Abstract
In the present study the authors attempt to experimentally produce the characteristic cerebriform appearance of Sézary cells from normal cells by stimulation in vitro with a variety of mitogens and antigens. Lymphocytes were stimulated with the mitogens phytohemagglutinin (PHA), concanavalin A, and pokeweed mitogen and the antigens spherulin, Candida, and herpes simplex virus Type I. In vitro blast transformation was measured in tritiated thymidine uptake microculture systems. Nuclear contours were morphometrically analyzed with the use of a computerized planimeter. An exhaustive search for Sézary-type cells did not reveal any nuclear profiles that were highly convoluted. The lowest form factor value obtained from the stimulated cultures was 0.14. This value represents 14% of a circle. All stimulated cultures analyzed morphometrically showed significant blast transformation. There was no correlation between the degree of stimulation and the nuclear contour values. An analysis of mean form factor values from ten separate lymphoid populations revealed that neither mitogens nor antigens had any effect on increasing the nuclear irregularity of lymphoid cells. One PHA-stimulated culture, in fact, had less irregular nuclear contours than the 3-day unstimulated control cells. The difference in these means was statistically significant (P less than 0.05). In addition, morphometric analysis of 73 published electron micrographs of human lymphoid cells from a variety of clinical and experimental conditions showing irregular nuclear contours revealed that only 5% had nuclei that were more irregular than the most convoluted nuclei analyzed from lymphocytes stimulated in vitro in the present study. The authors have shown that mitogen- and antigen-stimulated cells do not display the highly irregular nuclear outlines commonly seen in the Sézary syndrome and mycosis fungoides and emphasize the need for more objective analysis of Sézary-like nuclear profiles to determine their specificity.
Full text
PDF




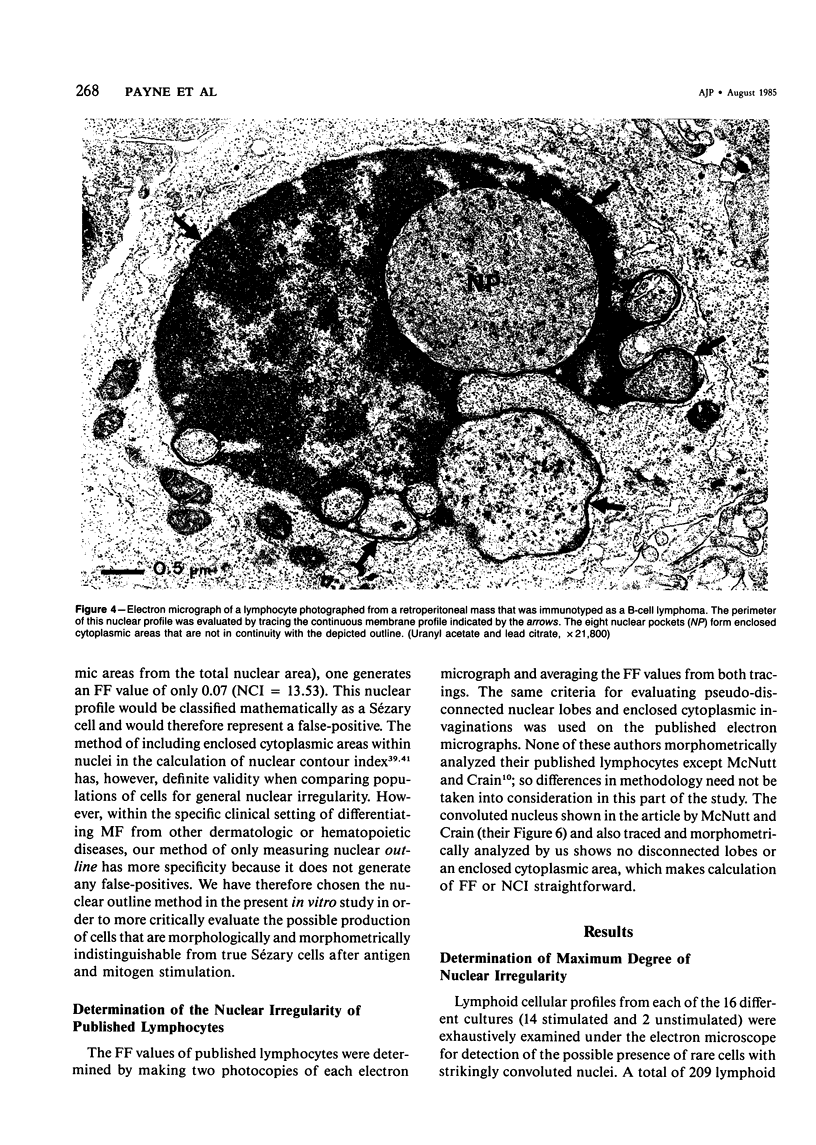







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger C. L., Warburton D., Raafat J., LoGerfo P., Edelson R. L. Cutaneous T-cell lymphoma: neoplasm of T cells with helper activity. Blood. 1979 Apr;53(4):642–651. [PubMed] [Google Scholar]
- Biberfeld P. Morphogenesis in blood lymphocytes stimulated with phytohaemagglutinin (PHA). A light and electron microscopic study. Acta Pathol Microbiol Scand Suppl. 1971;223(Suppl):1–70. [PubMed] [Google Scholar]
- Broder S., Edelson R. L., Lutzner M. A., Nelson D. L., MacDermott R. P., Durm M. E., Goldman C. K., Meade B. D., Waldmann T. A. The Sézary syndrome: a malignant proliferation of helper T cells. J Clin Invest. 1976 Dec;58(6):1297–1306. doi: 10.1172/JCI108585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee T. R., Murad T. M. Ultrastructure of mycosis fungoides. Cancer. 1970 Sep;26(3):686–698. doi: 10.1002/1097-0142(197009)26:3<686::aid-cncr2820260330>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Brunning R. D., Parkin J. Ultrastructural studies of parallel tubular arrays in human lymphocytes. Am J Pathol. 1975 Jan;78(1):59–70. [PMC free article] [PubMed] [Google Scholar]
- Burkhardt A., Bos I. R., Löning T., Gebbers J. O., Otto H. F., Seifert G. Interepithelial cells of the oral mucosa in mice. An ultrastructural classification with reflections on the origin of the Langerhans cell. Virchows Arch A Pathol Anat Histol. 1979;384(2):223–244. doi: 10.1007/BF00427258. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chapman J. A., Elves M. W., Gough J. An electron-microscope study of the in vitro transformation of human leucocytes. I. Transformation of lymphocytes to blastoid cells in the presence of phytohaemagglutinin. J Cell Sci. 1967 Sep;2(3):359–370. doi: 10.1242/jcs.2.3.359. [DOI] [PubMed] [Google Scholar]
- Dardick I., Setterfield G., Hall R., Bladon T., Little J., Kaplan G. Nuclear alterations during lymphocyte transformation: relationship to the heterogeneous morphologic presentations of non-Hodgkin's lymphomas. Am J Pathol. 1981 Apr;103(1):10–20. [PMC free article] [PubMed] [Google Scholar]
- Dardick I., Sinnott N. M., Hall R., Bajenko-Carr T. A., Setterfield G. Nuclear morphology and morphometry of B-lymphocyte transformation. Implications for follicular center cell lymphomas. Am J Pathol. 1983 Apr;111(1):35–49. [PMC free article] [PubMed] [Google Scholar]
- Douglas S. D., Cohnen G., Brittinger G. Ultrastructural comparison between phytomitogen transformed normal and chronic lymphocytic leukemia lymphocytes. J Ultrastruct Res. 1973 Jul;44(1):11–26. doi: 10.1016/s0022-5320(73)90013-0. [DOI] [PubMed] [Google Scholar]
- Douglas S. D. Human lymphocyte growth in vitro: morphologic, biochemical, and immunologic significance. Int Rev Exp Pathol. 1971;10:41–114. [PubMed] [Google Scholar]
- Edelson R. L. Cutaneous T cell lymphoma: mycosis fungoides, Sézary syndrome, and other variants. J Am Acad Dermatol. 1980 Feb;2(2):89–106. doi: 10.1016/s0190-9622(80)80385-9. [DOI] [PubMed] [Google Scholar]
- Fisher E. R., Horvat B. L., Wechsler H. L. Ultrastructural features of mycosis fungoides. Am J Clin Pathol. 1972 Aug;58(2):99–110. doi: 10.1093/ajcp/58.2.99. [DOI] [PubMed] [Google Scholar]
- Flaxman B. A., Zelazny G., Van Scott E. J. Nonspecificity of characteristic cells in mycosis fungoides. Arch Dermatol. 1971 Aug;104(2):141–147. [PubMed] [Google Scholar]
- Gisser S. D., Young I. Mycosis fungoides-like cells. Their presence in a case of pityriasic dermatitis with a comment on their significance as an indicator of primary T-cell dyscrasia. Am J Surg Pathol. 1978 Mar;2(1):97–101. [PubMed] [Google Scholar]
- Glasser L., Hicks M. J., Lindberg R. E., Jones J. F. The effect of in vivo dexamethasone on lymphocyte subpopulations: differential response of EAhu rosette-forming cells. Clin Immunol Immunopathol. 1981 Jan;18(1):22–31. doi: 10.1016/0090-1229(81)90003-9. [DOI] [PubMed] [Google Scholar]
- Hamburg A., Brynes R. K., Reese C., Golomb H. M. Human cord blood lymphocytes. Ultrastructural and immunologic surface marker characteristics; a comparison with B- and T-cell lymphomas. Lab Invest. 1976 Feb;34(2):207–215. [PubMed] [Google Scholar]
- Haneke E., Tulusan A. H., Weidner F. Histological features of "pagetoid reticulosis" (Woringer-Kolopp) in pre-mycosis fungoides. Arch Dermatol Res. 1977 May 27;258(3):265–273. doi: 10.1007/BF00561129. [DOI] [PubMed] [Google Scholar]
- Hicks M. J., Jones J. F., Thies A. C., Minnich L. L. The effect of lymphocyte recovery on lymphocyte typing results. Am J Clin Pathol. 1981 Dec;76(6):745–752. doi: 10.1093/ajcp/76.6.745. [DOI] [PubMed] [Google Scholar]
- Hicks M. J., Jones J. F., Thies A. C., Weigle K. A., Minnich L. L. Age-related changes in mitogen-induced lymphocyte function from birth to old age. Am J Clin Pathol. 1983 Aug;80(2):159–163. doi: 10.1093/ajcp/80.2.159. [DOI] [PubMed] [Google Scholar]
- Honma T., Saito T., Fujioka Y. Intraepithelial atypical lymphocytes in oral lesions of Behçet's syndrome. Arch Dermatol. 1981 Feb;117(2):83–85. [PubMed] [Google Scholar]
- Johnson S. C., Cripps D. J., Norback D. H. Actinic reticuloid. A clinical, pathologic, and action spectrum study. Arch Dermatol. 1979 Sep;115(9):1078–1083. doi: 10.1001/archderm.115.9.1078. [DOI] [PubMed] [Google Scholar]
- Laurent R., Agache P. Lymphomatoid papulosis. An ultrastructural study of 2 cases. Arch Dermatol Forsch. 1974;251(1):1–9. doi: 10.1007/BF00561704. [DOI] [PubMed] [Google Scholar]
- Litovitz T. L., Lutzner M. A. Quantitative measurements of blood lymphocytes from patients with chronic lymphocytic leukemia and the Sézary syndrome. J Natl Cancer Inst. 1974 Jul;53(1):75–77. doi: 10.1093/jnci/53.1.75. [DOI] [PubMed] [Google Scholar]
- Lutzner M. A., Hobbs J. W., Horvath P. Ultrastructure of abnormal cells in Sezary syndrome, mycosis fungoides, and parapsoriasis en plaque. Arch Dermatol. 1971 Apr;103(4):375–386. doi: 10.1001/archderm.103.4.375. [DOI] [PubMed] [Google Scholar]
- Lutzner M. A., Jordan H. W. The ultrastructure of an abnormal cell in Sézary's syndrome. Blood. 1968 Jun;31(6):719–726. [PubMed] [Google Scholar]
- Lutzner M., Edelson R., Schein P., Green I., Kirkpatrick C., Ahmed A. Cutaneous T-cell lymphomas: the Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975 Oct;83(4):534–552. doi: 10.7326/0003-4819-83-4-534. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Crain W. R. Quantitative electron microscopic comparison of lymphocyte nuclear contours in mycosis fungoides and in benign infiltrates in skin. Cancer. 1981 Feb 15;47(4):698–709. doi: 10.1002/1097-0142(19810215)47:4<698::aid-cncr2820470413>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Meijer C. J., van der Loo E. M., van Vloten W. A., van der Velde E. A., Scheffer E., Cornelisse C. J. Early diagnosis of mycosis fungoides and Sézary's syndrome by morphometric analysis of lymphoid cells in the skin. Cancer. 1980 Jun 1;45(11):2864–2871. doi: 10.1002/1097-0142(19800601)45:11<2864::aid-cncr2820451124>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Meyer C. J., van Leeuwen A. W., van der Loo E. M., van de Putte L. B., van Vloten W. A. Cerebriform (Sézary like) mononuclear cells in healthy individuals: a morphologically distinct population of T cells. Relationship with mycosis fungoides and Sézary's syndrome. Virchows Arch B Cell Pathol. 1977 Oct 27;25(2):95–104. doi: 10.1007/BF02889424. [DOI] [PubMed] [Google Scholar]
- Orfanos C. E., Schmidt H. W., Mahrle G., Gartmann H., Lever W. F. Retinoic acid in psoriasis: its value for topical therapy with and without corticosteroids. Clinical, histological nd electron microscopical studies on forty-four hospitalized patients with extensive psoriasis. Br J Dermatol. 1973 Feb;88(2):167–182. doi: 10.1111/j.1365-2133.1973.tb07522.x. [DOI] [PubMed] [Google Scholar]
- Payne C. M., Glasser L. The effect of steroids on peripheral blood lymphocytes containing parallel tubular arrays. Am J Pathol. 1978 Sep;92(3):611–618. [PMC free article] [PubMed] [Google Scholar]
- Payne C. M., Nagle R. B., Lynch P. J. Quantitative electron microscopy in the diagnosis of mycosis fungoides. A simple analysis of lymphocytic nuclear convolutions. Arch Dermatol. 1984 Jan;120(1):63–75. [PubMed] [Google Scholar]
- Payne C. M. Platelet satellitism: an ultrastructural study. Am J Pathol. 1981 Apr;103(1):116–128. [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. Current concepts in immunology: Regulation of the immune response--inducer and suppressor T-lymphocyte subsets in human beings. N Engl J Med. 1980 Aug 14;303(7):370–373. doi: 10.1056/NEJM198008143030704. [DOI] [PubMed] [Google Scholar]
- Rosenthal C. J., Noguera C. A., Coppola A., Kapelner S. N. Pseudolymphoma with mycosis fungoides manifestations, hyperresponsiveness to diphenylhydantoin, and lymphocyte disregulation. Cancer. 1982 Jun 1;49(11):2305–2314. doi: 10.1002/1097-0142(19820601)49:11<2305::aid-cncr2820491118>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Rüttner J. R., Pedio G. 'Sezary-like' cells in malignant and non-malignant diseases. Exp Cell Biol. 1977;45(3-4):141–146. [PubMed] [Google Scholar]
- Schrek R., Mayron L. W., Knospe W. H. Quantitative electron microscopy of normal and leukaemic lymphocytes. Lancet. 1971 Feb 13;1(7694):348–349. doi: 10.1016/s0140-6736(71)91076-2. [DOI] [PubMed] [Google Scholar]
- Schrek R. Ultrastructure of blood lymphocytes from chronic lymphocytic and lymphosarcoma cell leukemia. J Natl Cancer Inst. 1972 Jan;48(1):51–64. [PubMed] [Google Scholar]
- Setterfield G., Hall R., Bladon T., Little J., Kaplan J. G. Changes in structure and composition of lymphocyte nuclei during mitogenic stimulation. J Ultrastruct Res. 1983 Mar;82(3):264–282. doi: 10.1016/s0022-5320(83)80014-8. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Streilein J. W. Lymphocyte traffic, T-cell malignancies and the skin. J Invest Dermatol. 1978 Sep;71(3):167–171. doi: 10.1111/1523-1747.ep12547071. [DOI] [PubMed] [Google Scholar]
- Tan R. S., Butterworth C. M., McLaughlin H., Malka S., Samman P. D. Mycosis fungoides--a disease of antigen persistence. Br J Dermatol. 1974 Dec;91(6):607–616. doi: 10.1111/j.1365-2133.1974.tb12449.x. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K., Madden S. C., Zeldis L. J. Fine structural alterations of interphase nuclei of lymphocytes stimulated to grwoth activity in vitro. J Cell Biol. 1968 Dec;39(3):630–660. doi: 10.1083/jcb.39.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsambaos D., Orfanos C. E. Ultrastructural evidence suggesting an immunomodulatory activity of oral retinoid. Its effect on dermal components in psoriasis. Br J Dermatol. 1981 Jan;104(1):37–45. doi: 10.1111/j.1365-2133.1981.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Underwood J. C., Carr I. The ultrastructure of the lymphoreticular cells in non-lymphoid human neoplasms. Virchows Arch B Cell Pathol. 1972;12(1):39–50. doi: 10.1007/BF02893984. [DOI] [PubMed] [Google Scholar]
- Yeckley J. A., Weston W. L., Thorne E. G., Krueger G. G. Production of Sézary-like cells from normal human lymphocytes. Arch Dermatol. 1975 Jan;111(1):29–32. [PubMed] [Google Scholar]
- Zucker-Franklin D. Thymus-dependent lymphocytes in lymphoproliferative disorders of the skin (Sézary syndrome and mycosis fungoides). J Invest Dermatol. 1976 Sep;67(3):412–418. doi: 10.1111/1523-1747.ep12514715. [DOI] [PubMed] [Google Scholar]
- de Vries E. A., van Leeuwen A. W., van de Putte L. B., Lafeber G. J., Meyer C. J. Atypical T cells in rheumatoid synovial membranes. Virchows Arch B Cell Pathol. 1977 May 3;24(1):19–26. doi: 10.1007/BF02889263. [DOI] [PubMed] [Google Scholar]
- van Leeuwen A. W., Meyer C. J., van de Putte L. B., de Vries E., de Man J. C. Letter: Sezary type cells in rheumatoid synovial fluid. Lancet. 1976 Jan 31;1(7953):248–249. doi: 10.1016/s0140-6736(76)91367-2. [DOI] [PubMed] [Google Scholar]
- van der Loo E. M., Cnossen J., Meijer C. J. Morphological aspects of T cell subpopulations in human blood: characterization of the cerebriform mononuclear cells in healthy individuals. Clin Exp Immunol. 1981 Mar;43(3):506–516. [PMC free article] [PubMed] [Google Scholar]
- van der Loo E. M., van Vloten W. A., Cornelisse C. J., Scheffer E., Meijer C. J. The relevance of morphometry in the differential diagnosis of cutaneous T cell lymphomas. Br J Dermatol. 1981 Mar;104(3):257–269. doi: 10.1111/j.1365-2133.1981.tb00947.x. [DOI] [PubMed] [Google Scholar]






