Abstract
In men with metastatic hormone-refractory prostate cancer, androgen blockade produces dramatic and rapid declines in prostate-specific antigen (PSA), bone pain, and urinary tract obstruction. Nevertheless, there have been limited options with at best palliative results for patients who progress despite a castrate testosterone level. This paradigm changed in 2004 with the publication of 2 randomized clinical trials that demonstrated a 20% to 24% survival benefit for docetaxel-based therapy when compared to mitoxantrone and prednisone, data that supported US Food and Drug Administration approval of docetaxel-based therapy for the treatment of metastatic hormone-refractory prostate cancer. This article reviews the preliminary data and the timing and sequencing implications of ongoing clinical trials. Studies are evaluating the combination of docetaxel with agents that target bone, tumor vasculature, and the vitamin D receptor as well as second-line agents, such as satraplatin. The role of immune therapy is also evolving, and further studies will define the optimal timing of chemotherapy with immune therapy.
Key words: Prostate cancer, Hormone-refractory prostate cancer, Androgen-independent prostate cancer, Docetaxel
Dramatic and rapid declines in prostate-specific antigen (PSA), bone pain, and urinary tract obstruction characterize the initial response to androgen blockade in men with metastatic hormone-refractory prostate cancer. Historically, limited options are available for patients who progress despite a castrate testosterone level. Secondary hormonal manipulations, mitoxantrone-based chemotherapy, external beam radiation therapy, or radioisotope therapy demonstrate at best palliation. This paradigm changed in 2004 with the publication of 2 randomized clinical trials that demonstrated a 20% to 24% survival benefit for docetaxel-based therapy when compared to mitoxantrone and prednisone.1,2 These studies supported the approval of docetaxel-based therapy for the treatment of metastatic hormone-refractory prostate cancer by the US Food and Drug Administration (FDA) in May 2004. Clinical trials in hormonerefractory prostate cancer are now focused on building on the survival improvement seen with docetaxelbased therapy. This article will review the preliminary data supporting the approval of docetaxel for men with androgen-independent prostate cancer, as well as some of the approaches to combining docetaxel with targeted therapy. The potential approval of immune therapy for hormone-refractory prostate cancer also opens new avenues for combinations of mechanistically different treatments.3
Docetaxel-Based Therapy
Significant PSA declines, measurable soft-tissue responses, and relief of bone pain characterize the clinical activity observed in phase II studies when docetaxel was administered either weekly or every 3 weeks to men with androgen-independent prostate cancer. These observations supported the design and implementation of TAX 327, an international phase III trial comparing 2 different schedules of docetaxel combined with prednisone to mitoxantrone and prednisone. Patients with progression of metastatic prostate cancer despite surgical or medical castration were randomized among 3 arms: docetaxel 75 mg/m2 every 3 weeks with 5 mg prednisone twice daily, docetaxel 30 mg/m2 weekly with 5 mg prednisone twice daily, and mitoxantrone 12 mg/m2 every 3 weeks with 5 mg prednisone twice daily. Pretreatment stratifications included pain index ≥ 2, analgesic score ≥ 10 versus pain index < 2, analgesic score < 10, and Karofsky performance status ≤ 70 versus > 80. No dose escalation was incorporated into the study design, and treatment was limited to a total of 30 weeks for each arm. Patients were not permitted to have prior chemotherapy in TAX 327. (For the purposes of this trial, estramustine was not considered to be a chemotherapeutic agent.) The weekly docetaxel regimen was calculated to deliver a dose intensity equivalent to the every-3-week docetaxel regimen. The primary endpoint was overall survival with secondary endpoints including pain response, ≥ 50% PSA decline, measurable response, and quality of life.
Results of TAX 327
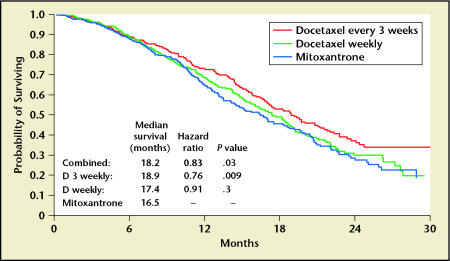
When compared to mitoxantrone/prednisone, improved survival was noted for patients treated with the every-3-week docetaxel regimen. Surprisingly, the weekly arm did not demonstrate a significant survival benefit. The initial reported median survivals were 18.9 months for every-3-week docetaxel/prednisone, 17.4 months for weekly docetaxel/prednisone, and 16.5 months for mitoxantrone/prednisone. A recent update demonstrates that after 213 more deaths, the median survivals remained essentially the same at 19.2, 17.8, and 16.3 months, respectively (Figure 1).1,4 After 3 years of follow-up, more patients (17.2% and 16.4%) were alive in the every-3-week and weekly docetaxel arms compared to the mitoxantrone arm (12.8%). The reduction in the risk of death, when compared to mitoxantrone and prednisone, was 24% for the every-3-week and 9% for the weekly docetaxel regimens. The hazard ratios were also similar in the most recent update of these data. PSA declines of ≥ 50% were noted in 45% of patients in the every-3-week arm, 48% of the weekly docetaxel arm, and 32% of the mitoxantrone arm. There was a non-significant trend toward improved objective response rate in patients treated with every-3-week docetaxel compared to mitoxantrone and prednisone.
Figure 1.
TAX 327 overall survival. Data from Tannock IF et al.1
Palliation of bone pain was superior in both docetaxel arms when compared to mitoxantrone and prednisone. The every-3-week and weekly docetaxel regimens had pain response rates of 35% and 31%, respectively. In mitoxantrone- treated patients, the pain response rate was significantly lower at 22%. One of the commonly preconceived notions about chemotherapy, worsening quality of life, is refuted by the data from TAX 327. Quality-of-life response favored both docetaxel arms compared to mitoxantrone using the Functional Assessment of Cancer Therapy-Prostate (FACT-P) instrument. Scores achieved in the docetaxel arms were 9 to 10 points higher than those noted in the mitoxantrone arm.
Rates of grade 3 or 4 neutropenia were highest in the every-3-week docetaxel arm (3%) with rates of febrile neutropenia at 2.7%. In comparison, grade 3 or 4 neutropenia was noted in 0.0% and 0.9% with weekly docetaxel or mitoxantrone. These relatively low rates of neutropenia were not supported by colony-stimulating factors. The rates of study discontinuation due to adverse events were similar in all 3 treatment arms. Although lacrimation, nail bed changes, neuropathy, and alopecia appeared more frequently in docetaxel-treated patients compared with mitoxantrone-treated patients, the toxicity patterns were not remarkably different.
One perplexing finding of TAX 327 was that the weekly regimen did not have an improved survival compared to mitoxantrone/prednisone. This has important implications for the design of future trials, because many of the newer signal transduction agents have been combined with weekly docetaxel rather than the every-3-week regimen. The statistical design of TAX 327 did not include a direct comparison of the weekly arm to the every-3-week arm, thus no valid comparisons can be made. Statistical variation, differences in dose and schedule, as well as unidentified biological mechanisms may in part account for the failure of weekly docetaxel. Despite the lack of survival benefit, weekly docetaxel still demonstrated significant improvements in palliation and quality-oflife indices when compared with mitoxantrone/prednisone.
Estramustine/Docetaxel-Based Therapy
Clinical trials were designed based on synergy observed between 2 agents, estramustine and docetaxel, both of which target tubulin in human prostate cancer cell lines. Estramustine, a synthetic nornitrogen mustard, has been demonstrated to interfere with microtubule associate proteins. This is in contrast with its designed mechanism of action, alkylation of DNA. Docetaxel stabilizes tubulin and thus prevents dissociation of the mitotic spindle; it is also known to phosphorylate Bcl-2. Preliminary phase I and II studies treating men with androgen-independent prostate cancer with docetaxel and estramustine demonstrated median survivals of 20 to 23 months. Based on these promising preliminary data, the Southwest Oncology Group designed a phase III study (SWOG 9916) that randomized 770 men to receive estramustine 280 mg PO 3 times daily on days 1-5 plus docetaxel 60 mg/m2 IV on day 2 every 21 days plus dexamethasone 60 mg PO in 3 divided doses prior to docetaxel or to receive mitoxantrone 12 mg/m2 IV every 21 days plus prednisone 5 mg PO twice daily. For study entry, patients were required to have progressive metastatic androgen-independent prostate cancer, demonstrated by a rising serum PSA, progression on bone scan, or progression on CT scan. If the patient tolerated the first cycle without grade 3 or 4 toxicities, dose escalation was permitted to 70 mg/m2 for docetaxel and 14 mg/m2 for mitoxantrone. The trial was powered to detect a 33% improvement in overall survival between the 2 treatment arms. To prevent vascular events, the protocol was amended in January 2001 to administer 2 mg of warfarin and 325 mg of aspirin per day in patients treated on the estramustine/docetaxel arm.
SWOG 9916 Study Results
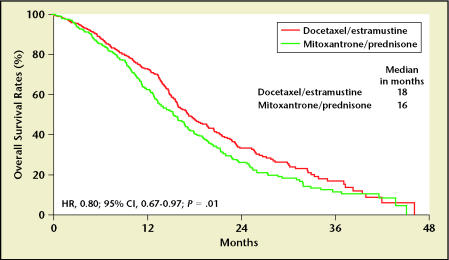
In an intent-to-treat analysis, patients receiving docetaxel/estramustine had a 20% reduction in the risk of death compared to those patients treated with mitoxantrone/prednisone (hazard ratio [HR], 0.80; 95% confidence interval [CI] 0.67–0.97) Longer median survivals were also noted in the docetaxel/estramustine-treated patients compared to the mitoxantrone/prednisone-treated patients (median 17.5 vs 15.6 months; logrank P = .020) (Figure 2).2 The median times to progression of the docetaxel/estramustine and the mitoxantrone/prednisone arms were 6 and 3 months, respectively (logrank P < .0001); PSA declines of ≥ 50% occurred in 50% of docetaxel/estramustine patients and 27% of mitoxantrone/prednisone patients (P < .0001). A trend toward improved objective responses in measurable soft tissue lesions was observed (17% docetaxel/estramustine vs 11% mitoxantrone/prednisone), but this was not statistically significant (P = .30). Grade 3/4 gastrointestinal and cardiac toxicity and neutropenic fevers were more common in docetaxel/estramustine-treated patients than in those treated with mitoxantrone/prednisone. The rates of cardiac ischemia appeared to be lower in those patients who received prophylactic anticoagulation, but no differences in deep venous thrombosis were observed. The evaluation of the use of prophylactic anticoagulation is limited; the trial was not initially designed to detect a difference in the rates of vascular events between estramustine/docetaxel patients who received prophylactic anticoagulation and those who did not.
Despite the fact that the mitoxantrone arm contained continuously administered prednisone, palliation of bone pain was not significantly different between the treatment arms. Again, despite the aforementioned toxicities, a global quality-of-life analysis showed a consistent lack of statistically significant differences between the docetaxel/estramustine arm and mitoxantrone/prednisone arm.5
Clinical Implications of Docetaxel Trials
SWOG 9916 and TAX 327 were the first trials to demonstrate improvements in survival for men treated with chemotherapy for androgen-independent prostate cancer. There are several findings in both these studies that have implications for patient management and future clinical trial designs. The median survival of the standard arm, mitoxantrone/prednisone, is higher than reported in other phase III studies. This could be attributed to a stage migration. Crossover could also account for the increased median survival of the control arm; 35% of patients who progressed on mitoxantrone and prednisone received second-line therapy on SWOG 9916, whereas 20% of patients failing mitoxantrone and prednisone received further chemotherapy. In a recent analysis of the crossover patterns of TAX 327, approximately 25% of patients who failed primary therapy crossed over to some form of second-line chemotherapy. The survivals were 10.8 months for patients on every-3-week docetaxel who then received mitoxantrone, 8.7 months for those on weekly docetaxel who crossed over to mitoxantrone, and 10.0 months for patients on mitoxantrone who later received docetaxel.6
Randomized trials have demonstrated an improved survival when vinblastine or paclitaxel is combined with estramustine, compared to the respective single agent alone.7,8 Is there a role for combining estramustine with docetaxel? This question cannot be answered by either TAX 327 or SWOG 9916. Although identical control arms were used in both TAX 327 and SWOG 9916, comparisons of the survivals obtained in the docetaxel/estramustine and docetaxel/prednisone arms cannot be validly made. This is due in part to slightly different entry criteria (prior chemotherapy vs no prior chemotherapy), different crossover patterns, and possible patient selection bias. Thus, only a randomized trial comparing docetaxel/estramustine to docetaxel/prednisone can properly evaluate the contribution of estramustine. The emergence of newer agents with potentially less toxicity and greater efficacy than estramustine makes the concept of such a large randomized trial impracticable. Based on lower rates of toxicity, as well as the FDA approval of docetaxel for hormone-refractory prostate cancer in 2004, the Cancer and Leukemia Group B (CALGB) and SWOG have accepted docetaxel/prednisone rather than docetaxel/estramustine as the standard of care for future phase III studies.
When in the course of androgenindependent prostate cancer should docetaxel-based therapy be administered? The approval of mitoxantrone and prednisone for men with hormone-refractory prostate cancer was restricted to those patients with symptomatic bone pain. The study populations of TAX 327 and SWOG 9916 comprised both symptomatic and asymptomatic patients. Thus, it is not known whether anticipating or waiting for the onset of symptoms will achieve a greater survival benefit. In TAX 327, the hazard ratios are similar for those patients who have bone pain compared with those who are asymptomatic. There are 2 ways that this data could be interpreted. Because more than 25% of patients treated on TAX 327 had at least 2 secondary hormonal manipulations, one could attempt a secondary manipulation first, then go forth with docetaxel at progression. Conversely, one could administer the only agent with a proven survival benefit up front. Clearly, better clinical and biological correlates are needed to determine the optimal timing of the initiation of docetaxel-based therapy.
Bone-Specific Targeted Therapy: Endothelin Receptor Antagonists
The endothelin axis is composed of 2 receptors (ETA and ETB) and 3 ligands (ET-1, ET-2, and ET-3) that control vasoconstriction, mitogenesis, nociception, and bone matrix formation. Binding of one of these ligands to its receptor results in cell proliferation, bone-matrix synthesis, and resistance to apoptosis. This ligand/receptor pathway can be found in a variety of human tumors, including prostate cancer. The endothelin A receptor is expressed in 71% of primary prostate cancers and at a higher rate in highgrade tumors and metastases. Osteoblasts also robustly express the ETA receptor. Atrasentan, an orally bioavailable specific ET-1A inhibitor, decreases mitogenic activity, osteoblastic activity, rates of bone metastases and angiogenesis, and blocks nociceptive effects. Side effects attributed to atrasentan include peripheral edema, rhinitis, headache, and dyspnea.9
Atrasentan has been evaluated in patients with hormone-refractory prostate cancer. M96-594 randomized 288 patients either to placebo, atrasentan 10 mg, or atrasentan 2.5 mg. The primary endpoint of this trial was time to disease progression, with PSA progression as a secondary endpoint. There was a significant difference in time to progression and survival for the evaluable patients who received atrasentan compared with placebo. Unfortunately, these differences in survival and time to progression were not observed in the intent-to-treat analysis.10 Bone alkaline phosphatase and PSA changed at a slower rate in those patients treated with atrasentan compared to placebo-treated patients. These results provided the justification for further studies.
A recently completed randomized trial, M00-211, compared atrasentan 10 mg to placebo in 811 hormonerefractory prostate cancer patients with asymptomatic progressive metastatic disease. The primary endpoint of the study was time to disease progression, as defined by the development of 2 or more new lesions on bone scan, development of extraskeletal metastases, worsening of prostate cancer pain, or skeletalrelated events. For all patients, the time to disease progression was not significantly different in the atrasentan arm compared to the placebo arm, but a significant difference was observed in favor of atrasentan for those patients with bone metastases only. Median changes in bone alkaline phosphatase, PSA, and quality-of-life parameters also favored the atrasentan- treated patients. A significant difference in favor of atrasentan was observed in the time to the 50% worsening of the PCS pain score, and there was a delay in time to development of bone pain in the atrasentan patients. Of note, a metaanalysis of 1097 patients in M00-211 and M96-594 found an improved time to disease progression in favor of atrasentan-treated patients compared to placebo-treated patients. One of the major issues that needs to be resolved regarding atrasentan treatment is the proper duration of therapy. In a meta-analysis, more than half of patients progressed at first evaluation. The separation of the progression curves occurs after this point. It is possible that the mechanism of action of atrasentan requires continuous administration of drugs to inhibit the target and bone scan progression may have been evaluated too early. Further studies are clearly needed to define response to progression in relationship to endothelin receptor inhibition.11
Preclinical studies have demonstrated that atrasentan combined with taxanes are synergistic in hormone- refractory prostate cancer cell lines. Phase I studies at Duke University School of Medicine as well as Columbia University Medical Center have demonstrated that atrasentan and docetaxel can be combined safely at full doses.12 To evaluate the combined efficacy of docetaxel and atrasentan, SWOG recently opened the Docetaxel and Atrasentan Hormone Refractory Prostate Cancer Trial (DAHRT). This trial compares docetaxel 75 mg/m2 every 3 weeks and prednisone 10 mg daily plus atrasentan 10 mg daily to docetaxel 75 mg/m2 every 3 weeks and prednisone 10 mg daily. The primary endpoint is progression-free survival. Secondary endpoints include overall survival, pain, quality-of-life, PSA responds, and objective response. The trial is designed to accrue 706 patients over a 4-year period and has 96% power to detect a 33% increase in progression-free survival from 6 to 8 months.
Angiogenesis in Prostate Cancer
New blood vessel growth and formation is critical to the metastatic process. In order to grow to sizes larger than 3 mm,6,13 cancer cells must generate new blood vessels. The process of neovascularization is regulated by a system of vascular growth factors, including vascular endothelial growth factor (VEGF), matrix metalloproteins, and integrins. Inhibition of these targets can arrest tumor growth and inhibit metastatic spread. These vascular growth factors are expressed in both the tissue and serum of patients with prostate cancer. A CALGB study found that circulating levels of VEGF were increased in patients with hormone-refractory prostate cancer and are prognostic of survival.14 Microvessel density has been found to be increased in patients who have metastatic disease in comparison to those who have clinically localized cancer. Thus, the tumor vasculature appears to be a rational therapeutic target for men with prostate cancer.
One of the first antiangiogenic agents to be evaluated in patients with prostate cancer was thalidomide. Thalidomide has single agent activity in hormone-refractory prostate cancer, as demonstrated in a study by Figg and colleagues15 in which 14% of patients treated with thalidomide at dosages of 200–1200 mg PO daily manifested a ≥ 50% PSA decline. The reported median survival for this heterogeneous group of patients, some of whom received prior chemotherapy, was 15.8 months. Thalidomide also appears to sensitize epithelial cells to the effects of chemotherapeutic agents. In addition to their ability to stabilize cytoplasmic microtubules, taxanes—both in vitro and in animal model systems—are antiangiogenic. To evaluate the possible interactions between docetaxel and thalidomide, a randomized phase II study designed by Reiter and colleagues16 compared weekly docetaxel to the combination of docetaxel and thalidomide. Although the primary endpoint of this trial was to evaluate the increase in toxicity of adding thalidomide to docetaxel rather than to detect a survival difference, the median survival of 28.9 months is the highest reported in a phase II study of a cytotoxic agent.16 Newer imide compounds have significantly higher levels of antitumor activity in animals and are being evaluated in men with androgen-independent prostate cancer. The antitumor activity of lenalidomide is approximately 5000 times more potent than thalidomide in animal models. Lenalidomide is being combined with docetaxel in an ongoing phase I study in patients with hormone-refractory prostate cancer. Activity has been noted in both chemotherapy-naïe and docetaxelresistant prostate cancer patients.17
Monoclonal antibodies can block the binding of VEGF to its receptor, thus inhibiting angiogenesis. A randomized trial found that a monoclonal antibody to VEGF, bevacizumab, can improve survival in colorectal cancer patients treated with irinotecan, 5-fluorouracil, and leucovorin when compared to those treated with irinotecan, 5-fluorouracil, and leucovorin alone.18 This antiangiogenesis approach is also being evaluated in prostate cancer. Picus and colleagues19 treated 79 men with hormone-refractory prostate cancer with docetaxel 70 mg/m2 every 3 weeks, estramustine 280 mg PO 3 times daily for 5 days, and bevacizumab 15 mg/kg day 2.19 This study found similar time to progression and survivals in patients with hormone-refractory prostate cancer as reported in previous estramustine-docetaxel based studies. These findings are being confirmed in a randomized trial comparing docetaxel/prednisone/bevacizumab to docetaxel and prednisone being performed by the CALGB. One thousand twenty patients will be randomized to detect an improvement in median survival from 19 to 24 months. Enrollment in this study has been brisk, with more than 700 patients to date.
Calcitriol Combined With Taxanes
Proliferation of human prostate cancer cell lines is inhibited by calcitriol, the biologically active form of vitamin D.20,21 Calcitriol also synergizes with chemotherapeutic agents such as docetaxel, paclitaxel, cisplatin, and carboplatin. This effect is independent of Bcl-2.22,23 Hypercalcemia is the dose-limiting side effect of continuous administration of high-dose calcitriol; this toxicity is minimized by weekly pulsed administration. A high-dose preparation of calcitriol, DN101, is being evaluated in hormone-refractory prostate cancer. A single institution phase II study of docetaxel 36 mg/m2 for 6 out of 8 weeks combined with DN101 in men with androgen-independent prostate cancer found a PSA decline rate of 50% in 81% of treated patients, with a median time to progression of 11.4 months. Fifty-three percent of patients with measurable disease had at least a partial response.24 To further evaluate this preliminary observation, a randomized phase II trial, Androgen Independent Prostate Cancer Study of Calcitriol Enhancing Taxotere (ASCENT) compared pulsed high-dose calcitriol, 45 mcg daily (DN101) plus weekly docetaxel 36 mg/m2 for 3 out of 4 weeks versus docetaxel alone. Although the primary endpoint was measuring a difference in 50% PSA decline rates at 6 months (power of 85% to detect a difference from 45% to 65%), an adjusted survival analysis demonstrated improved survival in patients treated with the combination over weekly docetaxel (HR, 0.67; 95% CI, 0.45–0.97).25 The rates of serious adverse events were significantly lower in the combination arm (27%) versus the docetaxel-only arm (47%). There were significantly fewer gastrointestinal events (9.6% vs 2.4%) and deep venous thrombosis (7.2% vs 1.5%) in those patients receiving combination therapy versus docetaxel alone. The exact mechanism of the decreased risk of deep venous thromboses is unknown but may be related to reductions in the level of tissue factor, a known procoagulant. Prospective confirmation is needed to determine if DN101 truly reduces docetaxel-based toxicity.26 A 900- patient phase III study, ASCENT II, will compare every-3-week docetaxel 75 mg/m2 combined with prednisone to weekly docetaxel combined with DN101. This study is open and actively accruing patients.
Second-Line Chemotherapy
Satraplatin (JM 27) is an orally bioavailable platinum compound that has activity in a variety of human solid tumors.27 In men with hormone-sensitive prostate cancer, Sternberg and colleagues randomized patients to the combination of satraplatin 100 mg/m2 for 5 days combined with prednisone 5 mg PO twice daily, or prednisone 5 mg PO twice daily combined with placebo.28 Treatment was administered every 5 weeks. A superior time to progression (5.2 vs 2.5 months) and PSA decline rate of > 50% (33% vs 9%) were found in men randomized to the satraplatin arm compared to placebo. The preliminary activity of this drug in men with chemotherapy-naïve hormone-refractory prostate cancer, as well as preclinical trials demonstrating activity of satraplatin in vitro against taxane-resistant or anthracycline-resistant cell lines, supported the design of the Satraplatin and Prednisone Against Hormone Refractory Prostate Cancer (SPARC) trial.29
At the time of the design of SPARC in 2003, mitoxantrone combined with prednisone was the only FDA-approved cytotoxic treatment for men with hormone-refractory prostate cancer. Recognizing that the standard of care could change with the maturation of SWOG 9916 and TAX 327, patients eligible for SPARC included those with progressive metastatic androgen-independent prostate cancer after a minimum of 2 courses of an unspecified chemotherapeutic regimen. Other entry requirements included adequate renal, hepatic, and hematologic function as well as an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Continuation of prior bisphosphonate therapy was permitted. The primary endpoints of the SPARC trial were progression-free survival and overall survival. Secondary endpoints included time to pain progression. For this trial, time to disease progression was a composite endpoint, based on the first occurrence of any of 5 criteria: (1) tumor progression by Response Evaluation Criteria in Solid Tumors (RECIST) scale or 2 or more new lesions on bone scan; (2) skeletalrelated events as evidenced by a fracture, requirement of radiation therapy or bone surgery, or the initiation of bisphosphonate therapy; (3) symptomatic progression based on an increase in the Present Pain Index (PPI) score based on patient pain diaries or increase in analgesic consumption, worsening of the patient’s ECOG performance status, or weight loss of more than 10%; (4) death. Most importantly, a rising PSA was not a criterion for progressive disease. In fact, the trial was powered for a 30% dropout rate due to PSA. Patients were randomized in a 2:1 ratio to either satraplatin 80 mg/m2 PO QD for 5 days with an antiemetic 1 mg PO twice daily combined with prednisone 5 mg PO twice daily continuously or to a placebo 80 mg/m2 PO daily for 5 days with placebo antiemetic 1 mg PO twice daily and prednisone 5 mg PO twice daily continuously. Crossover to the satraplatin arm from the placebo arm at progression was not permitted. The trial opened in late 2003 and accrued more than 900 patients. The median ages in the 2 arms were similar at 70 years for the satraplatin/prednisone arm and 68 years for placebo/prednisone. Nearly 90% of patients in both arms had ECOG performance scales of 0–1. Symptomatic bone pain was present in 63.2% of patients treated with satraplatin/prednisone and 66.2% of patients treated with placebo/prednisone. At entry, tumor progression by RECIST or bone scan was present in 61.7% of patients in the satraplatin/prednisone arm and 61.9% of patients in the placebo/prednisone arm. Fifty-one percent of patients received docetaxel and 20.2% received mitoxantrone as first-line therapy in the satraplatin/prednisone arm compared with 50.8% and 20.3%, respectively, in the control arm.
As adjudicated by an independent response review committee, median progression-free survival was 11.1 and 9.7 weeks, respectively, for the satraplatin/prednisone and placebo/prednisone arms. The time-to-progression curves separate after 10 weeks, and patients treated with satraplatin/prednisone overall had a 33% improvement in progression-free survival (HR, 0.67; 95% CI, 0.57–0.67; P = .0000003). This was also significantly different at the landmarks of 6 and 12 months post-treatment. The similarity of the medians is due to the fact that more than half of patients came off the study due to progression at or before the first evaluation. Most importantly, on an intent- to-treat basis, the hazard ratios seen in patients treated with docetaxel were identical to the overall population of treated patients (HR, 0.67; 95% CI, 0.54–0.83; P = .0006). Consistent with the phase I and II data, satraplatin was well tolerated. Grade 3 or 4 neutropenia was observed in 13.7% of patients, resulting in neutropenic sepsis in 0.06% of patients. Twenty-one percent of all patients experienced significant thrombocytopenia, and 3.8% of all patients required transfusions. A higher rate of anemia was observed in the satraplatin arm versus the placebo arm, requiring transfusions in 15.9% and 3.2% of patients, respectively. The rate of non-grade 3 or 4 non-hematologic toxicities were < 5%. Most importantly, there was no difference between the 2 arms in grade 3 or 4 nephrotoxicity and neurotoxicity. Based on these data, a new drug application was filed with the FDA in February 2007.
Immune Therapy
APC8015
APC8015 is an autologous CD54- positive dendritic cell vaccine loaded with a recombinant granulocyte macrophage-colony stimulating factor and a prostatic acid phosphatase fusion protein. In a phase III randomized placebo-controlled trial of 127 men with progressive asymptomatic androgen-independent prostate cancer, patients received APC8015 or placebo.30 The primary endpoint was time to disease progression. Secondary endpoints included time to onset of disease-related pain and overall survival. Although treatment with APC8015 did not result in a statistically significant delay in time to disease progression it did result in a statistically significant (P = .01) survival advantage of 4.5 months in an intent-to-treat analysis. Of note, subsequent chemotherapy with docetaxel was equally distributed in both arms. After adjusting for 20 prognostic factors, the overall treatment effect was significant at the P = .002 level. A second trial found similar results.29 It is clear that the traditional measures of outcome such as time to progression may not be appropriate for the evaluation of the efficacy of immune therapy. The observation of improved survival with APC8015 is being confirmed in the third randomized trial in men with hormone-refractory prostate cancer. Other populations are also under study, including the combination of APC8015 with bevacizumab in a phase II trial in men with hormone-sensitive prostate cancer.
One interesting subanalysis of the first 2 randomized trials evaluated the sequence of chemotherapy and immunotherapy. It has been known that T-cell activation can be observed as long as 33 months after the administration of APC8015. Thus, there can be a potential interaction between chemotherapy and immune therapy. Among the 51 patients from both studies who received APC8015 followed by docetaxel, the median survival was 34.5 months. Patients who were on placebo then docetaxel had a survival of 25.7 months, and patients treated with docetaxel alone had a median survival of 20.2 months. Thus it appears that APC8015 possibly enhances the effect of docetaxel. Further prospective studies defining the optimal treatment sequences are needed.31
GVAX® Vaccine
GVAX promotes granulocyte-macrophage colony-stimulating factor secretion through genetic modification of allogeneic prostate cancer cell lines LN CaP and PC-3.18 In a small phase II trial of men with metastatic hormone-resistant prostate cancer (N = 34), Simons and Sacks found that GVAX immunization was well tolerated.32 In a larger phase II trial (N = 80), Small and colleagues found that GVAX immunization stabilized or decreased levels of a biomarker of osteoblast activity in the majority of patients with metastatic disease.33 Two phase III trials are in progress in symptomatic and asymptomatic men with metastatic prostate cancer. The first Vaccine Immunotherapy with Allogeneic Prostate Cancer Cell Lines (VITAL) trial compares GVAX to docetaxel and prednisone in men with asymptomatic hormone-refractory prostate cancer. VITAL II will be performed in symptomatic patients and will compare GVAX combined with docetaxel and prednisone to docetaxel.
Conclusions
Docetaxel-based therapy is the FDA-approved standard of care for men with androgen-independent prostate cancer. New combinations are showing promising activity in this disease, and the optimal sequences and timing of treatment are undergoing evaluation. Ongoing phase III studies combine docetaxel with agents that target bone, tumor vasculature, and the vitamin D receptor. Second-line agents such as satraplatin have been evaluated in phase III studies. The role of immune therapy is evolving, and further studies will define the optimal timing of chemotherapy with immune therapy.
Main Points.
Limited treatment options for men with metastatic hormone-refractory prostate cancer expanded in 2004 when 2 clinical trials demonstrated a 20% to 24% survival benefit for docetaxel-based therapy compared to mitoxantrone/prednisone. The US Food and Drug Administration has approved docetaxel-based therapy, which is now the standard of care for phase III studies.
The endothelin A receptor is expressed in 71% of primary prostate cancers. A recent trial observed a significant difference in favor of the ET-1A inhibitor atrasentan versus placebo for patients with bone metastases. Proper duration of therapy remains an issue.
Thalidomide was one of the first antiangiogenic agents to be evaluated in patients with prostate cancer. A trial to evaluate the increase in toxicity of adding thalidomide to docetaxel reported median survival of 28.9 months, the highest in a phase II study of a cytotoxic agent. Monoclonal antibodies can also inhibit angiogenesis.
Proliferation of human prostate cancer cell lines is inhibited by calcitriol, the biologically active form of vitamin D, which also synergizes with chemotherapeutic agents such as docetaxel, paclitaxel, cisplatin, and carboplatin.
The platinum compound satraplatin has activity in a variety of human solid tumors. Encouraging results from Satraplatin and Prednisone Against Hormone Refractory Prostate Cancer trial have supported the filing of a new drug application with the FDA in February 2007.
Immune therapy has shown promising initial results, but its role is evolving, especially in terms of optimal timing in conjunction with chemotherapy with immune therapy.
Figure 2.
SWOG 9916 overall survival. HR, hazard ratio; CI, confidence interval. Adapted from Petrylak DP et al,2
References
- 1.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP. Chemotherapy for androgen-independent prostate cancer. Semin Urol Oncol. 2002;20(3 suppl 1):31–35. doi: 10.1053/suro.2002.35052. [DOI] [PubMed] [Google Scholar]
- 4.Berthold DR, Pond G, DeWit R, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival of the TAX 327 study [abstract 147] Proc ASCO Prostate Cancer Symposium; 2007 doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 5.Berry DL, Moinpour CM, Jiang CS, et al. Quality of life and pain in advanced stage prostate cancer: results of a Southwest Oncology Group randomized trial comparing docetaxel and estramustine to mitoxantrone and prednisone. J Clin Oncol. 2006;24:2828–2835. doi: 10.1200/JCO.2005.04.8207. [DOI] [PubMed] [Google Scholar]
- 6.Berthold DR, Pond G, DeWitt R, et al. Survival and PSA response of patients in the TAX 327 study who crossed over to recieve docetaxel after mitoxantrone or vice versa [abstract 225] Proc ASCO Prostate Cancer Symposium. 2007 doi: 10.1093/annonc/mdn288. [DOI] [PubMed] [Google Scholar]
- 7.Hudes G, Einhorn L, Ross E, et al. Vinblastine versus vinblastine plus oral estramustine phosphate for patients with hormone-refractory prostate cancer: a Hoosier Oncology Group and Fox Chase Network phase III trial. J Clin Oncol. 1999;17:3160–3166. doi: 10.1200/JCO.1999.17.10.3160. [DOI] [PubMed] [Google Scholar]
- 8.Berry W, Friedland D, Fleagle J, et al. A phase II study of weekly paclitaxel/estramustine/carboplatin in hormone-refractory prostate cancer. Clin Genitourin Cancer. 2006;5:131–137. doi: 10.3816/CGC.2006.n.029. [DOI] [PubMed] [Google Scholar]
- 9.Carducci MA, Nelson JB, Bowling MK, et al. Atrasentan, an endothelin-receptor antagonist for refractory adenocarcinomas: safety and pharmacokinetics. J Clin Oncol. 2002;20:2171–2180. doi: 10.1200/JCO.2002.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Carducci MA, Padley RJ, Breul J, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormonerefractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol. 2003;21:679–689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 11.Carducci MA, Jimeno A. Targeting bone metastasis in prostate cancer with endothelin receptor antagonists. Clin Cancer Res. 2006;12(20 Pt 2):6296s–6300s. doi: 10.1158/1078-0432.CCR-06-0929. [DOI] [PubMed] [Google Scholar]
- 12.Moore CN, Creel P, Petros W, et al. Phase I/II study of docetaxel and atrasentan in men with metastatic hormone-refractory prostate cancer (HRPC) J Clin Oncol. 2006 (Abstract 14504) [Google Scholar]
- 13.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 14.George DJ, Halabi S, Shepard TF, et al. Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormonerefractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clin Cancer Res. 2001;7:1932–1936. [PubMed] [Google Scholar]
- 15.Figg WD, Dahut W, Duray P, et al. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clin Cancer Res. 2001;7:1888–1893. [PubMed] [Google Scholar]
- 16.Reiter AS, Ando DK, Price JL, et al. Follow-up analysis of a randomized phase II study of docetaxel and thalidomide in androgen-independent prostate cancer. Updated survival and CYP2C19 mutation status [abstract 265] Proc ASCO Prostate Cancer Symposium. 2005 [Google Scholar]
- 17.Moss RA, Mohile SG, G. S, Melia J, Petrylak DP. Phase I open-label study using lenalidomide and docetaxel in androgen independent prostate cancer (AIPC) [abstract 89] Proc ASCO Prostate Cancer Symposium. 2007 [Google Scholar]
- 18.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 19.Picus J, Halabi S, Rini B, et al. The use of bevacizumab (B) with docetaxel (D) and estramustine (E) in hormone refractory prostate cancer (HRPC): Initial results of CALGB 90006. Proc Am Soc Clin Oncol. 2003;22 (Abstract 1578) [Google Scholar]
- 20.Wang YR, Wigington DP, Strugnell SA, Knutson JC. Growth inhibition of cancer cells by an active metabolite of a novel vitamin D prodrug. Anticancer Res. 2005;25:4333–4339. [PubMed] [Google Scholar]
- 21.Getzenberg RH, Light BW, Lapco PE, et al. Vitamin D inhibition of prostate adenocarcinoma growth and metastasis in the Dunning rat prostate model system. Urology. 1997;50:999–1006. doi: 10.1016/S0090-4295(97)00408-1. [DOI] [PubMed] [Google Scholar]
- 22.Blutt SE, Polek TC, Stewart LV, et al. A calcitriol analogue, EB1089, inhibits the growth of LNCaP tumors in nude mice. Cancer Res. 2000;60:779–782. [PubMed] [Google Scholar]
- 23.Hershberger PA, Yu WD, Modzelewski RA, et al. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin Cancer Res. 2001;7:1043–1051. [PubMed] [Google Scholar]
- 24.Beer TM, Hough KM, Garzotto M, et al. Weekly high-dose calcitriol and docetaxel in advanced prostate cancer. Semin Oncol. 2001;28(4 suppl 15):49–55. doi: 10.1016/s0093-7754(01)90155-1. [DOI] [PubMed] [Google Scholar]
- 25.Beer TM, Ryan CW, Venner PM, et al. Double-blinded randomized study of high-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT Investigators. J Clin Oncol. 2007;25:669–674. doi: 10.1200/JCO.2006.06.8197. [DOI] [PubMed] [Google Scholar]
- 26.Beer TM, Venner PM, Ryan CW, et al. High dose calcitriol may reduce thrombosis in cancer patients. Br J Haematol. 2006;135:392–394. doi: 10.1111/j.1365-2141.2006.06322.x. [DOI] [PubMed] [Google Scholar]
- 27.Sternberg CN. Satraplatin in the treatment of hormone-refractory prostate cancer. BJU Int. 2005;96:990–994. doi: 10.1111/j.1464-410X.2005.05799.x. [DOI] [PubMed] [Google Scholar]
- 28.Sternberg CN, Whelan P, Hetherington J, et al. Phase III trial of satraplatin, an oral platinum plus prednisone vs. prednisone alone in patients with hormone-refractory prostate cancer. Oncology. 2005;68:2–9. doi: 10.1159/000084201. [DOI] [PubMed] [Google Scholar]
- 29.Petrylak DP, Sartor O, Witjes F, et al. A phase III, randomized, double-blind trial of satraplatin and prednisone vs placebo and prednisone for patients with hormone refractory prostate cancer (HRPC) [abstract 145] Proc ASCO Prostate Cancer Symposium. 2007 [Google Scholar]
- 30.Small EJ, Schellhammer PF, Higano CS, et al. Results of a placebo-controlled phase III trial of immunotherapy with APC8015 for patients with hormone refractory prostate cancer (HRPC) Proc Am Soc Clin Oncol. 2005;23 doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 31.Petrylak DP, Small E, Schellhammer PF. Androgen independent prostate cancer (AIPC) patients who receive sipuleucel-T followed by docetaxel have prolonged survival. Proc Am Urol Association. 2007 [Google Scholar]
- 32.Simons JW, Sacks N. Granulocyte-macrophage colony-stimulating factor-transduced allogeneic cancer cellular immunotherapy: the GVAX vaccine for prostate cancer. Urol Oncol. 2006;24:419–424. doi: 10.1016/j.urolonc.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 33.Small E, Higano C, Smith D, et al. Analysis of prognostic variables in phase II trials of GVAX vaccine for prostate cancer in metastatic hormone refractory prostate cancer [abstract 254] Proc ASCO Prostate Cancer Symposium. 2006 [Google Scholar]