not modify the transepithelial potential of rat renal distal convoluted tubules. Nephron. 1991;58:225–228. doi: 10.1159/000186419. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Alles WP. Urinary kallikrein: a physiological regulator of epithelial Na absorption. Proc Natl Acad Sci USA. 1986;83:5345–5348. doi: 10.1073/pnas.83.14.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SA, Clausen C. Urinary proteases degrade epithelial sodium channels. J Membr Biol. 1991;122:77–88. doi: 10.1007/BF01872741. [DOI] [PubMed] [Google Scholar]
- Marunaka Y, Eaton DC. Effects of vasopressin and cAMP on single amiloride-blockable Na channels. Am J Physiol. 1991;260:C1071–C1084. doi: 10.1152/ajpcell.1991.260.5.C1071. [DOI] [PubMed] [Google Scholar]
- Methfessel C, Witzemann V, Takahashi T, Mishina M, Numa S, Sakmann B. Patch clamp measurements on Xenopus laevis oocytes: currents through endogenous channels and implanted acetylcholine receptor and sodium channels. Pflügers Arch. 1986;407:577–588. doi: 10.1007/BF00582635. [DOI] [PubMed] [Google Scholar]
- Orce GG, Castillo GA, Margolius HS. Inhibition of short-circuit current in toad urinary bladder by inhibitors of glandular kallikrein. Am J Physiol. 1980;239:F459–F465. doi: 10.1152/ajprenal.1980.239.5.F459. [DOI] [PubMed] [Google Scholar]
- Palmer LG, Frindt G. Conductance and gating of epithelial Na channels from rat cortical collecting tubule. J Gen Physiol. 1988;92:121–138. doi: 10.1085/jgp.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer LG, Frindt G. Gating of Na channels in the rat cortical collecting tubule: effects of voltage and membrane stretch. J Gen Physiol. 1996;107:35–45. doi: 10.1085/jgp.107.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puoti A, May A, Canessa CM, Horisberger J-D, Schild L, Rossier BC. The highly selective low conductance epithelial Na channel of Xenopus laevisA6 kidney cells. Am J Physiol. 1995;38:C188–C197. doi: 10.1152/ajpcell.1995.269.1.C188. [DOI] [PubMed] [Google Scholar]
- Schafer JA, Hawk CT. Regulation of Na+channels in the cortical collecting duct by AVP and mineralocorticoids. Kidney Int. 1992;41:255–268. doi: 10.1038/ki.1992.37. [DOI] [PubMed] [Google Scholar]
- Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the α, β, and γ subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichter L, Sidell N, Hagiwara S. K channels are expressed early in human T-cell development. Proc Natl Acad Sci USA. 1986;83:5623–5629. doi: 10.1073/pnas.83.15.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet V, Chraibi A, Gaeggeler H-P, Horisberger J-D, Rossier BC. An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature. 1997;389:607–610. doi: 10.1038/39329. [DOI] [PubMed] [Google Scholar]
- Verrey F. Antidiuretic hormone action in A6 cells: effect on apical Cl and Na conductances and synergism with aldosterone for NaCl reabsorption. J Membr Biol. 1994;138:65–76. doi: 10.1007/BF00211070. [DOI] [PubMed] [Google Scholar]
- Zhou H, Tate SS, Palmer LG. Primary structure and functional properties of an epithelial K channel. Am J Physiol. 1994;35:C809–C824. doi: 10.1152/ajpcell.1994.266.3.C809. [DOI] [PubMed] [Google Scholar]
- Zweifach A, Lewis SA. Characterization of a partially degraded Na+ channel from urinary tract epithelium. J Membr Biol. 1988;101:49–56. doi: 10.1007/BF01872819. [DOI] [PubMed] [Google Scholar]