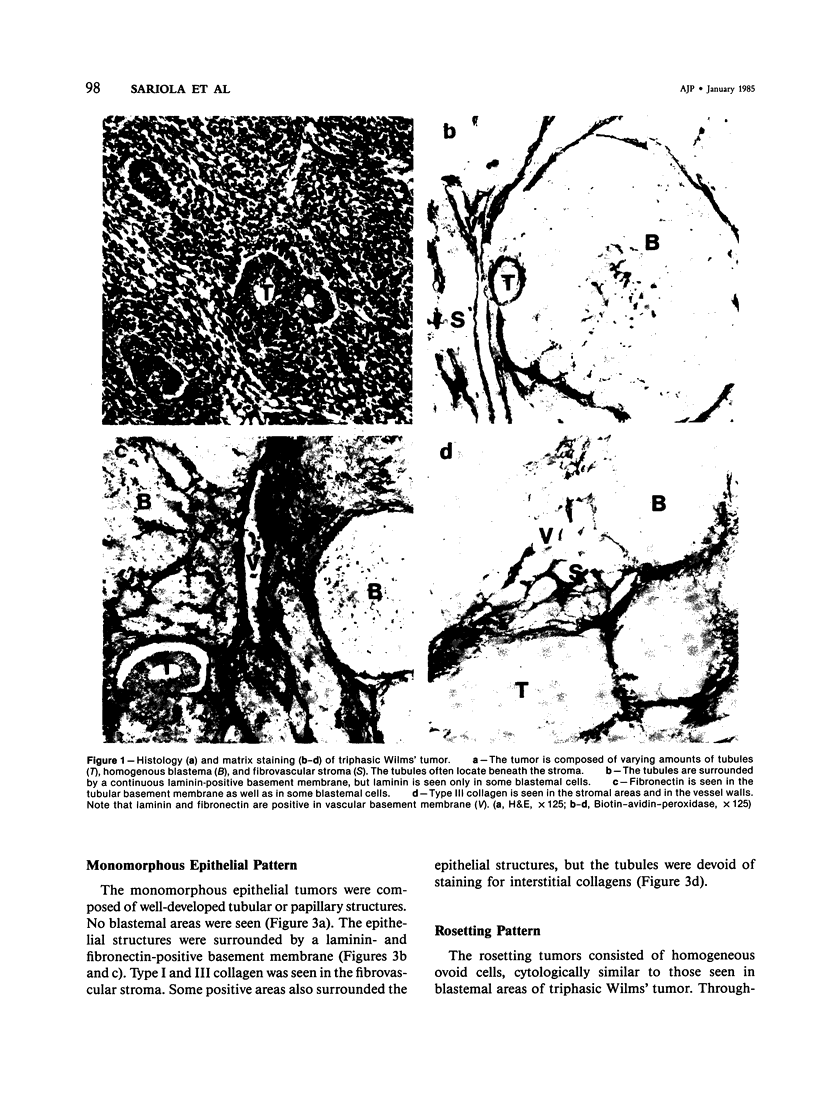
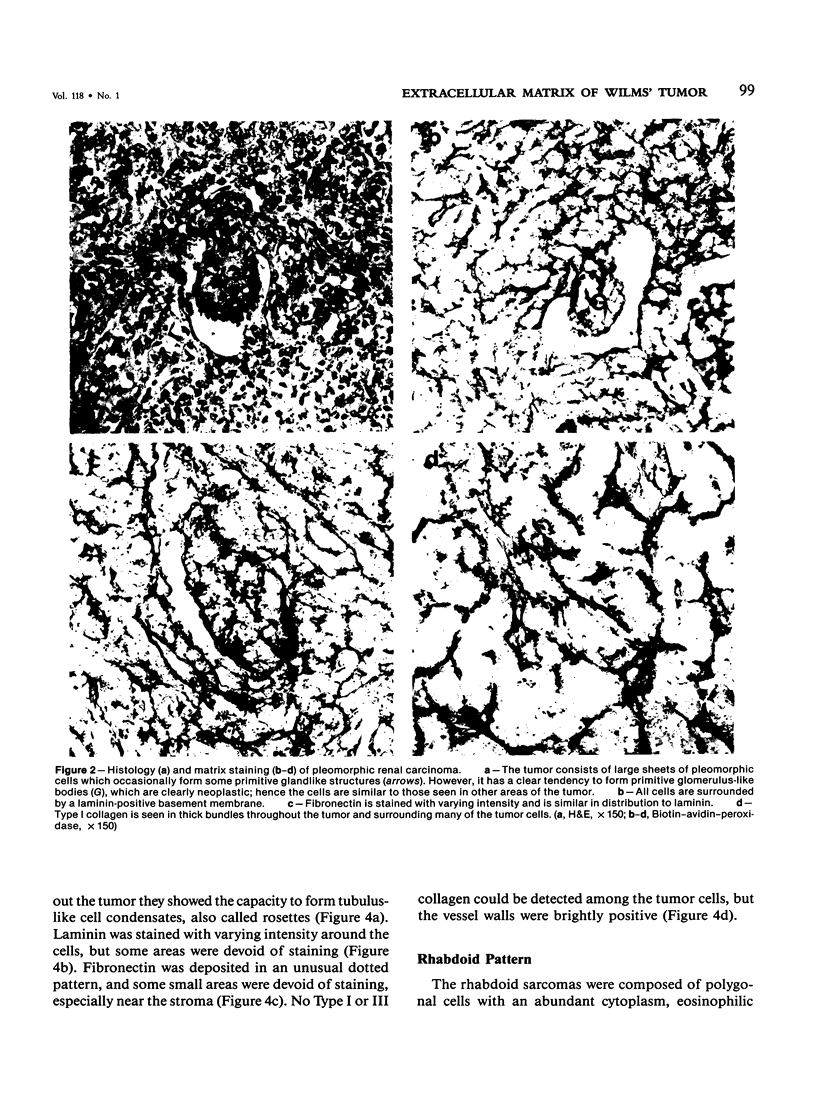
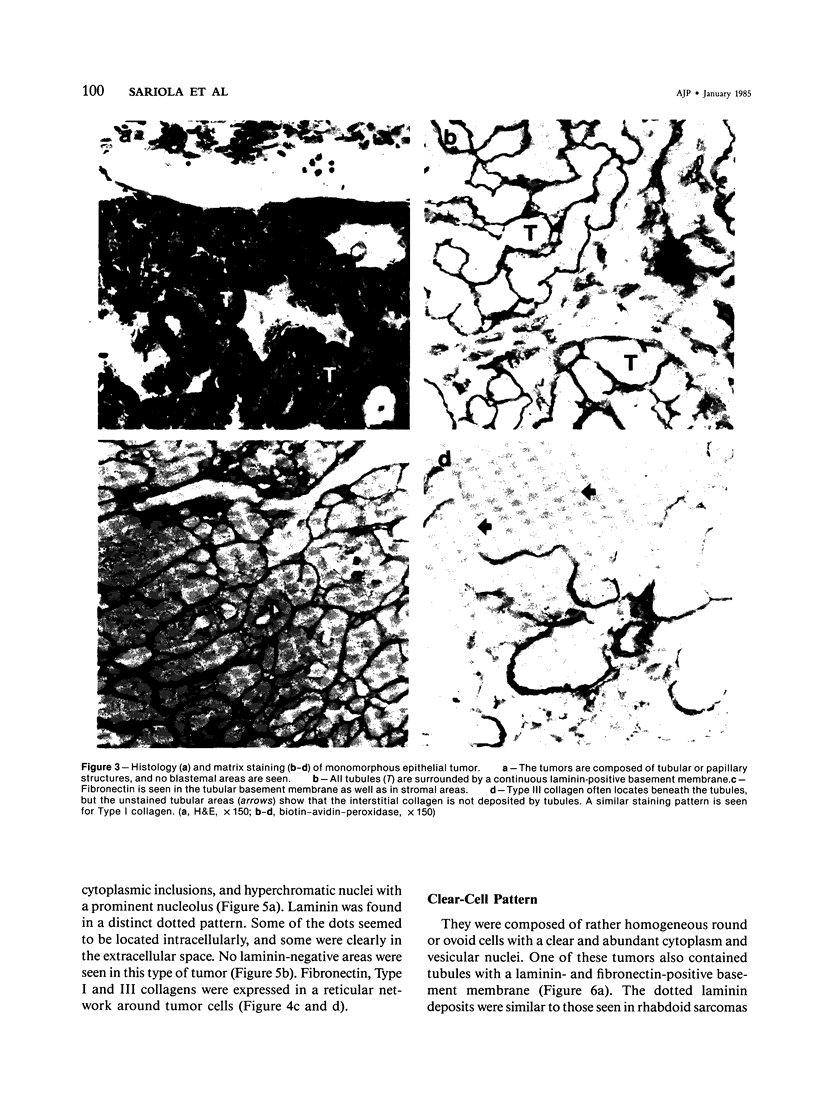
Abstract
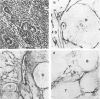
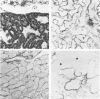
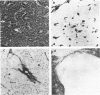
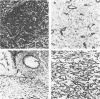
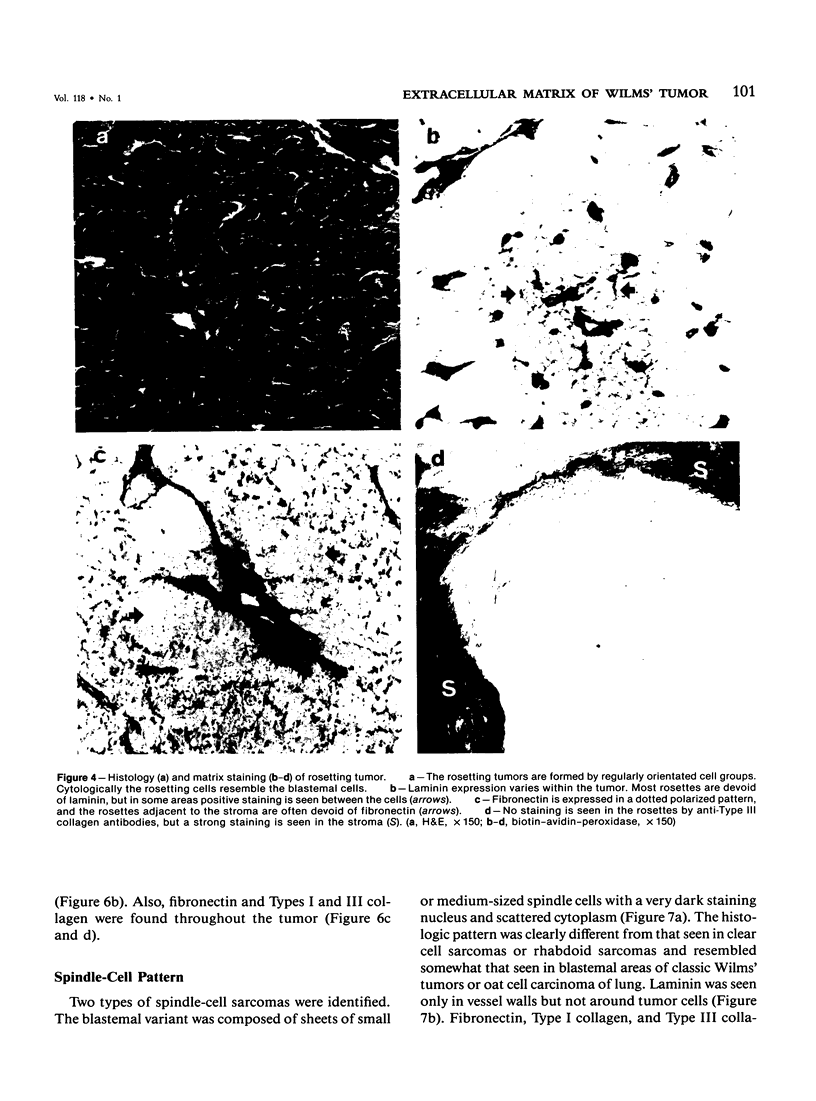
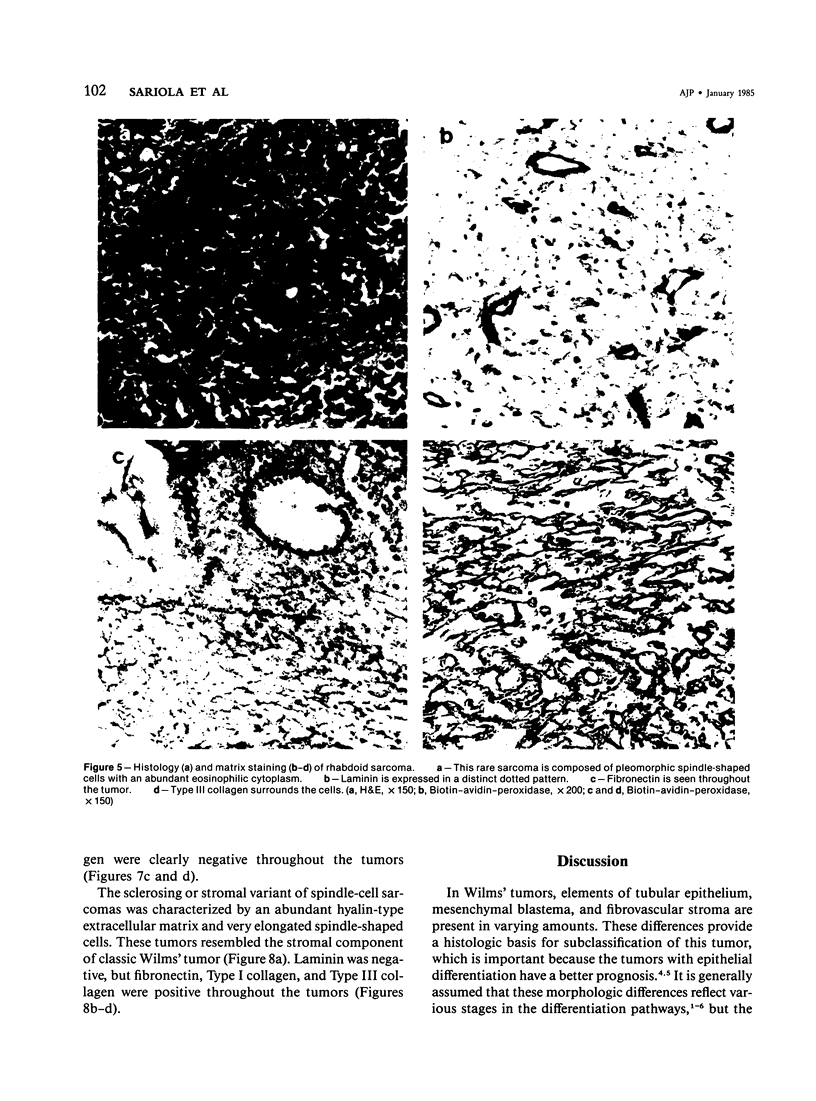
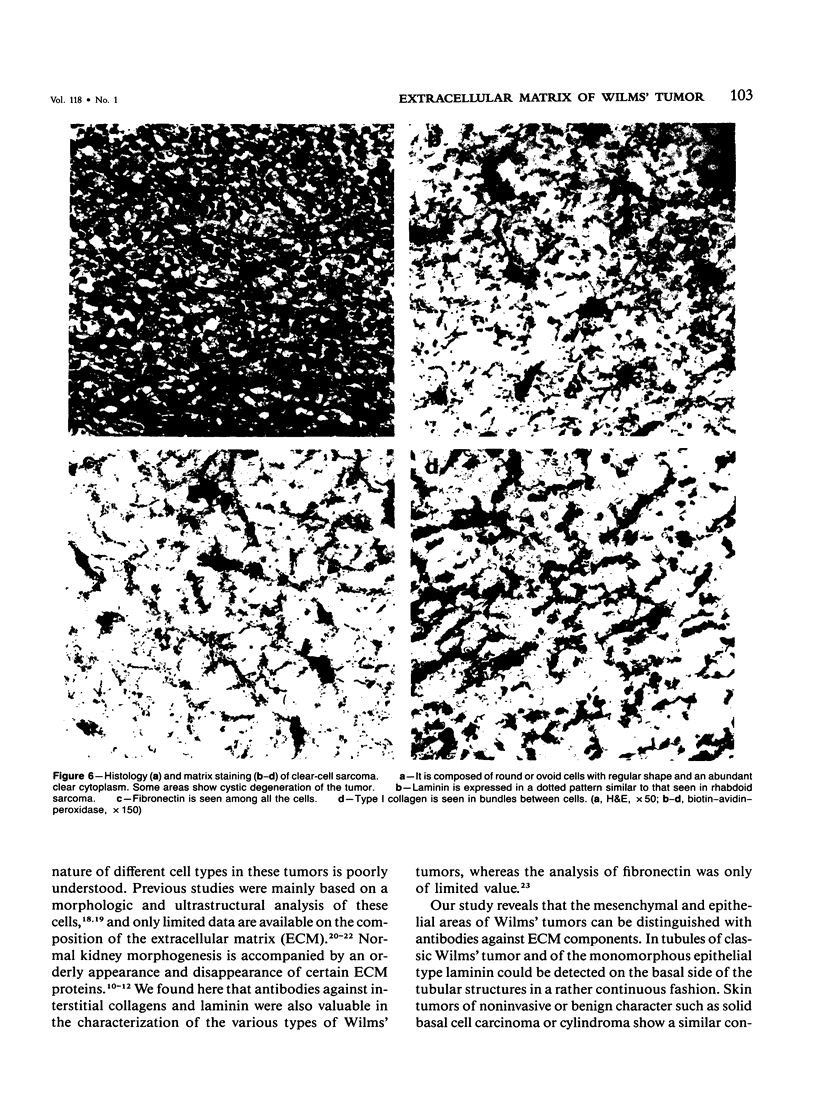
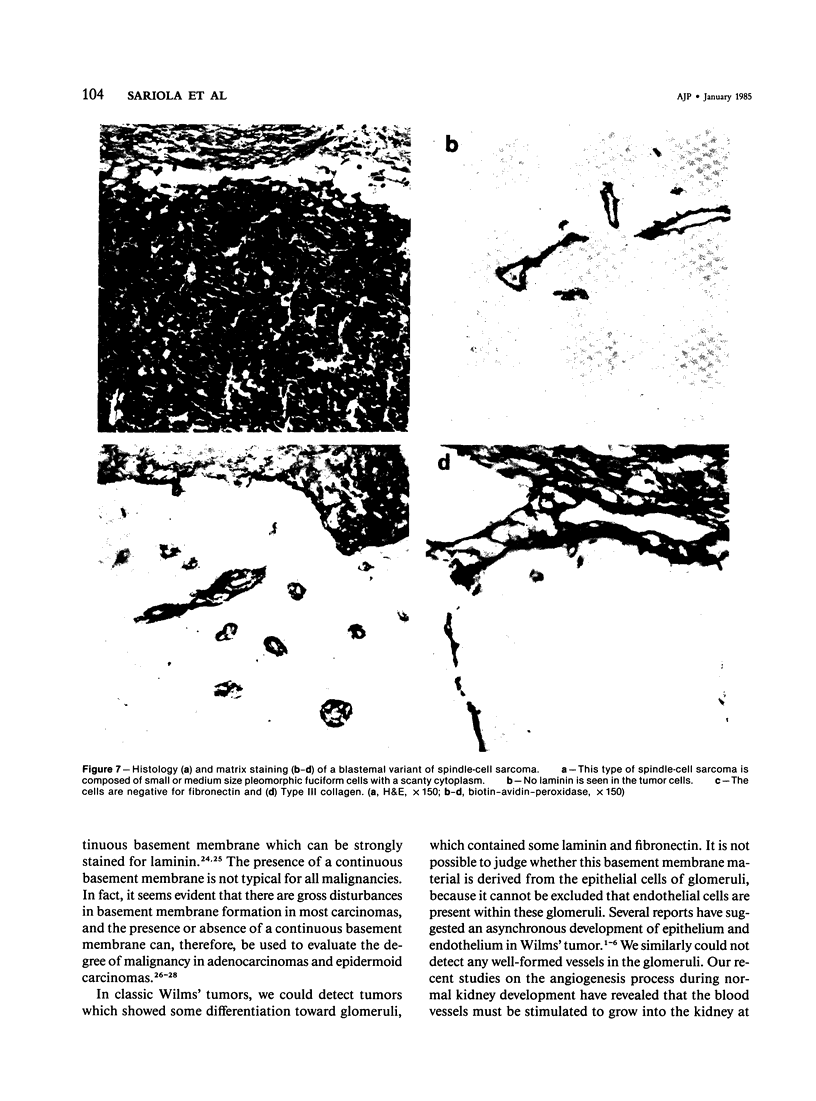
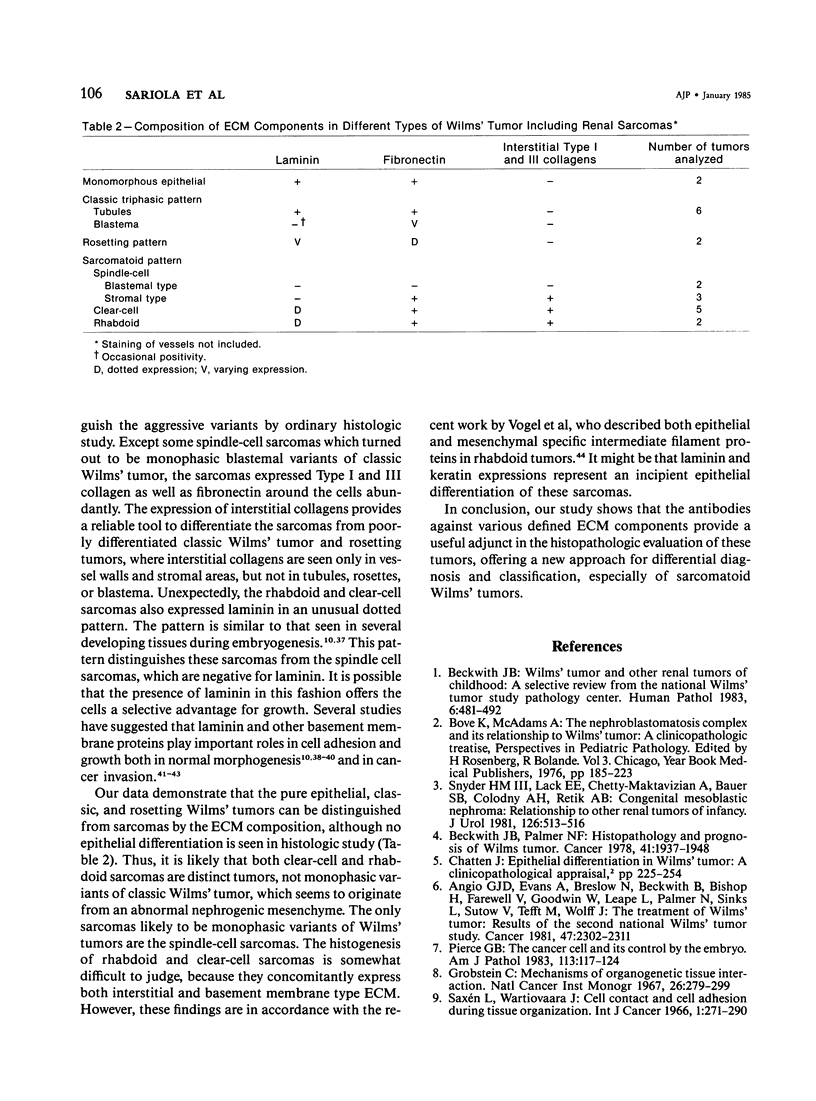
Different histologic types of 82 Wilms' tumors were graded on the basis of the histologic pattern. Representative tumors of each group were analyzed by the immunoperoxidase method for evaluation of the histogenesis of Wilms' tumor and the value of antibodies against extracellular matrix (ECM) components (collagens I and III, laminin, fibronectin) in differential diagnosis of different types of Wilms' tumors. The tubules of classic Wilms' tumor expressed laminin, which could be seen also in and around some blastemal cells. Blastema and tubules were negative for interstitial collagens, but Type I and III collagen were prominent in the fibrovascular stroma. The monomorphous tubular, psammomatous and rosetting tumors expressed laminin, but no interstitial collagens. In sarcomas, only the blastemal variant of spindle-cell sarcomas was negative for interstitial collagens, which were abundantly seen in all other sarcomas. While spindle-cell sarcomas were devoid of laminin, the highly malignant rhabdoid and clear-cell sarcomas expressed laminin in a characteristic dotted fashion. Staining for fibronectin gave varying results and had therefore only a limited value in distinguishing different types of Wilms' tumors. However, the antibodies against interstitial collagens and against the basement membrane glycoprotein laminin turned out to be a useful adjunct in differential diagnosis and classification, especially of sarcomatoid Wilms' tumors. The basement membrane of normal nephrons is similar to that in tubules of triphasic Wilms' tumor, but the ECM of blastemas is different. This transformed phenotype might represent a maturation arrest of the blastemal cell when compared with the expression of proteins during normal nephrogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrechtsen R., Nielsen M., Wewer U., Engvall E., Ruoslahti E. Basement membrane changes in breast cancer detected by immunohistochemical staining for laminin. Cancer Res. 1981 Dec;41(12 Pt 1):5076–5081. [PubMed] [Google Scholar]
- Alitalo K., Keski-Oja J., Vaheri A. Extracellular matrix proteins characterize human tumor cell lines. Int J Cancer. 1981 Jun 15;27(6):755–761. doi: 10.1002/ijc.2910270605. [DOI] [PubMed] [Google Scholar]
- Allerton S. E., Beierle J. W., Powars D. R., Bavetta L. A. Abnormal extracellular components in Wilms' tumor. Cancer Res. 1970 Mar;30(3):679–683. [PubMed] [Google Scholar]
- Barsky S. H., Siegal G. P., Jannotta F., Liotta L. A. Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab Invest. 1983 Aug;49(2):140–147. [PubMed] [Google Scholar]
- Beckwith J. B., Palmer N. F. Histopathology and prognosis of Wilms tumors: results from the First National Wilms' Tumor Study. Cancer. 1978 May;41(5):1937–1948. doi: 10.1002/1097-0142(197805)41:5<1937::aid-cncr2820410538>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Beckwith J. B. Wilms' tumor and other renal tumors of childhood: a selective review from the National Wilms' Tumor Study Pathology Center. Hum Pathol. 1983 Jun;14(6):481–492. doi: 10.1016/s0046-8177(83)80003-3. [DOI] [PubMed] [Google Scholar]
- Bove K. E., McAdams A. J. The nephroblastomatosis complex and its relationship to Wilms' tumor: a clinicopathologic treatise. Perspect Pediatr Pathol. 1976;3:185–223. [PubMed] [Google Scholar]
- D'Angio G. J., Evans A., Breslow N., Beckwith B., Bishop H., Farewell V., Goodwin W., Leape L., Palmer N., Sinks L. The treatment of Wilms' tumor: results of the Second National Wilms' Tumor Study. Cancer. 1981 May 1;47(9):2302–2311. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Ekblom P., Alitalo K., Vaheri A., Timpl R., Saxén L. Induction of a basement membrane glycoprotein in embryonic kidney: possible role of laminin in morphogenesis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):485–489. doi: 10.1073/pnas.77.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P. Formation of basement membranes in the embryonic kidney: an immunohistological study. J Cell Biol. 1981 Oct;91(1):1–10. doi: 10.1083/jcb.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P., Lehtonen E., Saxén L., Timpl R. Shift in collagen type as an early response to induction of the metanephric mesenchyme. J Cell Biol. 1981 May;89(2):276–283. doi: 10.1083/jcb.89.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P., Miettinen M., Rapola J., Foidart J. M. Demonstration of laminin, a basement membrane glycoprotein, in routinely processed formalin-fixed human tissues. Histochemistry. 1982;75(3):301–307. doi: 10.1007/BF00496733. [DOI] [PubMed] [Google Scholar]
- Franklin W. A., Ringus J. C. Basement membrane antigen in Wilms' tumor. Lab Invest. 1981 Apr;44(4):375–380. [PubMed] [Google Scholar]
- Fung C. H., Gonzalez-Crussi F., Yonan T. N., Martinez N. 'Rhabdoid' Wilms' tumor: an ultrastructural study. Arch Pathol Lab Med. 1981 Oct;105(10):521–523. [PubMed] [Google Scholar]
- Gonzalez-Crussi F., Baum E. S. Renal sarcomas of childhood. A clinicopathologic and ultrastructural study. Cancer. 1983 Mar 1;51(5):898–912. doi: 10.1002/1097-0142(19830301)51:5<898::aid-cncr2820510524>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Mechanisms of organogenetic tissue interaction. Natl Cancer Inst Monogr. 1967 Sep;26:279–299. [PubMed] [Google Scholar]
- Haas J. E., Palmer N. F., Weinberg A. G., Beckwith J. B. Ultrastructure of malignant rhabdoid tumor of the kidney. A distinctive renal tumor of children. Hum Pathol. 1981 Jul;12(7):646–657. doi: 10.1016/s0046-8177(81)80050-0. [DOI] [PubMed] [Google Scholar]
- Hard G. C., Fox R. R. Histologic characterization of renal tumors (nephroblastomas) induced transplacentally in IIIVO/J and WH/J rabbits by N-ethylnitrosourea. Am J Pathol. 1983 Oct;113(1):8–18. [PMC free article] [PubMed] [Google Scholar]
- Leivo I., Vaheri A., Timpl R., Wartiovaara J. Appearance and distribution of collagens and laminin in the early mouse embryo. Dev Biol. 1980 Apr;76(1):100–114. doi: 10.1016/0012-1606(80)90365-6. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Amenta P. S. The basement membrane in pathology. Lab Invest. 1983 Jun;48(6):656–677. [PubMed] [Google Scholar]
- Novak R. W., Caces J. N., Johnson W. W. Sarcomatous renal tumor of childhood. An electron microscopic study. Am J Clin Pathol. 1980 May;73(5):622–625. doi: 10.1093/ajcp/73.5.622. [DOI] [PubMed] [Google Scholar]
- Pierce G. B. The cancer cell and its control by the embryo. Rous-Whipple Award lecture. Am J Pathol. 1983 Oct;113(1):117–124. [PMC free article] [PubMed] [Google Scholar]
- Rousseau-Merck M. F., Nogues C., Nezelof C., Marin-Cudraz B., Paulin D. Infantile renal tumors associated with hypercalcemia. Characterization of intermediate-filament clusters. Arch Pathol Lab Med. 1983 Jun;107(6):311–314. [PubMed] [Google Scholar]
- Sariola H., Ekblom P., Lehtonen E., Saxén L. Differentiation and vascularization of the metanephric kidney grafted on the chorioallantoic membrane. Dev Biol. 1983 Apr;96(2):427–435. doi: 10.1016/0012-1606(83)90180-x. [DOI] [PubMed] [Google Scholar]
- Sariola H., Timpl R., von der Mark K., Mayne R., Fitch J. M., Linsenmayer T. F., Ekblom P. Dual origin of glomerular basement membrane. Dev Biol. 1984 Jan;101(1):86–96. doi: 10.1016/0012-1606(84)90119-2. [DOI] [PubMed] [Google Scholar]
- Saxen L., Wartiovaara J. Cell contact and cell adhesion during tissue organization. Int J Cancer. 1966 May 15;1(3):271–290. doi: 10.1002/ijc.2910010307. [DOI] [PubMed] [Google Scholar]
- Schmidt D., Dickersin G. R., Vawter G. F., Mackay B., Harms D. Wilms' tumor: review of ultrastructure and histogenesis. Pathobiol Annu. 1982;12:281–300. [PubMed] [Google Scholar]
- Snyder H. M., 3rd, Lack E. E., Chetty-Baktavizian A., Bauer S. B., Colodny A. H., Retik A. B. Congenital mesoblastic nephroma: relationship to other renal tumors of infancy. J Urol. 1981 Oct;126(4):513–516. doi: 10.1016/s0022-5347(17)54601-7. [DOI] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Fibronectin in human solid tumors. Int J Cancer. 1981;27(4):427–435. doi: 10.1002/ijc.2910270403. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Liotta L. A., Russo R. G., Martin G. R. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982 Jun;42(6):2265–2269. [PubMed] [Google Scholar]
- Thesleff I., Ekblom P. Distribution of keratin and laminin in ameloblastoma. Comparison with developing tooth and epidermoid carcinoma. J Oral Pathol. 1984 Feb;13(1):85–96. doi: 10.1111/j.1600-0714.1984.tb01404.x. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., Martin G. R. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979 Oct 10;254(19):9933–9937. [PubMed] [Google Scholar]
- Timpl R., Wick G., Gay S. Antibodies to distinct types of collagens and procollagens and their application in immunohistology. J Immunol Methods. 1977;18(1-2):165–182. doi: 10.1016/0022-1759(77)90168-5. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi A., Hayashi K., Donahoe P. K. The epithelial-mesenchymal interface of the male rate Mullerian duct: loss of basement membrane integrity and ductal regression. Dev Biol. 1982 Jul;92(1):27–40. doi: 10.1016/0012-1606(82)90147-6. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Mosher D. F. High molecular weight, cell surface-associated glycoprotein (fibronectin) lost in malignant transformation. Biochim Biophys Acta. 1978 Sep 18;516(1):1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- Varani J., Lovett E. J., 3rd, McCoy J. P., Jr, Shibata S., Maddox D. E., Goldstein I. J., Wicha M. Differential expression of a lamininlike substance by high- and low-metastatic tumor cells. Am J Pathol. 1983 Apr;111(1):27–34. [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Gospodarowicz D. Respective roles of laminin and fibronectin in adhesion of human carcinoma and sarcoma cells. Nature. 1981 Jan 22;289(5795):304–306. doi: 10.1038/289304a0. [DOI] [PubMed] [Google Scholar]
- Vogel A. M., Gown A. M., Caughlan J., Haas J. E., Beckwith J. B. Rhabdoid tumors of the kidney contain mesenchymal specific and epithelial specific intermediate filament proteins. Lab Invest. 1984 Feb;50(2):232–238. [PubMed] [Google Scholar]
- Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974 Nov;77(2):314–346. [PMC free article] [PubMed] [Google Scholar]
- Weber L., Krieg T., Müller P. K., Kirsch E., Timpl R. Immunofluorescent localization of type IV collagen and laminin in human skin and its application in junctional zone pathology. Br J Dermatol. 1982 Mar;106(3):267–273. doi: 10.1111/j.1365-2133.1982.tb01722.x. [DOI] [PubMed] [Google Scholar]