Abstract
Cells use polar molecules in the membrane to sense changes in the transmembrane potential. The opening of voltage-gated ion channels and membrane bending due to the inverse flexoelectric effect are two examples of such electromechanical coupling. We have looked for membrane motions in an electric field using atomic (or scanning) force microscopy (AFM) with the intent of studying voltage-dependent conformational changes of ion channels. Voltage-clamped HEK293 cells were either untransfected controls or transfected with Shaker K+ channels. Using a ± 10-mV peak–peak AC carrier stimulus, untransfected cells moved 0.5–15 nm normal to the plane of the membrane. These movements tracked the voltage at frequencies >1 kHz with a phase lead of 60–120°, as expected of a displacement current. The movement was outward with depolarization, but the holding potential only weakly influenced the amplitude of the movement. In contrast, cells transfected with a noninactivating mutant of Shaker K+channels showed similar movements, but these were sensitive to the holding potential; decreasing with depolarization between −80 and 0 mV. Searching for artifactual origins of these movements, we used open or sealed pipettes and AFM cantilever placements just above the cells. These results were negative, suggesting that the observed movements were produced by the cell membrane rather than by movement of the patch pipette, or by acoustic or electrical interactions of the membrane with the AFM tip. In control cells, the electrical motor may arise from the flexoelectric effect, where changes in potential induce changes in curvature. In transfected cells, it appears that channel-specific movements also occurred. These experiments demonstrate that the AFM may be able to exploit voltage-dependent movements as a source of contrast for imaging membrane components. The electrically induced motility will cause twitching during action potentials, and may have physiological consequences.
Keywords: scanning microscopy, K channels, gating, flexoelectric, mechanical
introduction
Many membrane proteins transduce chemical, optical, mechanical, and electrical signals. Specific components undergo structural changes to provide an output signal such as the activation of nucleotide binding proteins or the opening of ion channels. The membrane itself, particularly in connection with the underlying cytoskeleton, is also sensitive to mechanical or electrical stimuli. Changes in the transmembrane electric field may cause changes in the conformation of the lipid bilayer or polar molecules embedded in the bilayer. Transmembrane electric fields can induce changes in curvature of simple black-lipid membranes (Todorov et al., 1994). While transduction of mechanical inputs into electrical outputs are quite common for channels (see Guharay and Sachs, 1984), the reverse process is rarely observed outside auditory outer hair cells (Ashmore, 1989). However, there is a wide range of experiments that indirectly demonstrate conformational changes of voltage-sensitive ion channels during electrical stimulation (see Mannuzzu et al., 1996; Yang et al., 1996).
Voltage-gated ion channels sense the transmembrane electric field using a highly charged transmembrane segment called S4 (Perozo et al., 1993; Bezanilla and Stefani, 1994). Using fluorescently labeled cysteine-substituted amino acids in the S4 region of Shaker potassium channels, Mannuzzu et al. (1996) showed that depolarization caused a region of more than seven residues to move outward and become exposed to the extracellular space. Similar results have been obtained for Na channels using cysteine scanning of S4 to assess accessibility to extracellular sulfhydryl reagents (Yang and Horn, 1995; Larsson et al., 1996).
Similar results were obtained by French et al. (1997) using a more indirect method. They examined the interaction between sodium channels and a positively charged μ-conotoxin derivative whose binding caused a voltage-dependent shift in the activation curve. Electrostatic calculations suggested that this data could be accounted for if the S4 segment moved outward ≈0.5 nm during the field-sensing transition.
The estimated size of these gating movements lies well within the height resolution range of atomic (or scanning) force microscopes (AFMs)1 (Binnig et al., 1986). In these instruments, a flexible cantilever with a final tip diameter of ∼20 nm is used to detect height differences of surfaces with high lateral resolution. The height resolution is only limited, in principle, by the thermal noise of the cantilever, and in practice can reach <0.1 nm on biological membranes under physiological conditions (Häberle et al., 1992). With a cluster of ion channels in contact with the cantilever tip, a concerted outward movement of S4 segments could push the cantilever away from the cell. This displacement should only occur in the voltage range where the voltage sensors are expected to move, thereby providing a measure of specificity for the detected signal.
In previous experiments, we found mechanical responses of excised membrane patches to voltage pulses (Mosbacher et al., 1996). But those records were made from membrane vesicles where the electric field distribution was unclear. Additionally, those excised patches were not well supported by the cytoskeleton, so the mechanical noise level was relatively high. In the current experiments, our goal was to investigate electromechanical transduction of ion channels in intact cells. Much to our surprise, we found voltage-dependent movements in untransfected control cells (HEK293), as well as those transfected with Shaker K+ channels. Given the unexpected nature of the control response, much of our effort was diverted from measurements of the transfected cells to the response of controls. Additional detailed studies of the transfected cells are in progress.
materials and methods
Electrophysiology
HEK293 cells were plated on glass coverslips 3 d before recording and some were transiently transfected with a noninactivating (Δ 1–60) mutant of the Shaker H4 potassium channel (a kind gift of F. Bezanilla, University of California, Los Angeles) as described previously (Burnashev et al., 1995). Green fluorescent protein was cotransfected to signal expression of individual cells. 3–4 d after transfection, cells were placed in a recording chamber and superfused with Normal Rat Ringer containing 135 mM NaCl, 5.4 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM HEPES, pH = 7.2 (NaOH). Thick-wall borosilicate glass pipettes filled with a solution containing 140 mM KCl, 10 mM HEPES, 10 mM EGTA, 2 mM MgCl2, 2 mM Na-ATP, pH 7.3 (KOH), had tip resistances between 1 and 3 MΩ. Cells were clamped in whole-cell mode, lifted from the coverslip, and placed in front of the AFM cantilever (see Fig. 1 A). Only cells with a seal resistance of >2 GΩ were used for quantitative experiments to be sure that the applied electric field dropped primarily across the cell membrane. Since it took ∼20 s to record a power spectrum of cantilever displacements, cells were held at each potential for ∼30 s when recording the effects of the holding potential (Vh).
Figure 1.
A shows a schematic top view drawing (not to scale) of the set up. The patch pipette holding the HEK cell was attached to the tubular piezo ceramic used for x, y, z scanning. The holding potential, Vh, and the AC carrier voltage were applied to the cell by the patch-clamp amplifier. The cantilever movement was translated into a voltage, Vdet, by the laser and quadrant detector. The output was proportional to the height difference Δh of the surface. In B, force–distance plots are shown for a cantilever approaching glass and an (untransfected) HEK293 cell. Forward and backward movements of the cantilever are indicated by arrows. C shows a calibration curve for the cantilever. The piezo holding a sealed, stiff pipette was moved over a defined distance with an AC signal, and the corresponding peak of the PSD was the displacement measured. A linear regression line gave a slope of 16 μVrms/√ Hz)/nm. A PSD of a typical experiment is shown in C. The (transfected) cell was held at Vh = −60 mV and an AC voltage of ± 10 mV at 66 Hz (vertical arrow) was applied by the patch amplifier. The corresponding peak in the PSD of the detector signal had an amplitude of 2.8 nm. E shows a PSD without an electrical stimulus (same cell). F shows the effect of restoring the AC carrier but placing the cantilever tip just above the surface of the cell.
Electrophysiological recordings were carried out using an EPC 7 patch-clamp amplifier, driven by a sine wave generator (Hameg, Frankfurt, Germany). The EPC 7 output was followed by a low pass eight pole Bessel filter (Frequency Devices Inc., Haverhill, MA) set to either 10 or 1 kHz. Data were digitized using ITC 16 (Instrutech Corp., Great Neck, NY) and analyzed using Pulse++ (Ulix, Tuebingen, Germany) and Igor (Wavemetrics, Lake Oswego, OR) software.
AFM
The AFM we used was described in detail in a previous publication (Hoerber et al., 1995). To calibrate the AFM height sensitivity, we used an in situ measurement. For each cantilever, a power spectrum of the freely moving cantilever was recorded in air and under physiological buffer before the experiment. The spring constant of the cantilever was calculated according to Hutter et al. (1993) after calibrating the sensitivity of the quadrant photodiode by displacing the cantilever with a sealed pipette for a known distance. The voltage-displacement relation of the piezo was calibrated with a Michaelson interferometer and checked optically by measuring the distance that a motor-driven micromanipulator had to move to compensate for the displacement of the piezo. The sensitivity of the photodiode was ≈100 μVrms/nm, and the calculated spring constants were either 0.008–0.009 or 0.017– 0.018 N/m, corresponding closely to the values provided by the manufacturer (0.01 N/m for the cantilever with 320 μm length and 0.02 N/m for the cantilever with 220 μm length; Park Scientific Instruments, Sunnyvale, CA). All cantilevers were taken from one wafer to minimize variations in the spring constant.
To increase the height resolution, the x/y scan of the AFM was stopped and we measured height differences at a single location using sine wave voltage stimulation to permit narrow band detection of the movement. This allowed us to detect signals above the low frequency noise caused by mechanical instabilities of the set-up. The phase shift of the signal relative to the stimulus provided information about the possible nature of the mechanical responses.
Displacement data were either stored by an HP34570 spectrum analyzer (Hewlett Packard Co., Palo Alto, CA) on floppy disk or read out from a lock-in amplifier (Stanford Scientific Instruments, Stanford, CA). The power density spectra were calculated with the spectrum analyzer usually set to a span of 800 Hz and a frequency resolution of 3.6 Hz. Typically, 20 spectra were averaged to produce the output.
results
A voltage-clamped cell was lifted from the cover slip by the clamping pipette and pressed gently against the fixed cantilever of the AFM (for a schematic overview, see Fig. 1 A). The loading force was set low (0.5–3 nN) so the force–distance curve was essentially linear over ∼100 nm (Fig. 1 B). The pipette was attached to a tube-type piezo translator that provided x, y, and z displacements. Before each experiment, the sensitivity of the detection system was checked by placing the patch pipette in contact with the cantilever, which was driven a known distance with a sine wave. From the amplitude of the relevant frequency of the power spectral density (PSD) of the detector output (Fig. 1 C), we could measure the deflection sensitivity.
A typical spectrum from our experiments on transfected cells is shown in Fig. 1 D. At a holding potential of −60 mV, stimulation of the cell with a ± 10-mV peak–peak AC carrier signal induced a narrow-band peak, Vdet, at the same frequency, corresponding to a cantilever movement of ∼3 nm. The carrier was set at 66 Hz to avoid the line frequency of 50 Hz and was usually located in a low noise region of the background noise spectra. (In what follows, all movement amplitudes are noted as the peak–peak values.) No movement was detectable at this frequency when (a) the AC command voltage was turned off (Fig. 1 E), (b) the membrane-tip contact was released (Fig. 1 F), or (c) the cell was removed and the cantilever touched the tip of the open patch pipette (not shown). Therefore, we concluded that acoustic coupling to the cantilever, electromagnetic induction, or piezo-electric behavior of the patch pipette were not the origin of the observed signal.
Assuming the movement did arise from the membrane itself, we examined its properties. First, we found an almost linear dependence of the movement on the carrier voltage amplitude, ≈0.4 nm/mV, over the range of ± 50 mV (as far as we examined, data not shown). We observed an inverse relationship between membrane conductance and movement, suggesting that current-driven electroosmotic effects were not responsible for the movement. With loose seals of ≈50 MΩ, we were only able to detect movement using large AC voltages, >∼25 mV, as expected from the ratio of patch to total current.
Frequency Dependence
We tested the frequency dependence of the movement at a fixed holding potential. To examine the extent of viscoelastic coupling of any local pipette movements to the cantilever, we induced a second detector signal by moving the patch pipette axially with the piezo element. Fig. 2 A shows this experiment. The AC voltage stimulating the cell was kept at 66 Hz (±15 mV) while the patch pipette movement (±11 nm) was varied between 80 and 700 Hz. The movement of the membrane induced by movement of the patch pipette was damped. Even with frequencies as low as 84 Hz, a peak-to-peak movement of only 2 nm was detectable. The cantilever movement induced by shaking the patch pipette dropped by two thirds between 84 and 700 Hz and vanished completely above 1,000 Hz, while the simultaneously recorded electrical motility remained essentially constant in amplitude. In contrast, Fig. 2 B shows that the electrically induced movement acted over a wider bandwidth, being independent of frequency up to 1,000 Hz. The system reached its resonance frequency at ∼1,500 Hz, above which the signal as well as the noise level dropped significantly. (In this part of the experiment, the pipette movement was fixed at 66 Hz. Because of the increased bandwidth of the stimulus during the experiment shown in Fig. 2 B, the spectrum analyzer range had to be increased, causing an equivalent decrease in the frequency resolution. This caused the 66 Hz peak to appear broader and shorter.)
Figure 2.
The voltage-induced movements had a higher bandwidth than did movements coupled from movement of the patch pipette. In A, a series of spectra are superimposed that show the frequency dependence of cantilever movement induced by 11-nm oscillations of the patch pipette (± 5 mV sinusoidal stimulation applied to the piezo). The cell was simultaneously voltage clamped with ± 30 mV at 66 Hz and Vh = −45 mV (untransfected cell). For comparison, B shows several superimposed spectra where the frequency of the voltage clamp carrier was changed while the movement of the clamping pipette was kept at 66 Hz. This series was recorded subsequent to the one in A. (To permit the measurement at higher frequencies, B was recorded with three different PSD bandwidths, which are printed in different grey scales. This accounts for the varying width of the peaks at 66 Hz.)
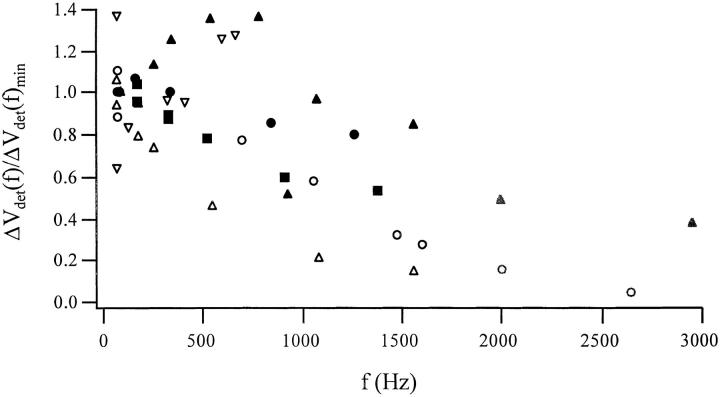
Fig. 3 summarizes the frequency dependence of the voltage-clamp–induced movement in six cells. The movement of different cells varied between 0.1 and 0.4 nm/mV of the AC carrier voltage at the lowest frequency tested (in most cases 66 Hz; although we occasionally changed this frequency because the background noise was too high at 66 Hz.). If the stimulus were an action potential, we would expect the membrane to move between 10 and 40 nm. This is a rather large movement that may affect the local mass transport of diffusible species and perhaps also be capable of sending a mechanical signal to other cells.
Figure 3.
Frequency dependence of the voltage-induced movement of six cells normalized to the amplitude at the lowest frequency tested (66 [3 cells], 85 [2 cells], and 166 [1 cell] Hz). In three experiments (bold markers), we used a stiffer cantilever with k = 0.02 N/m (normal cantilever, k = 0.01 N/m). The mean sensitivity at the lowest frequency was (0.15 ± 0.05) nm/mVpp (mean ± SEM, n = 6). Points measured above the resonance frequency of the set-up (∼2 kHz) are drawn in grey to indicate that the decrease in signal amplitude is also affected by the detection system.
We saw a fourfold scatter in the absolute amplitude of the movement between individual cells. To investigate whether this might be caused by varying mechanical properties of the cantilever, we used two types of levers possessing different spring constants, and thus different resonant frequencies (fres). Below the resonance frequency, we found no significant difference in the amplitude of the movement using hard or soft cantilevers (Fig. 3, open symbols, fres of the freely moving cantilever at ∼700 Hz; bold symbols, fres at ∼2.1 kHz). This indicates that the smaller response at higher frequencies came primarily from properties of the detection system rather than the underlying molecular motor. The average voltage-dependent signal was depressed by 3 dB only above 1,000 Hz, so the motor must have a relaxation time <150 μs.
Differences between Transfected and Untransfected Cells
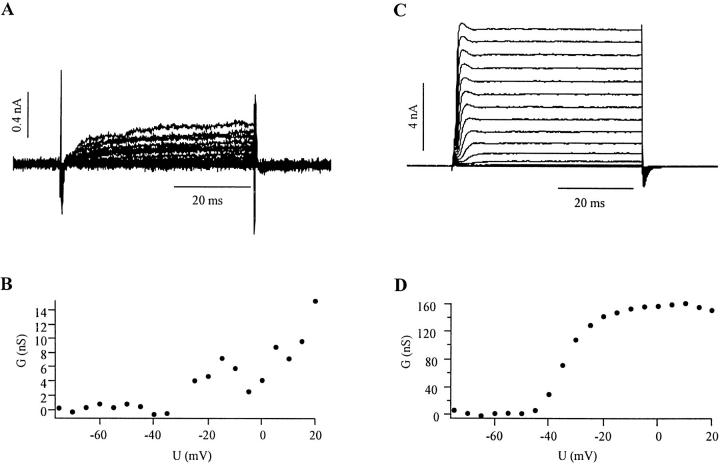
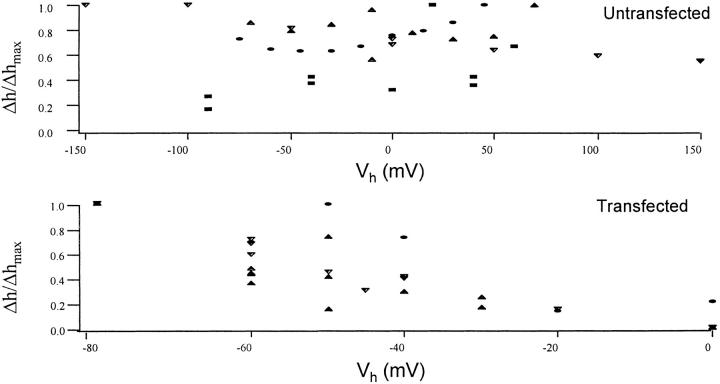
As mentioned above, the voltage-dependent movement was approximately linear with the carrier amplitude between ∼5 and 50 mV. We also looked for a dependence of the movement on the holding potential. For untransfected cells that showed a slightly outward rectifying current–voltage relation (see Fig. 5 A), there was no significant correlation between the holding potential and the amplitude of the movement (Fig. 4 A, pooled data from four cells). However, when the cells were transfected with a noninactivating mutant of the Shaker K+ channel (Hoshi et al., 1990; Bezanilla et al., 1991), we found an inverse relationship between the holding potential and the size of the modulated movement (Fig. 4 B). At negative holding potentials, the movement was 0.5–0.9 nm/mV (n = 3), and dropped to almost zero near 0 mV. The outward current at 0 mV ranged between 1 and 4 nA with Normal Rat Ringer in the bath and high potassium internal dialysis (Fig. 5 C). The maximal movement of transfected cells was larger than that of untransfected ones (0.2–0.7 nm/mV), but this difference was not statistically significant due to the large variations within the group.
Figure 5.
Currents and conductance-voltage relation of untransfected control cells and cells transfected with a noninactivating mutant of Shaker H4 K+ channel. A and C show 20 superimposed traces of currents for 50-ms step changes in potential from a holding potential of −80 mV in steps of 5 mV for untransfected and transfected HEK293 cells, respectively. B and D show the chord-conductance/voltage behavior for both cells as calculated by the steady state current during the last 20 ms of the 50-ms depolarizing pulse.
Figure 4.
The effect of holding potential on the movement of untransfected versus transfected cells. Movement of the transfected cells had a unique dependence on the holding potential. (A) Pooled and normalized data from four untransfected cells. The displacements were normalized to their largest value (16.7 nm at 45 mV ± 50 mV, 10.4 nm at 20 mV ± 15 mV, 4.5 nm at 70 mV ± 10 mV, and 3.7 nm at −100 mV ± 25 mV AC stimulus) and plotted against the holding potential of the cell. (B) Data for three transfected cells. Maximal values were 3.0 nm at −80 mV (± 5 mV AC stimulus), 5.9 nm at −80 mV (± 10 mV AC stimulus), and 9.9 nm at −80 mV (± 10 mV AC stimulus). (The holding potential of the cells was only changed from hyperpolarized to depolarized because the slow recovery of the movement signal after activation of the current in transfected cells interfered with running the experiment.)
Interestingly, after 30-s depolarizations, the voltage-induced movement of transfected cells only reappeared when we held the cell for several minutes at −80 mV. Correlated with the depolarization-induced loss of modulation, we found a steady outward displacement of the membrane. Upon return to −80 mV, this DC displacement disappeared while the modulation signal returned to control values (data not shown). This dependence of the DC displacement on the potential in transfected cells was not seen with untransfected cells, so we presume that it resulted from the current flow, possibly linked to volume changes caused by the large K+ efflux.
The Search for Artifacts
To investigate whether the movement of the cantilever arose artifactually from a movement of the patch pipette or some other source, we repeated the experiments without cells. No signal could be detected when the cell was removed from the pipette by exposure to air, and the same pipette, under the same conditions, was then placed in contact with the cantilever. Additionally, neither sealed nor unpulled pipettes filled with saline showed any signal, even when we applied much higher AC voltages. It is unlikely that the patch pipette itself moved when a voltage was applied. This view is supported by the difference in frequency dependence of the voltage-induced and directly coupled mechanical stimulation described above.
To look for possible electroosmotic effects, we placed the cantilever in front of an open pipette while passing the AC carrier signal. No movement was detectable. As a further control, we applied hydraulic pressure to the pipette to cause a flow of solution onto the cantilever. This steady flow was readily detectable.
It has been suggested that an electrostatic effect between the membrane and the cantilever could be a source for an AFM signal (Butt, 1991; Levadny et al., 1996). Both studies showed that, as expected from the value of the Debye length, the electrostatic interaction drops with a space constant of a few angstroms in the normal physiological environment. We tested one electrostatic interaction by touching the cantilever directly to the silver wire used to contact the patch pipette. The bath electrode was placed in a patch pipette to maintain a resistance of ∼3 MΩ between the electrodes. We could not detect any significant signal at a noise level of 0.1 nm. This makes it unlikely that surface charge effects are responsible for the observed motion.
We only found one condition where we could induce a lever movement without a cell on the patch pipette: when we replaced the normal bath solution by distilled H2O and we touched the cantilever to the tip of a patch pipette containing normal intracellular saline. The large Debye length in distilled water may explain this movement (Butt, 1991).
discussion
We have found a significant electromechanical coupling in cell membranes. One of our most surprising observations was a large movement in control cells that lacked strong voltage-dependent conductances. The fact that the movement was essentially independent of the holding potential implies that the system was heavily damped, regardless of the type of motor.
The observed movement was, in fact, a minimum estimate for the motor itself, since any displacements of the motor were shared between the elasticity of the cantilever and the underlying cytoskeleton (see Fig. 6 A). The observed displacement should be approximately the true displacement times (1 + kcant/kcyt)−1, where kcant is the stiffness of the cantilever and kcyt is the stiffness of the cytoskeleton. This attenuation factor is frequency dependent since the motion of the cell and cantilever involve viscous losses. The frequency dependence can be clearly seen in the variation of cantilever movement when the cell was pushed with the patch pipette (see Fig. 2 A). At high frequencies, the cantilever was moved substantially less (∼2 nm) than the pipette (∼10 nm). In addition, the linewidth of the cantilever movement was broader and the center frequency was slightly below that of the driving frequency, as expected from a heavily damped harmonic oscillator. The fact that the cantilever movement decreased with frequency indicates that the effective viscosity was not in series with the pipette/cantilever axis. We suspect that attempting to push the cell against the drag of the 1-centipoise bath environment led to increased indentation at the site of pushing, and consequently less movement at the cantilever.
Figure 6.

(A) Mechanical equivalent model showing the effect of cytoskeletal (kcyt) and cantilever (kcant) stiffness to reduce the true voltage-induced displacement (d) to the observed displacement (see text). (B–D) Cartoons of an AFM tip indenting the membrane and moving with the applied voltage. B is for the dipole rotation model, C is for the Shaker channel model, and D is for the flexoelectric effect where the minus signs represent fixed charges on the outer monolayer.
In apparent contradiction, the stiffness of heart cells was reported to increase with frequency (Shroff et al., 1995). This discrepancy can be explained by instrumental differences. In Shroff et al.'s work, a cover slip with attached cells was vibrated in the z direction by a piezoelectric stage so that there was no drag between the cells and the media. The stiffness of the heart cells measured by AFM was in the range of 0.1 N/m at 100 Hz (Shroff et al., 1995). If we assume that Shroff et al.'s figures apply to our cells, a 0.01 N/m cantilever would have underestimated the true movement by ∼15%.
The molecular nature of the membrane motor is not yet clear. As the applied voltage drops mainly across the membrane, the electromechanical transduction must be located in the cell membrane. One possibility is that membrane proteins, whose dipoles are not oriented parallel to the imposed field, reorient in the field. However, to obtain movements of 5 nm requires that the proteins extend far from the membrane (Fig. 6 B). The vertical displacement, Δh = l sinθ, where l is the horizontally projected length of the protein extending above the center of rotation, and θ is the angle of rotation in the field. For small rotations (an assumption required to keep the movement independent of the holding potential), l = Δh /sinθ. If, for example, θ = 10° and Δh = 5 nm, then l = 29 nm, a large but not unreasonable length if one allows for the possibility of attached extracellular matrix. It is also possible that the relevant motors are actually fibrils or cilia of macroscopic dimensions that rotate in the field (Barber et al., 1995).
A problem with the dipole explanation is that (a) the dipoles should make significant contributions to the membrane capacitance, which is already comparable to bilayers (Sokabe and Sachs, 1990; Sokabe et al., 1991; Raicu et al., 1996), and (b) they will probably have a distinctive dispersion near 1 kHz that has not been observed (compare Palti and Adelman, 1969). Because of their linearity, these dipolar movements would not show up in gating current experiments where only the nonlinear components are emphasized.
Another possibility for explaining the large movement of untransfected cells is the flexoelectric effect (Petrov et al., 1989). This effect is a result of membrane bending causing a change in surface charge density, and thereby a change in the membrane field. The inverse of this effect, whereby changes in voltage cause a change in bending (Todorov et al., 1994), may account for the movement (see Fig. 6 D). In their experiments with polar black lipid membranes, Todorov et al. (1994) found movements of ∼1 nm/mV. The magnitude of the bending force can be calculated from the change in electrostatic energy as a function of curvature.
The energy in a capacitor is given by U = CV 2/2, where C is the capacitance and V the voltage. The derivative of the energy with respect to the radius of curvature is the force exerted on the AFM tip, F = dU/dr = CVdV/dr, where the capacitance is taken to be independent of curvature. In a spherical cap, with symmetrical changes of curvature of ± r, the induced flexoelectric voltage is ΔVpp = 2f/(εε0 r), or dΔVpp/dr = −2f/ (εε0 r 2), where f is the flexoelectric coefficient (in coulombs), ε is the dielectric constant, and ε0 is the permittivity of free space (8.85 pF/m). Using Cs = 1 μF/cm2 to represent the specific capacitance of the membrane, and for simplicity considering that the indented membrane is a hemisphere, F = 2VCs f/ε0. The coefficient f has been estimated to range from 10−18 to 10−21 C, depending upon the source of membrane, with the sign of f depending upon the symmetry of the charge distribution (compare Petrov et al., 1993). Taking ε = 2 and V = ± 10 mV, the peak–peak force exerted on the AFM tip could range from 0.7 to 70 pN. At the maximum force, a cantilever like ours, with a stiffness of 0.01 N/m, would deflect 7 nm, a distance comparable to our observed displacements. Since our basal indentation was ∼200 nm, the flexoelectric movement would not be sufficient to significantly alter the radius of curvature of the membrane around the tip, and hence the force would be expected to be independent of the holding potential. It is perhaps significant that Petrov and coworkers have observed in bilayers a flexoelectric sensitivity similar to what we observed here, ≈2 nm/mV (Todorov et al., 1994). In apparent contradiction to the above argument, we observed similar movements when we pushed the flat part of the cantilever against the cell. It may be that in this case, the irregularities of the cell surface were pushed inward, causing the necessary local curvature. Ion channels, with their strong dipole moment, should increase the inverse flexoelectric effect, in accordance with our observation of the small increase in membrane movement in transfected cells. The opening of voltage-gated ion channels appears to be strongly influenced by the flexoelectric response of a membrane patch taken from locust muscles (Petrov et al., 1993).
The distinctive effect of transfection with Shaker K+ channels was that the movement became sensitive to the holding potential. It would appear that we detected the channels moving in the field (superimposed on the control response of untransfected cells that were independent of holding potential). The total movement remained in phase with the displacement current to high frequencies, suggesting that we were observing movement of the voltage sensor regions rather than changes in the pore gating transition (see Fig. 6 C). Supporting this hypothesis is the observation that the transfected cells moved further than the untransfected cells at negative potentials. Taking the average maximal movements used to normalize the data in Fig. 4, the transfected cells were more sensitive than the untransfected cells by ≈0.2 nm/mV, or 40%.
The dependence of the excess movement (that over control cells) on the holding potential is in basic agreement with gating current measurements (Olcese et al., 1997). The prediction is that as a function of the holding potential, the movement curve should be bell shaped, peaking at ∼−80 mV. While we have seen the reduced response at depolarized potentials, we have not yet explored the hyperpolarized side. The shape of the movement/potential curve is not accurate because of the substantial ionic current flow. K+ efflux induced by depolarization can cause shrinkage of the cell, thereby pulling the cell surface away from the cantilever and damping the AC signal. We estimate that the observed K+ currents could shrink the cell diameter by ≈200 nm in 20 s. The influence of such currents can be removed using K+ channel blockers, nonconducting mutants, or by varying the command pulse duration to minimize flux in favor of gating movements.
A further experimental detail has to be taken into account in interpreting the results: AFM images contain information about both the height and the stiffness of the substrate. In our experiments, the peaks in the PSD could reflect a height difference and/or a difference in compliance. Results similar to what we observed could result if the cell membrane changed its bending stiffness with the transmembrane field. However, since the compliance of the cantilever and the cell are probably comparable, and the results seem nearly independent of cantilever stiffness, this seems an unlikely conclusion.
Independently of the underlying mechanism of the observed electromechanical coupling, this interaction might be used as a tool for providing contrast for imaging rather than the intermolecular forces that are normally used (Ludwig et al., 1997). If the observed voltage-dependent movements prove to arise from K+ channels, or for that matter from some other defined membrane protein, one could imagine using ultrafine AFM tips to make images of the channels with the movement serving as the basis of contrast—truly functional imaging.
Acknowledgments
We thank B. Sakmann and the Max Planck Institute, Heidelberg, Germany, for financial and moral support, S. Gruenewald, W. Oeffner, and W. Häberle for technical advice, S. Jeney and A. Pralle for helpful discussions, and F. Bezanilla for supplying the Shaker clone and providing unpublished data.
Footnotes
J. Mosbacher acknowledges an EMBO fellowship ALTF 599/1994, and F. Sachs acknowledges the United States Army Research Office and National Institutes of Health for financial support.
Abbreviations used in this paper: AFM, atomic force microscopy; PSD, power spectral density.
Dr. Mosbacher's present address is Novartis Pharma AG, K-125.7.12, Ch-4002 Basel, Switzerland.
references
- Ashmore, J.F. 1989. Transducer motor coupling in cochlear outer hair cells. In Cochlear Mechanisms, Structure, Function and Models. J.P. Wilson and D.T. Kemp, editors. Plenum Publishing Corp., New York. 109–114.
- Barber K, Enam SA, Bodovitz S, Falduto M, Frail D, Klein WL. Particulate forms of APP in the extracellular milieu of cultured cells. Exp Neurol. 1995;132:42–53. doi: 10.1016/0014-4886(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Perozo E, Papazian DM, Stefani E. Molecular basis of gating charge immobilization in Shaker potassium channels. Science. 1991;254:679–683. doi: 10.1126/science.1948047. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Stefani E. Voltage-dependent gating of ionic channels. Annu Rev Biophys Biomol Struct. 1994;23:819–846. doi: 10.1146/annurev.bb.23.060194.004131. [DOI] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Physical Review Letters. 1986;56:930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Zhou Z, Neher E, Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol (Camb) 1995;485:403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt H-J. Measuring local surface-charge densities in electrolyte solutions with a scanning force microscope. Biophys J. 1992;63:578–582. doi: 10.1016/S0006-3495(92)81601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French RJ, Prusaksochaczewski E, Zamponi GW, Becker S, Kularatna AS, Horn R. Interactions between a pore-blocking peptide and the voltage sensor of the sodium channel—an electrostatic approach to channel geometry. Neuron. 1997;18:407–413. doi: 10.1016/s0896-6273(00)80058-6. [DOI] [PubMed] [Google Scholar]
- Guharay F, Sachs F. Stretch-activated single ion channel currents in tissue-cultured embryonic chick skeletal muscle. J Physiol (Camb) 1984;352:685–701. doi: 10.1113/jphysiol.1984.sp015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häberle, W., J.K.H. Hörber, F. Ohnesorge, D.P. Smith, and G. Binnig. 1992. In situ investigations of single living cells infected by viruses. Ultramicroscopy. 42–44:1161–1167. [DOI] [PubMed]
- Hörber JKH, Mosbacher J, Häberle W, Ruppersberg JP, Sakmann B. A look at membrane patches with a scanning force microscope. Biophys J. 1995;68:1687–1693. doi: 10.1016/S0006-3495(95)80346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Hutter JL, Bechhofer J. Calibration of atomic-force microscope tips. Rev Sci Instrum. 1993;64:1868–1873. [Google Scholar]
- Larsson HP, Baker OS, Dhillon DS, Isacoff EY. Transmembrane movement of the Shaker K+channel S4. Neuron. 1996;16:387–397. doi: 10.1016/s0896-6273(00)80056-2. [DOI] [PubMed] [Google Scholar]
- Levadny VG, Belaya ML, Pink DA, Jericho MH. Theory of electrostatic effects in soft biological interfaces using atomic force microscopy. Biophys J. 1996;70:1745–1752. doi: 10.1016/S0006-3495(96)79737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig M, Dettmann W, Gaub HE. Atomic force microscope imaging contrast based on molecular recognition. Biophys J. 1997;72:445–448. doi: 10.1016/S0006-3495(97)78685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzzu LM, Moronne MM, Isacoff EY. Direct physical measure of conformational rearrangement underlying potassium channel gating. Science. 1996;271:213–216. doi: 10.1126/science.271.5246.213. [DOI] [PubMed] [Google Scholar]
- Mosbacher J, Häberle W, Hörber JKH. Studying membranes with scanning force microscopy and patch-clamp technique. J Vac Sci Tech. 1996;14:1449–1454. [Google Scholar]
- Olcese R, Latorre R, Toro L, Bezanilla F, Stefani E. Correlation between charge movement and ionic current during slow inactivation in Shaker K+ channels. J Gen Physiol. 1997;100:1–12. doi: 10.1085/jgp.110.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palti Y, Adelmann WJJ. Measurement of axonal membrane conductances and capacity by means of a varying potential control voltage clamp. J Membr Biol. 1969;1:431–458. doi: 10.1007/BF01869791. [DOI] [PubMed] [Google Scholar]
- Perozo E, Mackinnon R, Bezanilla F, Stefani E. Gating currents from a nonconducting mutant reveal open-closed conformations in Shaker K+ channels. Neuron. 1993;11:353–358. doi: 10.1016/0896-6273(93)90190-3. [DOI] [PubMed] [Google Scholar]
- Petrov AG, Ramsey RL, Usherwood PN. Curvature-electric effects in artificial and natural membranes studied using patch-clamp techniques. Eur Biophys J. 1989;17:13–17. doi: 10.1007/BF00257141. [DOI] [PubMed] [Google Scholar]
- Petrov AG, Miller BA, Hristova K, Usherwood PN. Flexoelectric effects in model and native membranes containing ion channels. Eur Biophys J. 1993;22:289–300. doi: 10.1007/BF00180263. [DOI] [PubMed] [Google Scholar]
- Raicu V, Raicu G, Turcu G. Dielectric properties of yeast cells as simulated by the two-shell model. Biochim Biophys Acta. 1996;1274:143–148. doi: 10.1016/0005-2728(96)00024-2. [DOI] [PubMed] [Google Scholar]
- Shroff SG, Saner DR, Lal R. Dynamic micromechanical properties of cultured rat atrial myocytes measured by atomic force microscopy. Am J Physiol. 1995;269:C286–C292. doi: 10.1152/ajpcell.1995.269.1.C286. [DOI] [PubMed] [Google Scholar]
- Sokabe M, Sachs F, Jing ZQ. Quantitative video microscopy of patch clamped membranes stress, strain, capacitance, and stretch channel activation. Biophys J. 1991;59:722–728. doi: 10.1016/S0006-3495(91)82285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe M, Sachs F. The structure and dynamics of patch-clamped membranes: a study using differential interference contrast light microscopy. J Cell Biol. 1990;111:599–606. doi: 10.1083/jcb.111.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorov AT, Petrov AG, Fendler JH. First observation of the converse flexoelectric effect in bilayer lipid membranes. J Phys Chem. 1994;98:3076–3079. [Google Scholar]
- Yang N, George AL, Jr, Horn R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron. 1996;16:113–122. doi: 10.1016/s0896-6273(00)80028-8. [DOI] [PubMed] [Google Scholar]
- Yang N, Horn R. Evidence for voltage-dependent S4 movement in sodium channels. Neuron. 1995;15:213–218. doi: 10.1016/0896-6273(95)90078-0. [DOI] [PubMed] [Google Scholar]