Abstract
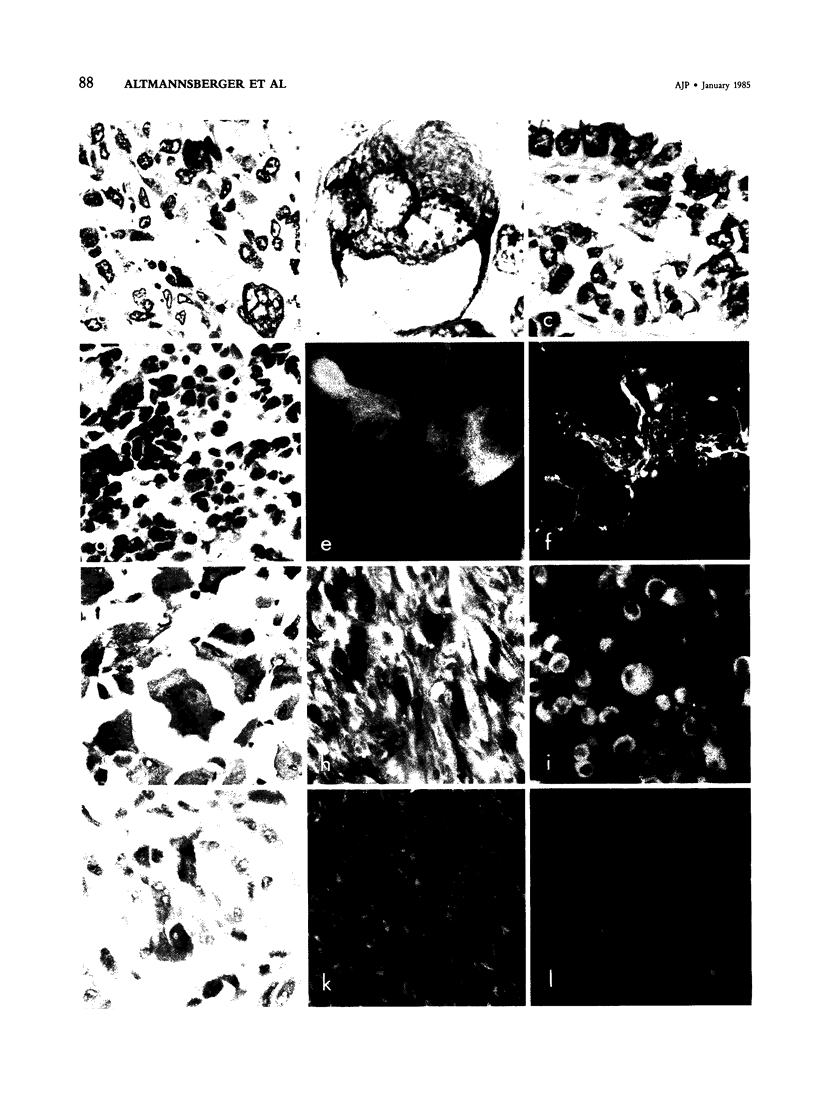
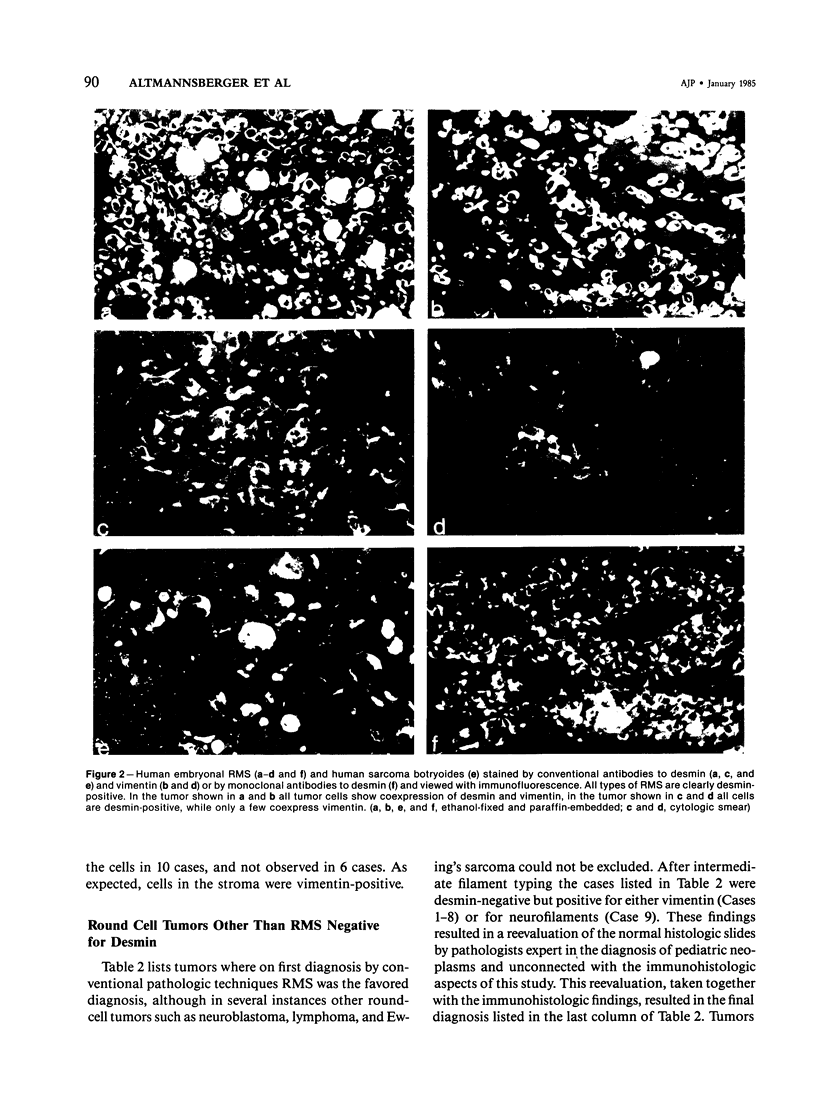
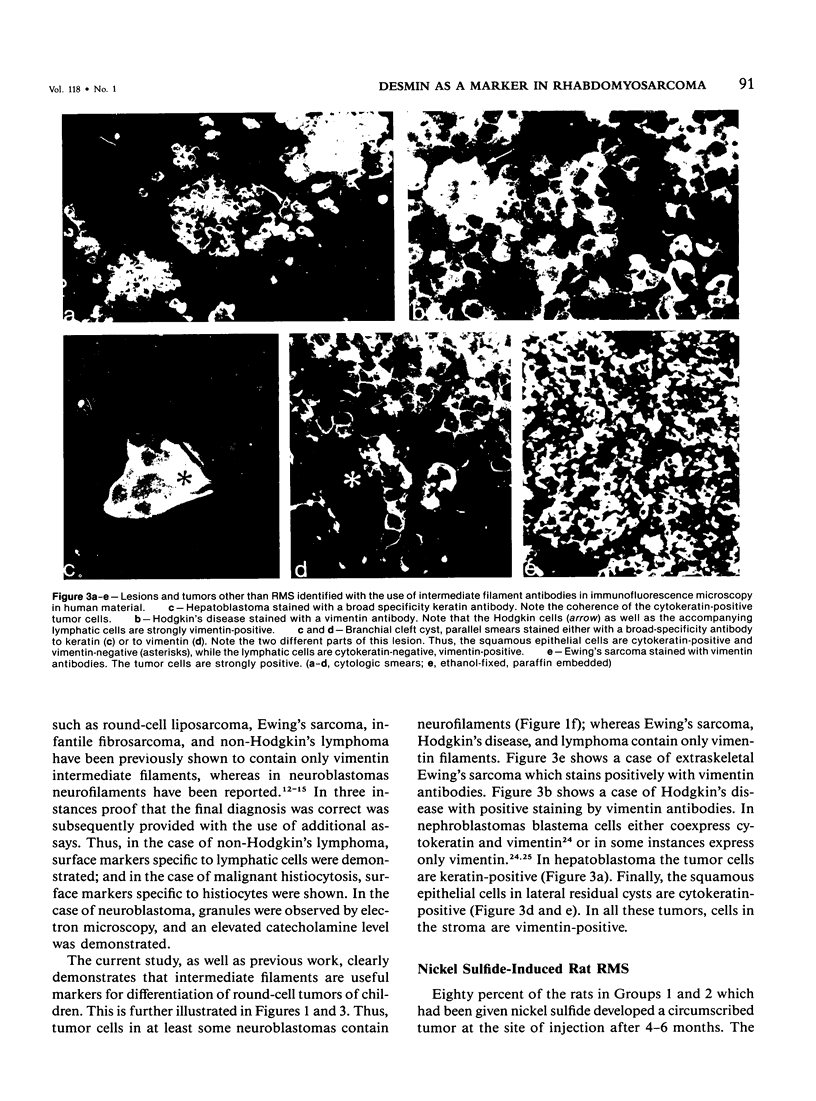
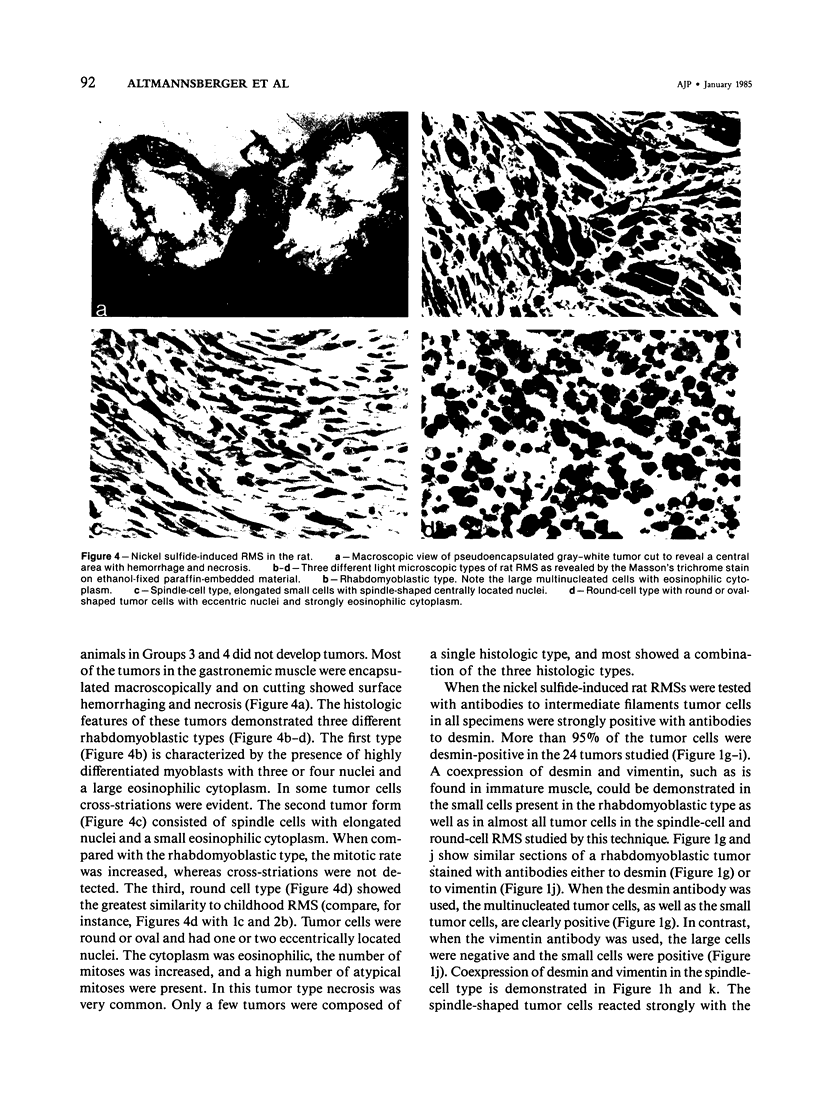
Putative human rhabdomyosarcoma (RMS) has been divided into two groups according to desmin content. Twenty-five tumors with histologic features consistent with but not necessarily sufficient to prove a diagnosis of RMS were desmin-positive. More than 95% of the tumor cells were desmin-positive, suggesting a muscle origin and supporting the diagnosis of RMS. Nine tumors for which the preferred first histologic diagnosis was also RMS were desmin-negative. Reexamination of the original histologic slides together with results from intermediate filament typing resulted in a diagnosis other than RMS for all tumors in this second group, and in several instances other tests were used to prove the correctness of the final diagnosis. The results on human material were extended to a rat model system in which RMS was induced by nickel sulfide. Again, all 24 tumors tested were desmin-positive. Vimentin was coexpressed in a varying percentage of tumor cells in RMS of human and rat origin. The results show that desmin is an excellent marker for rhabdomyosarcoma, yielding few if any false-positive or false-negative results in frozen or alcohol-fixed material.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmannsberger M., Osborn M., Droese M., Weber K., Schauer A. Diagnostic value of intermediate filament antibodies in clinical cytology. Klin Wochenschr. 1984 Feb 1;62(3):114–123. doi: 10.1007/BF01738701. [DOI] [PubMed] [Google Scholar]
- Altmannsberger M., Osborn M., Schäfer H., Schauer A., Weber K. Distinction of nephroblastomas from other childhood tumors using antibodies to intermediate filaments. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(2):113–124. doi: 10.1007/BF02889858. [DOI] [PubMed] [Google Scholar]
- Altmannsberger M., Osborn M., Treuner J., Hölscher A., Weber K., Shauer A. Diagnosis of human childhood rhabdomyosarcoma of antibodies to desmin, the structural protein of muscle specific intermediate filaments. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;39(2):203–215. doi: 10.1007/BF02892848. [DOI] [PubMed] [Google Scholar]
- Bale P. M., Parsons R. E., Stevens M. M. Diagnosis and behavior of juvenile rhabdomyosarcoma. Hum Pathol. 1983 Jul;14(7):596–611. doi: 10.1016/s0046-8177(83)80203-2. [DOI] [PubMed] [Google Scholar]
- Bruni C., Rust J. N. Fine structure of dividing cells and of nondividing, differentiating cells of nickel sulfide-induced rhabdomyosarcomas. J Natl Cancer Inst. 1975 Mar;54(3):687–696. [PubMed] [Google Scholar]
- Bussolati G., Alfani V., Weber K., Osborn M. Immunocytochemical detection of actin on fixed and embedded tissues: its potential use in routine pathology. J Histochem Cytochem. 1980 Feb;28(2):169–173. doi: 10.1177/28.2.6986431. [DOI] [PubMed] [Google Scholar]
- Corson J. M., Pinkus G. S. Intracellular myoglobin--a specific marker for skeletal muscle differentiation in soft tissue sarcomas. An immunoperoxidase study. Am J Pathol. 1981 Jun;103(3):384–389. [PMC free article] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies to desmin, the muscle-specific intermediate filament protein. EMBO J. 1983;2(12):2305–2312. doi: 10.1002/j.1460-2075.1983.tb01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk H., Krepler R., Artlieb U., Gabbiani G., Rungger-Brändle E., Leoncini P., Franke W. W. Proteins of intermediate filaments. An immunohistochemical and biochemical approach to the classification of soft tissue tumors. Am J Pathol. 1983 Feb;110(2):193–208. [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Lampert I. A., Jacobs M. Intermediate filaments in smooth muscle tumours. J Clin Pathol. 1983 Jan;36(1):57–61. doi: 10.1136/jcp.36.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Kapanci Y., Barazzone P., Franke W. W. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol. 1981 Sep;104(3):206–216. [PMC free article] [PubMed] [Google Scholar]
- Gilman J. P. Muscle tumourigenesis. Proc Can Cancer Conf. 1966;6:209–223. [PubMed] [Google Scholar]
- Hildebrand H. F., Biserte G. Ultrastructural investigation of NI3S2-induced rhabdomyosarcoma in Wistar rat: comparative study with emphasis on myofibrillar differentiation and ciliar formation. Cancer. 1978 Aug;42(2):528–554. doi: 10.1002/1097-0142(197808)42:2<528::aid-cncr2820420222>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Bennett G. S., Tapscott S. J., Croop J. M., Toyama Y. Intermediate-size filaments: changes in synthesis and distribution in cells of the myogenic and neurogenic lineages. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):317–329. doi: 10.1101/sqb.1982.046.01.033. [DOI] [PubMed] [Google Scholar]
- Kahn H. J., Yeger H., Baumal R., Thom H., Phillips J. M. Categorization of pediatric neoplasms by immunostaining with antiprekeratin and antivimentin antisera. Cancer. 1983 Feb 15;51(4):645–653. doi: 10.1002/1097-0142(19830215)51:4<645::aid-cncr2820510417>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Kahn H. J., Yeger H., Kassim O., Jorgensen A. O., MacLennan D. H., Baumal R., Smith C. R., Phillips M. J. Immunohistochemical and electron microscopic assessment of childhood rhabdomyosarcoma. Increased frequency of diagnosis over routine histologic methods. Cancer. 1983 May 15;51(10):1897–1903. doi: 10.1002/1097-0142(19830515)51:10<1897::aid-cncr2820511023>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Koh S. J., Johnson W. W. Antimyosin and antirhabdomyoblast sera: their use for the diagnosis of childhood rhabdomyosarcoma. Arch Pathol Lab Med. 1980 Mar;104(3):118–122. [PubMed] [Google Scholar]
- Lazarides E., Granger B. L., Gard D. L., O'Connor C. M., Breckler J., Price M., Danto S. I. Desmin- and vimentin-containing filaments and their role inthe assembly of the Z disk in muscle cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):351–378. doi: 10.1101/sqb.1982.046.01.036. [DOI] [PubMed] [Google Scholar]
- Maurer H. M., Moon T., Donaldson M., Fernandez C., Gehan E. A., Hammond D., Hays D. M., Lawrence W., Jr, Newton W., Ragab A. The intergroup rhabdomyosarcoma study: a preliminary report. Cancer. 1977 Nov;40(5):2015–2026. doi: 10.1002/1097-0142(197711)40:5<2015::aid-cncr2820400505>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Badley R. A., Virtanen I. Alveolar rhabdomyosarcoma. Demonstration of the muscle type of intermediate filament protein, desmin, as a diagnostic aid. Am J Pathol. 1982 Aug;108(2):246–251. [PMC free article] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Badley R. A., Virtanen I. Expression of intermediate filaments in soft-tissue sarcomas. Int J Cancer. 1982 Nov 15;30(5):541–546. doi: 10.1002/ijc.2910300502. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Virtanen I. Histogenesis of Ewing's sarcoma. An evaluation of intermediate filaments and endothelial cell markers. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;41(3):277–284. [PubMed] [Google Scholar]
- Moloney J. B. A virus-induced rhabdomyosarcoma of mice. Natl Cancer Inst Monogr. 1966 Sep;22:139–142. [PubMed] [Google Scholar]
- Mukai K., Rosai J., Hallaway B. E. Localization of myoglobin in normal and neoplastic human skeletal muscle cells using an immunoperoxidase method. Am J Surg Pathol. 1979 Aug;3(4):373–376. doi: 10.1097/00000478-197908000-00008. [DOI] [PubMed] [Google Scholar]
- Mukai K., Schollmeyer J. V., Rosai J. Immunohistochemical localization of actin: applications in surgical pathology. Am J Surg Pathol. 1981 Jan;5(1):91–97. doi: 10.1097/00000478-198101000-00013. [DOI] [PubMed] [Google Scholar]
- Osborn M., Geisler N., Shaw G., Sharp G., Weber K. Intermediate filaments. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):413–429. doi: 10.1101/sqb.1982.046.01.040. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- SAXEN E. A. On the factor of age in the production of subcutaneous sarcomas in mice by 20-methylcholanthrene. J Natl Cancer Inst. 1953 Dec;14(3):547–569. [PubMed] [Google Scholar]
- SCHLUMBERGER H. G., ZACK G. Neoplasia in the parakeet. IV. Transplantable methylcholanthrene-induced rhabdomyosarcoma. Cancer Res. 1959 Oct;19:954–958. [PubMed] [Google Scholar]
- Shaw G., Weber K. The distribution of the neurofilament triplet proteins within individual neurones. Exp Cell Res. 1981 Nov;136(1):119–125. doi: 10.1016/0014-4827(81)90043-4. [DOI] [PubMed] [Google Scholar]
- Webb M., Heath J. C., Hopkins T. Intranuclear distribution of the inducing metal in primary rhabdomyosarcomata induced in the rat by nickel, cobalt and cadmium. Br J Cancer. 1972 Aug;26(4):274–278. doi: 10.1038/bjc.1972.37. [DOI] [PMC free article] [PubMed] [Google Scholar]