Abstract
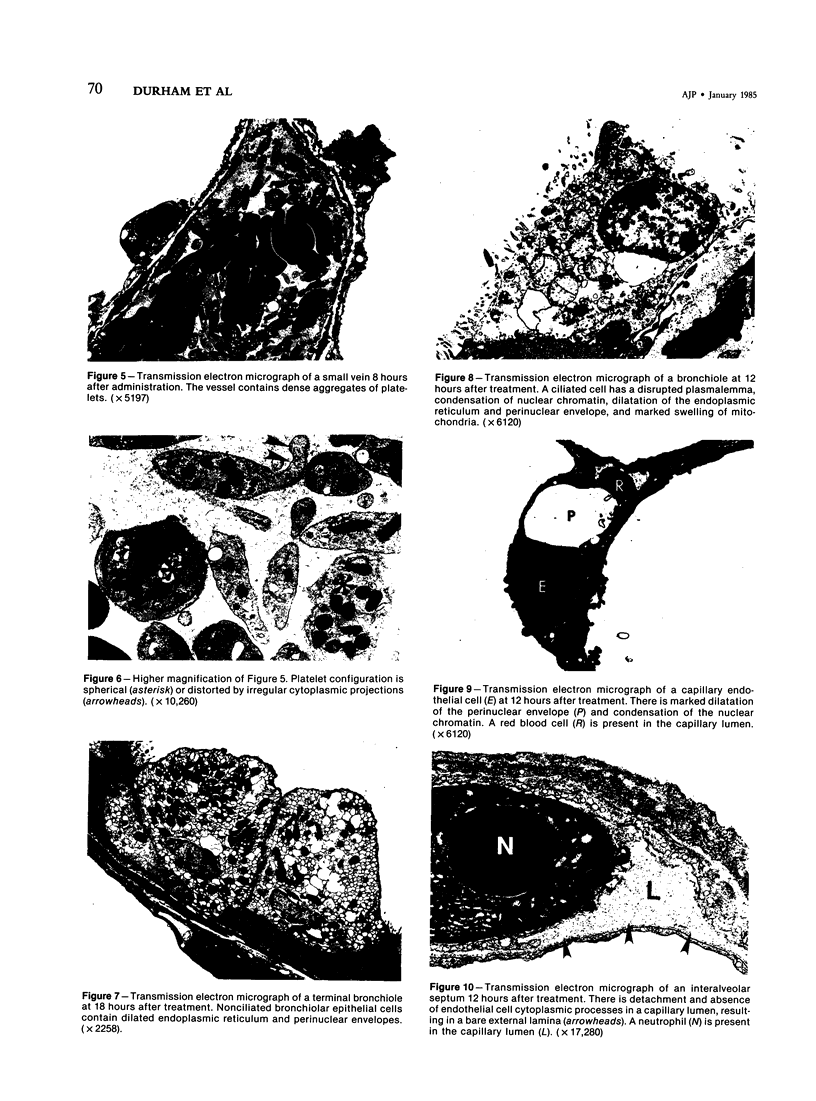
The morphogenesis of pulmonary edema and bronchiolar injury induced by the toxic furan, 4-ipomeanol, was studied by combined light and transmission electron microscopy. Weanling male CD-1 mice received 47 mg 4-ipomeanol/kg body weight by intraperitoneal injection and were studied at intervals from 2 to 360 hours after treatment. Interstitial edema associated with damaged endothelial cells was observed as early as 2 hours after treatment. The most severe endothelial damage was observed from 12 to 24 hours after treatment and occurred in association with alveolar edema. Capillary endothelial cell damage was characterized by marked dilation of endoplasmic reticulum and perinuclear envelopes, marked swelling of mitochondria, separation of cytoplasmic processes from other endothelial cells and their basal laminae, and occasional disruptions in the plasmalemma. Endothelial cell lesions in small caliber veins were similar but less pronounced as compared with the alterations observed in capillary endothelium. Minimal changes were present in alveolar epithelial cells. Damage to nonciliated bronchiolar epithelial cells was first observed at 4 hours after injection. The most severe changes in nonciliated cells occurred from 36 to 48 hours after treatment and included swelling of endoplasmic reticulum, necrosis, and sloughing. There was also necrosis and sloughing of ciliated bronchiolar epithelial cells. Endothelial and bronchiolar epithelial repair and resolution of the alveolar edema were complete by 240 hours after treatment. It is concluded that the endothelium lining capillaries and small veins, in addition to the nonciliated bronchiolar epithelial cell, are early targets in the development of 4-ipomeanol toxicity in the mouse and that the endothelial cell injury plays a major role in the development of pulmonary edema in this species. The results further suggest the possibility that pulmonary endothelial cells have the capability of metabolizing xenobiotic compounds such as 4-ipomeanol to form ultimate toxins.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman C. M., Butler E. N., Repine J. E. Hyperoxia damages cultured endothelial cells causing increased neutrophil adherence. Am Rev Respir Dis. 1983 Sep;128(3):469–472. doi: 10.1164/arrd.1983.128.3.469. [DOI] [PubMed] [Google Scholar]
- Boyd M. R. Biochemical mechanisms in chemical-induced lung injury: roles of metabolic activation. Crit Rev Toxicol. 1980 Aug;7(2):103–176. doi: 10.3109/10408448009037487. [DOI] [PubMed] [Google Scholar]
- Boyd M. R., Burka L. T., Harris T. M., Wilson B. J. Lung-toxic furanoterpenoids produced by sweet potatoes (Ipomoea batatas) following microbial infection. Biochim Biophys Acta. 1974 Feb 25;337(2):184–195. doi: 10.1016/0005-2760(74)90200-8. [DOI] [PubMed] [Google Scholar]
- Boyd M. R., Burka L. T. In vivo studies on the relationship between target organ alkylation and the pulmonary toxicity of a chemically reactive metabolite of 4-ipomeanol. J Pharmacol Exp Ther. 1978 Dec;207(3):687–697. [PubMed] [Google Scholar]
- Boyd M. R., Dutcher J. S. Renal toxicity due to reactive metabolites formed in situ in the kidney: investigations with 4-ipomeanol in the mouse. J Pharmacol Exp Ther. 1981 Mar;216(3):640–646. [PubMed] [Google Scholar]
- Boyd M. R. Effects of inducers and inhibitors on drug-metabolizing enzymes and on drug toxicity in extrahepatic tissues. Ciba Found Symp. 1980;76:43–66. doi: 10.1002/9780470720592.ch4. [DOI] [PubMed] [Google Scholar]
- Boyd M. R. Evidence for the Clara cell as a site of cytochrome P450-dependent mixed-function oxidase activity in lung. Nature. 1977 Oct 20;269(5630):713–715. doi: 10.1038/269713a0. [DOI] [PubMed] [Google Scholar]
- Boyd M. R. Role of metabolic activation in the pathogenesis of chemically induced pulmonary disease: mechanism of action of the lung-toxic furan, 4-ipomeanol. Environ Health Perspect. 1976 Aug;16:127–138. doi: 10.1289/ehp.7616127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd M. R., Wilson B. J., Harris T. M. Confirmation by chemical synthesis of the structure of 4-ipomeanol, a lung-toxic metabolite of the sweet potato, Ipomoea batatas. Nat New Biol. 1972 Apr 5;236(66):158–159. doi: 10.1038/newbio236158a0. [DOI] [PubMed] [Google Scholar]
- Buckpitt A. R., Statham C. N., Boyd M. R. In vivo studies on the target tissue metabolism, covalent binding, glutathione depletion, and toxicity of 4-ipomeanol in birds, species deficient in pulmonary enzymes for metabolic activation. Toxicol Appl Pharmacol. 1982 Aug;65(1):38–52. doi: 10.1016/0041-008x(82)90360-x. [DOI] [PubMed] [Google Scholar]
- Cockerell G. L., Gilmartin J. E., Albern W. F., Losikoff A. M., Sansone E. B. Design and testing of a biocontainment system for chemical carcinogens. J Toxicol Environ Health. 1981 Jan;7(1):1–7. doi: 10.1080/15287398109529953. [DOI] [PubMed] [Google Scholar]
- Cottrell T. S., Levine O. R., Senior R. M., Wiener J., Spiro D., Fishman A. P. Electron microscopic alterations at the alveolar level in pulmonary edema. Circ Res. 1967 Dec;21(6):783–797. doi: 10.1161/01.res.21.6.783. [DOI] [PubMed] [Google Scholar]
- Cunningham A. L., Hurley J. V. Alpha-naphthyl-thiourea-induced pulmonary oedema in the rat: a topographical and electron-microscope study. J Pathol. 1972 Jan;106(1):25–35. doi: 10.1002/path.1711060103. [DOI] [PubMed] [Google Scholar]
- DeFouw D. O. Ultrastructural changes in type I and type II alveolar epithelial cells in isolated perfused dog lungs after production of moderate and severe hydrostatic edema. Am J Anat. 1980 Jul;158(3):355–364. doi: 10.1002/aja.1001580309. [DOI] [PubMed] [Google Scholar]
- DeFouw D. O. Ultrastructure of the pulmonary alveolar septa after hemodynamic edema. Ann N Y Acad Sci. 1982;384:44–53. doi: 10.1111/j.1749-6632.1982.tb21360.x. [DOI] [PubMed] [Google Scholar]
- Devereux T. R., Jones K. G., Bend J. R., Fouts J. R., Statham C. N., Boyd M. R. In vitro metabolic activation of the pulmonary toxin, 4-ipomeanol, in nonciliated bronchiolar epithelial (Clara) and alveolar type II cells isolated from rabbit lung. J Pharmacol Exp Ther. 1982 Jan;220(1):223–227. [PubMed] [Google Scholar]
- Doster A. R., Farrell R. L., Wilson B. J. An ultrastructural study of bronchiolar lesions in rats induced by 4-ipomeanol, a product from mold-damaged sweet potatoes. Am J Pathol. 1983 Apr;111(1):56–61. [PMC free article] [PubMed] [Google Scholar]
- Doster A. R., Mitchell F. E., Farrell R. L., Wilson B. J. Effects of 4-ipomeanol, a product from mold-damaged sweet potatoes, on the bovine lung. Vet Pathol. 1978 May;15(3):367–375. doi: 10.1177/030098587801500312. [DOI] [PubMed] [Google Scholar]
- Dutcher J. S., Boyd M. R. Species and strain differences in target organ alkylation and toxicity by 4-ipomeanol. Predictive value of covalent binding in studies of target organ toxicities by reactive metabolites. Biochem Pharmacol. 1979 Dec 1;28(23):3367–3372. doi: 10.1016/0006-2952(79)90074-1. [DOI] [PubMed] [Google Scholar]
- Heffner J. E., Shoemaker S. A., Canham E. M., Patel M., McMurtry I. F., Morris H. G., Repine J. E. Acetyl glyceryl ether phosphorylcholine-stimulated human platelets cause pulmonary hypertension and edema in isolated rabbit lungs. Role of thromboxane A2. J Clin Invest. 1983 Feb;71(2):351–357. doi: 10.1172/JCI110776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Larsen G. L., Webster R. O., Mitchell B. C., Goins A. J., Henson J. E. Pulmonary microvascular alterations and injury induced by complement fragments: synergistic effect of complement activation, neutrophil sequestration, and prostaglandins. Ann N Y Acad Sci. 1982;384:287–300. doi: 10.1111/j.1749-6632.1982.tb21379.x. [DOI] [PubMed] [Google Scholar]
- Hislop A., Reid L. Normal structure and dimensions of the pulmonary arteries in the rat. J Anat. 1978 Jan;125(Pt 1):71–83. [PMC free article] [PubMed] [Google Scholar]
- Hurley J. V. Types of pulmonary microvascular injury. Ann N Y Acad Sci. 1982;384:269–286. doi: 10.1111/j.1749-6632.1982.tb21378.x. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Brigham K. L. Acute effects of Escherichia coli endotoxin on the pulmonary microcirculation of anesthetized sheep structure:function relationships. Lab Invest. 1983 Apr;48(4):458–470. [PubMed] [Google Scholar]
- Meyrick B., Miller J., Reid L. Pulmonary oedema induced by ANTU, or by high or low oxygen concentrations in rat--an electron microscopic study. Br J Exp Pathol. 1972 Aug;53(4):347–358. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Reid L. Ultrastructural features of the distended pulmonary arteries of the normal rat. Anat Rec. 1979 Jan;193(1):71–97. doi: 10.1002/ar.1091930106. [DOI] [PubMed] [Google Scholar]
- Minchin R. F., Boyd M. R. Localization of metabolic activation and deactivation systems in the lung: significance to the pulmonary toxicity of xenobiotics. Annu Rev Pharmacol Toxicol. 1983;23:217–238. doi: 10.1146/annurev.pa.23.040183.001245. [DOI] [PubMed] [Google Scholar]
- Peckham J. C., Mitchell F. E., Jones O. H., Jr, Doupnik B., Jr Atypical interstitial pneumonia in cattle fed moldy sweet potatoes. J Am Vet Med Assoc. 1972 Jan 15;160(2):169–172. [PubMed] [Google Scholar]
- Robin E. D., Cross C. E., Zelis R. Pulmonary edema. 2. N Engl J Med. 1973 Feb 8;288(6):292–304. doi: 10.1056/NEJM197302082880606. [DOI] [PubMed] [Google Scholar]
- Ryan J. W., Ryan U. S. Metabolic functions of the pulmonary vascular endothelium. Adv Vet Sci Comp Med. 1982;26:79–98. [PubMed] [Google Scholar]
- Said S. I. The lung as a metabolic organ. N Engl J Med. 1968 Dec 12;279(24):1330–1334. doi: 10.1056/NEJM196812122792409. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Fox R. B., Harada R. N., Repine J. E. Reduction of the edema of acute hyperoxic lung injury by granulocyte depletion. J Appl Physiol Respir Environ Exerc Physiol. 1982 May;52(5):1237–1244. doi: 10.1152/jappl.1982.52.5.1237. [DOI] [PubMed] [Google Scholar]
- Staub N. C. The pathogenesis of pulmonary edema. Prog Cardiovasc Dis. 1980 Jul-Aug;23(1):53–80. doi: 10.1016/0033-0620(80)90005-5. [DOI] [PubMed] [Google Scholar]