Abstract
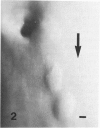
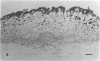
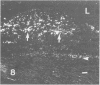
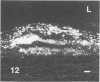
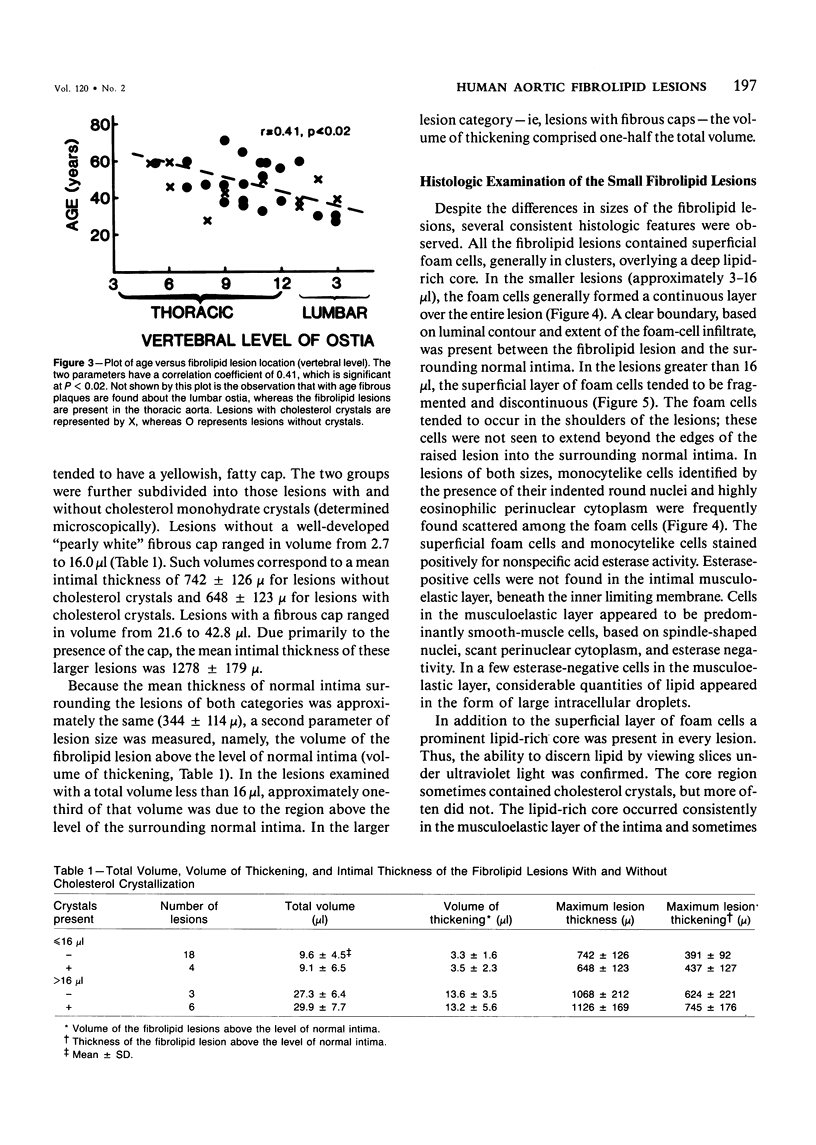
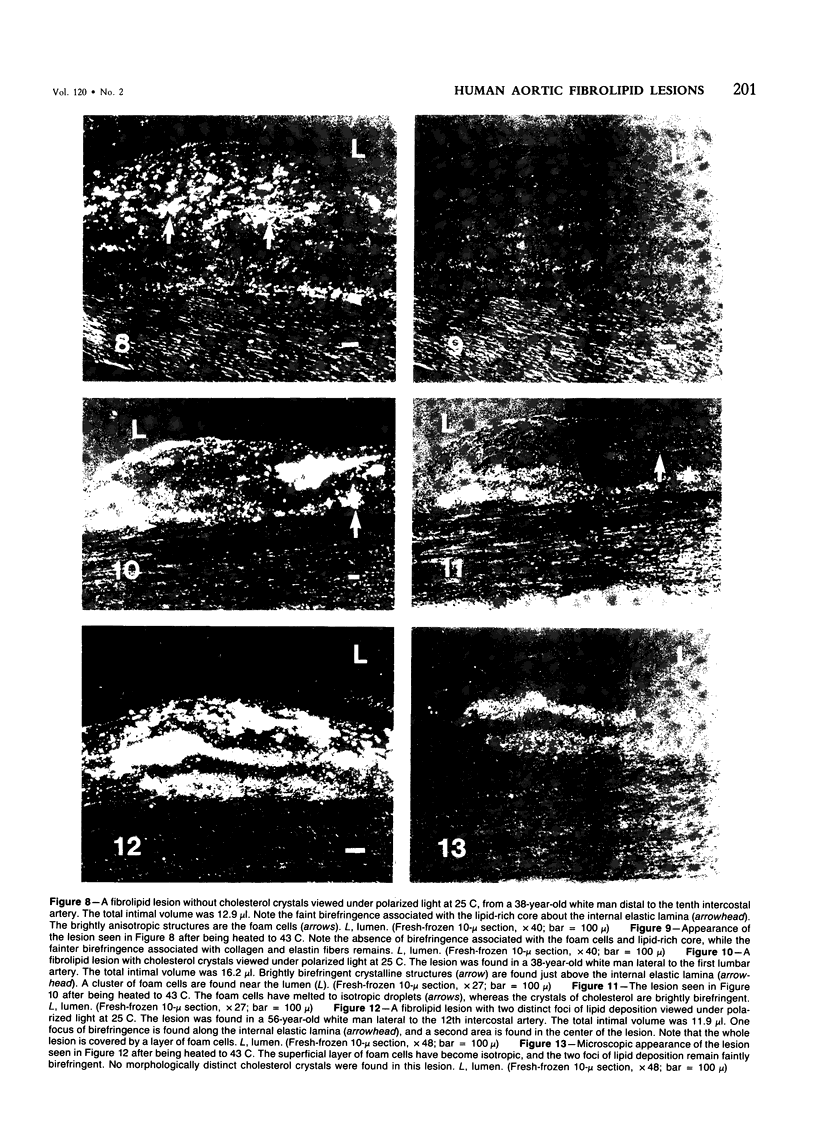
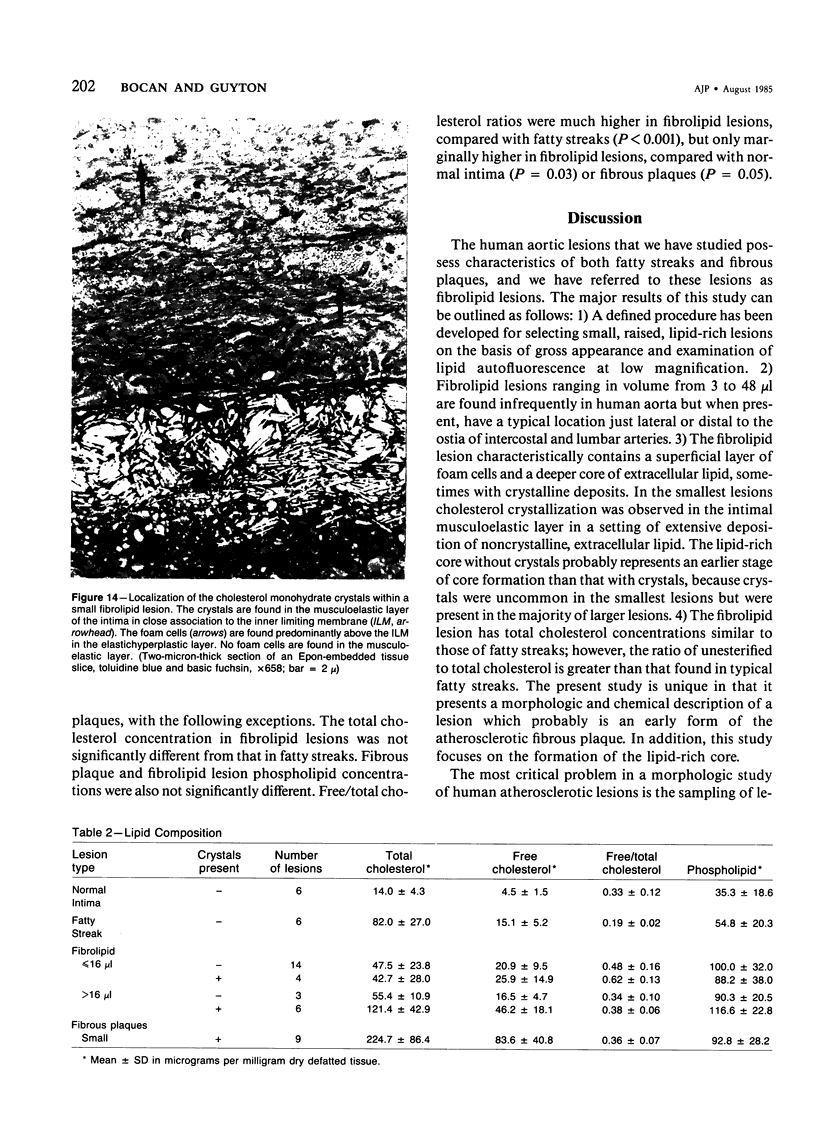
The early development of the lipid-rich core and other features of atherosclerotic fibrous plaques has been elucidated by examining discrete, small regions of raised intima in human aorta, which often bear a resemblance to both fatty streaks and fibrous plaques. Approximately one-fourth of small raised lesions (less than 16 sq mm of surface area) contained little or no stainable lipid, while three-fourths had a characteristic appearance, which included a superficial layer of foam cells, a core of noncrystalline and/or crystalline lipid, and a developed or developing collagenous cap. Total intimal volumes of the lipid-containing lesions, termed "fibrolipid lesions," ranged from 3 to 43 microliters, with the majority less than 16 microliters. Core lipid in the smallest lesions was located in the musculoelastic layer of the intima. In larger lesions the core extended luminally into the elastic hyperplastic layer, and cholesterol crystals were found more frequently. Total cholesterol concentration in fibrolipid lesions was similar to that in fatty streaks; however, the ratio of unesterified to total cholesterol was relatively high, similar to that found in fibrous plaques. It is concluded that 1) the formation of a lipid-rich core and cholesterol crystallization are early events in the development of many raised lesions; 2) the consistent association between the superficial layer of foam cells and the deep-lying lipid-rich core raises the possibility of an influence, possibly indirect, of foam-cell lipid metabolism on core formation; and 3) the fibrolipid lesion may represent one stage in a potential transitional morphologic sequence between fatty streak and fibrous plaque.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baginski E. S., Foà P. P., Zak B. Microdetermination of inorganic phosphate, phospholipids, and total phosphate in biologic materials. Clin Chem. 1967 Apr;13(4):326–332. [PubMed] [Google Scholar]
- DAOUD A., JARMOLYCH J., ZUMBO A., FANI, FLORENTIN R. PREATHEROMA PHASE OF CORONARY ATHEROSCLEROSIS IN MAN. Exp Mol Pathol. 1964 Oct;90:475–484. doi: 10.1016/0014-4800(64)90028-0. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Hata Y., Hower J., Insull W., Jr Cholesteryl ester-rich inclusions from human aortic fatty streak and fibrous plaque lesions of atherosclerosis. I. Crystalline properties, size and internal structure. Am J Pathol. 1974 Jun;75(3):423–456. [PMC free article] [PubMed] [Google Scholar]
- Haust M. D. The morphogenesis and fate of potential and early atherosclerotic lesions in man. Hum Pathol. 1971 Mar;2(1):1–29. doi: 10.1016/s0046-8177(71)80019-9. [DOI] [PubMed] [Google Scholar]
- Katz S. S., Shipley G. G., Small D. M. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976 Jul;58(1):200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramsch D. M., Franzblau C., Hollander W. The protein and lipid composition of arterial elastin and its relationship to lipid accumulation in the atherosclerotic plaque. J Clin Invest. 1971 Aug;50(8):1666–1677. doi: 10.1172/JCI106656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruth H. S. Localization of unesterified cholesterol in human atherosclerotic lesions. Demonstration of filipin-positive, oil-red-O-negative particles. Am J Pathol. 1984 Feb;114(2):201–208. [PMC free article] [PubMed] [Google Scholar]
- Malcom G. T., Strong J. P., Restrepo C. Atherosclerosis and lipid composition of the abdominal aorta. Comparison of autopsied New Orleans and Guatemalan men. Lab Invest. 1984 Jan;50(1):79–86. [PubMed] [Google Scholar]
- Ridolfi R. L., Hutchins G. M. The relationship between coronary artery lesions and myocardial infarcts: ulceration of atherosclerotic plaques precipitating coronary thrombosis. Am Heart J. 1977 Apr;93(4):468–486. doi: 10.1016/s0002-8703(77)80410-9. [DOI] [PubMed] [Google Scholar]
- Rothblat G. H., Phillips M. C. Mechanism of cholesterol efflux from cells. Effects of acceptor structure and concentration. J Biol Chem. 1982 May 10;257(9):4775–4782. [PubMed] [Google Scholar]
- SMITH E. B. THE INFLUENCE OF AGE AND ATHEROSCLEROSIS ON THE CHEMISTRY OF AORTIC INTIMA. 1. THE LIPIDS. J Atheroscler Res. 1965 Mar-Apr;5(2):224–240. doi: 10.1016/s0368-1319(65)80064-3. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Ross R. Cellular proliferation in atherosclerosis and hypertension. Prog Cardiovasc Dis. 1984 Mar-Apr;26(5):355–372. doi: 10.1016/0033-0620(84)90010-0. [DOI] [PubMed] [Google Scholar]
- Smith E. B., Evans P. H., Downham M. D. Lipid in the aortic intima. The correlation of morphological and chemical characteristics. J Atheroscler Res. 1967 Mar-Apr;7(2):171–186. doi: 10.1016/s0368-1319(67)80079-6. [DOI] [PubMed] [Google Scholar]
- Smith E. B., Slater R. S., Chu P. K. The lipids in raised fatty and fibrous lesions in human aorta. A comparison of the changes at different stages of development. J Atheroscler Res. 1968 May-Jun;8(3):399–419. doi: 10.1016/s0368-1319(68)80097-3. [DOI] [PubMed] [Google Scholar]
- Smith E. B. The relationship between plasma and tissue lipids in human atherosclerosis. Adv Lipid Res. 1974;12(0):1–49. doi: 10.1016/b978-0-12-024912-1.50008-9. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Colombo M., Gonzales J. J., Hollander W., Schmid K. The glycosaminoglycans of the human artery and their changes in atherosclerosis. J Clin Invest. 1976 Aug;58(2):470–481. doi: 10.1172/JCI108491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy R. E., Kissling G. E., Devaney K. O., Lopez C. R., Toca V. T., Gandia M. Spatial dispersion of foam cells in the human thoracic aorta. Lab Invest. 1983 Dec;49(6):693–701. [PubMed] [Google Scholar]
- Tracy R. E., Strong J. P., Toca V. T., Lopez C. R. Atheronecrosis and its fibroproliferative base and cap in the thoracic aorta. Lab Invest. 1979 Dec;41(6):546–552. [PubMed] [Google Scholar]
- Velican C., Velican D. Heterogeneity in the composition and aggregation patterns of coronary intima acid mucopolysaccharides (glycosaminoglycans). Atherosclerosis. 1978 Feb;29(2):141–159. doi: 10.1016/0021-9150(78)90004-7. [DOI] [PubMed] [Google Scholar]