Abstract
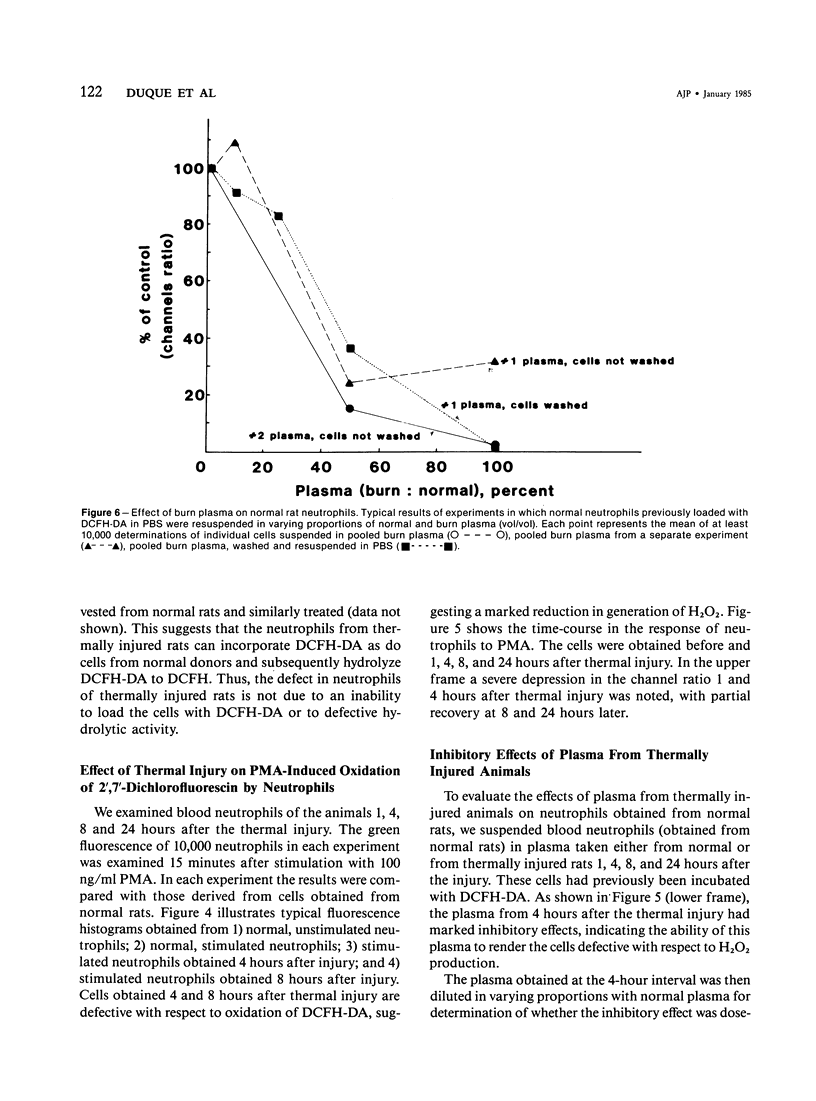
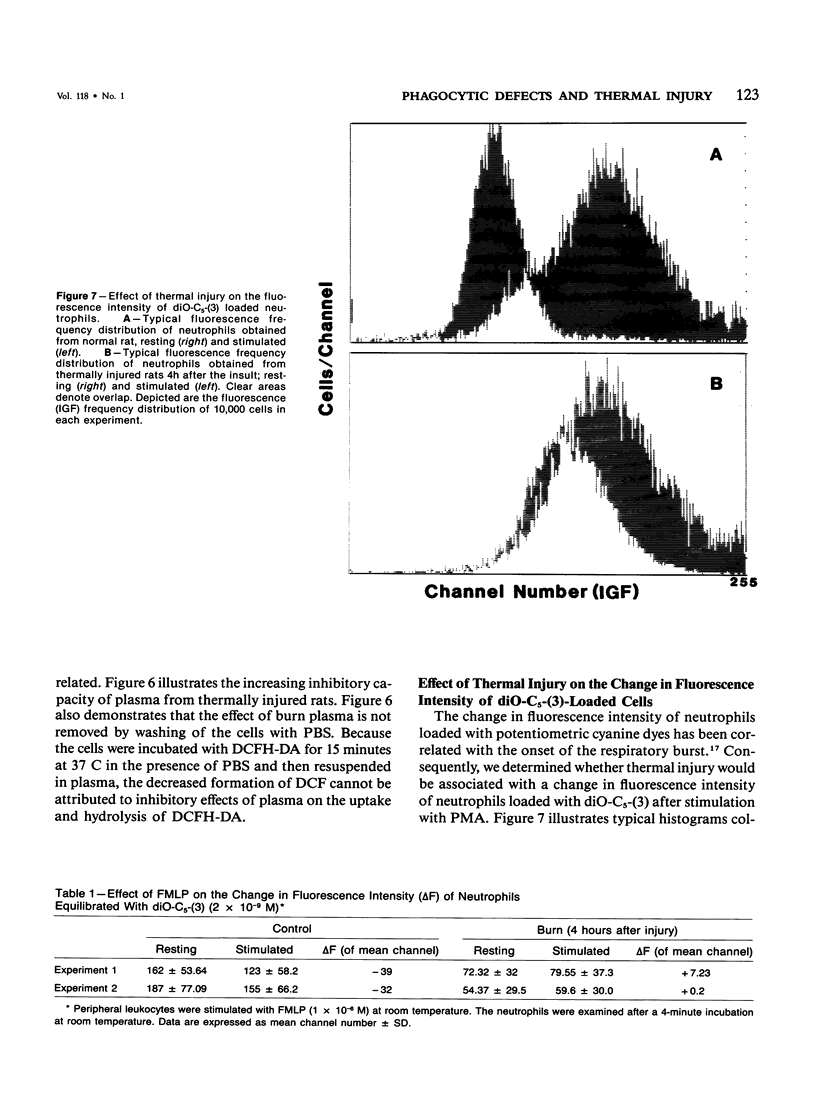
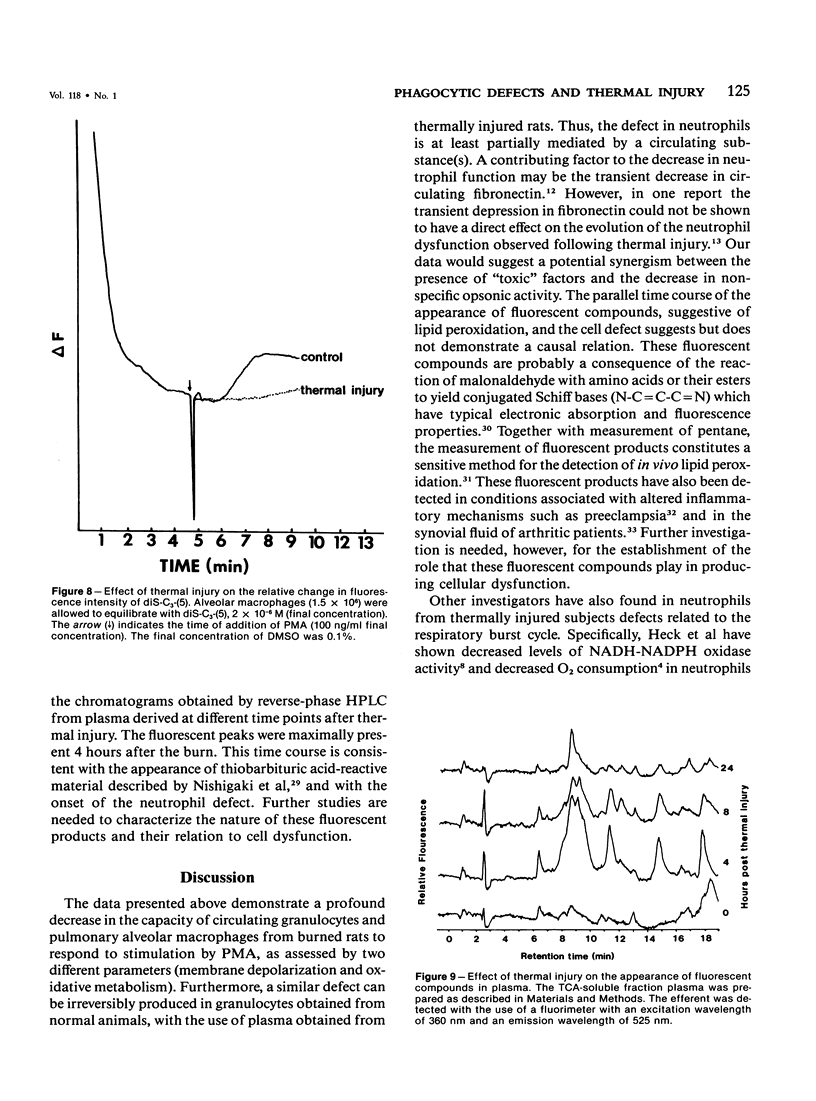
Defective phagocytic cell function may partially account for the morbidity and mortality associated with thermal injury. In experimental thermal injury in the rat, small circulating blood volumes increase the difficulty in obtaining significant data. Furthermore, purification and or elicitation procedures have the potential for altering the cell surface characteristics and/or the functional response of the cell in question. We have examined the circulating neutrophils and pulmonary alveolar macrophages of anesthetized rats following a 16-20% body surface area scald injury to the shaved back. The circulating neutrophils of thermally injured rats were examined by flow cytometry following stimulation with phorbol myristate acetate (PMA) (100 ng/ml) in terms of the change in fluorescence intensity of the potentiometric cyanine dye, dipentyloxocarboxyanine and the formation of the oxidized product of 2',7'-dichlorofluorescin diacetate-loaded cells. The alveolar macrophages were examined after stimulation with PMA (100 ng/ml) in terms of the change in fluorescence intensity of the potentiometric dye, dipropylthiodicarbocyanine and the generation of superoxide production, as assessed by the superoxide dismutase inhibitable reduction of cytochrome c. Both cells exhibited a profound inhibition of cell function 4 hours after the insult, with partial return toward control values at later time points. Furthermore, the plasma of thermally injured rats, 4 hours after the burn was inhibitory to normal rat neutrophils. Fluorescent compounds suggestive of in vivo lipid peroxidation were maximally detectable at this time point. Further research is needed to establish the role of these products in the induction of phagocytic cell dysfunction.
Full text
PDF

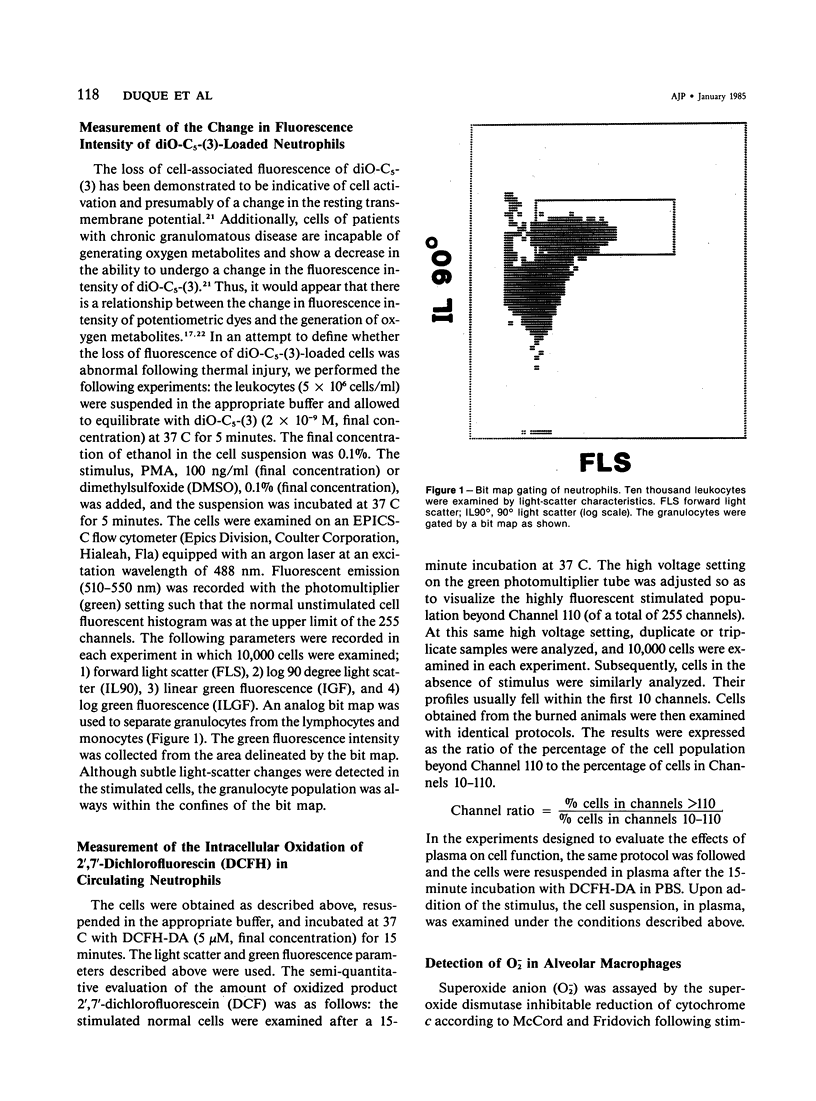
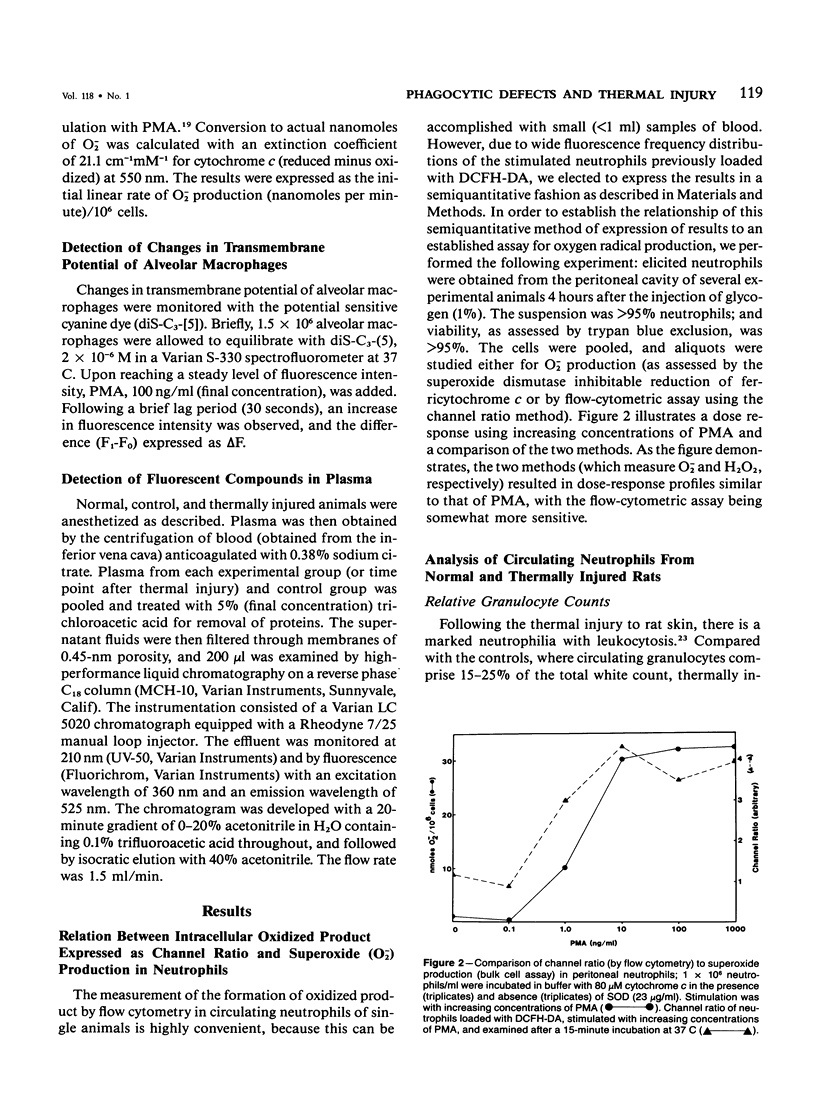
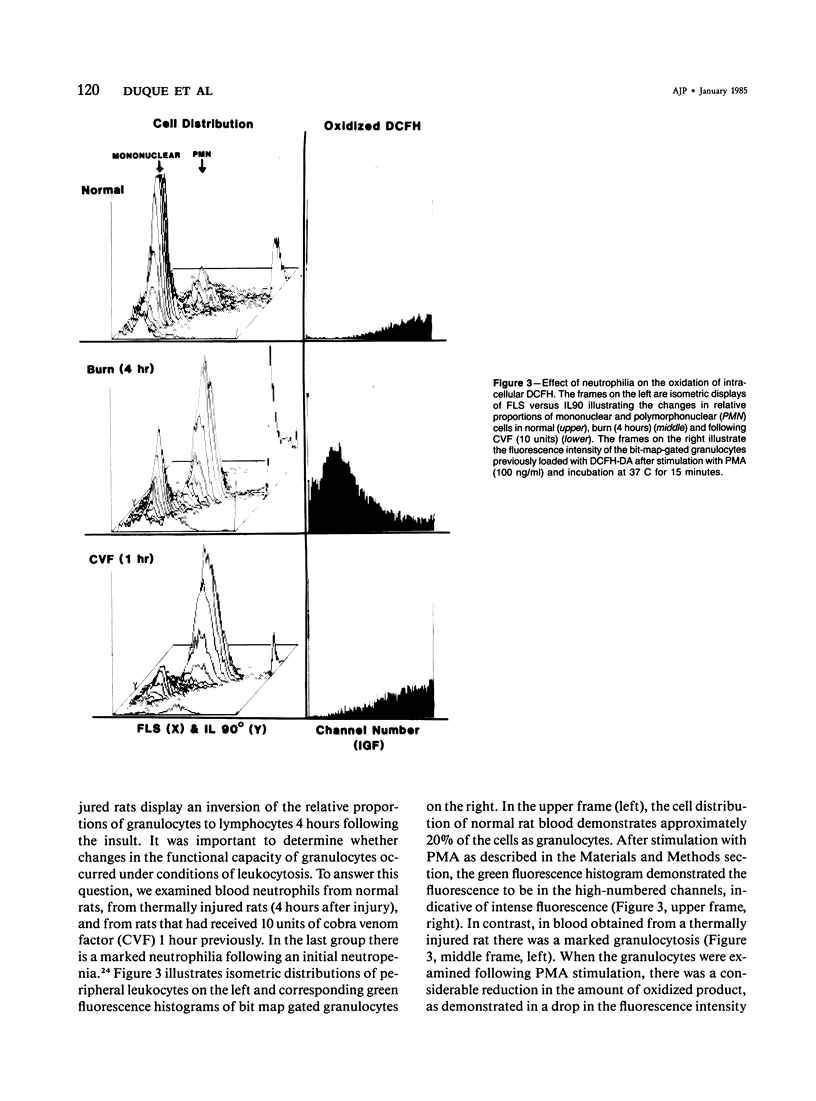
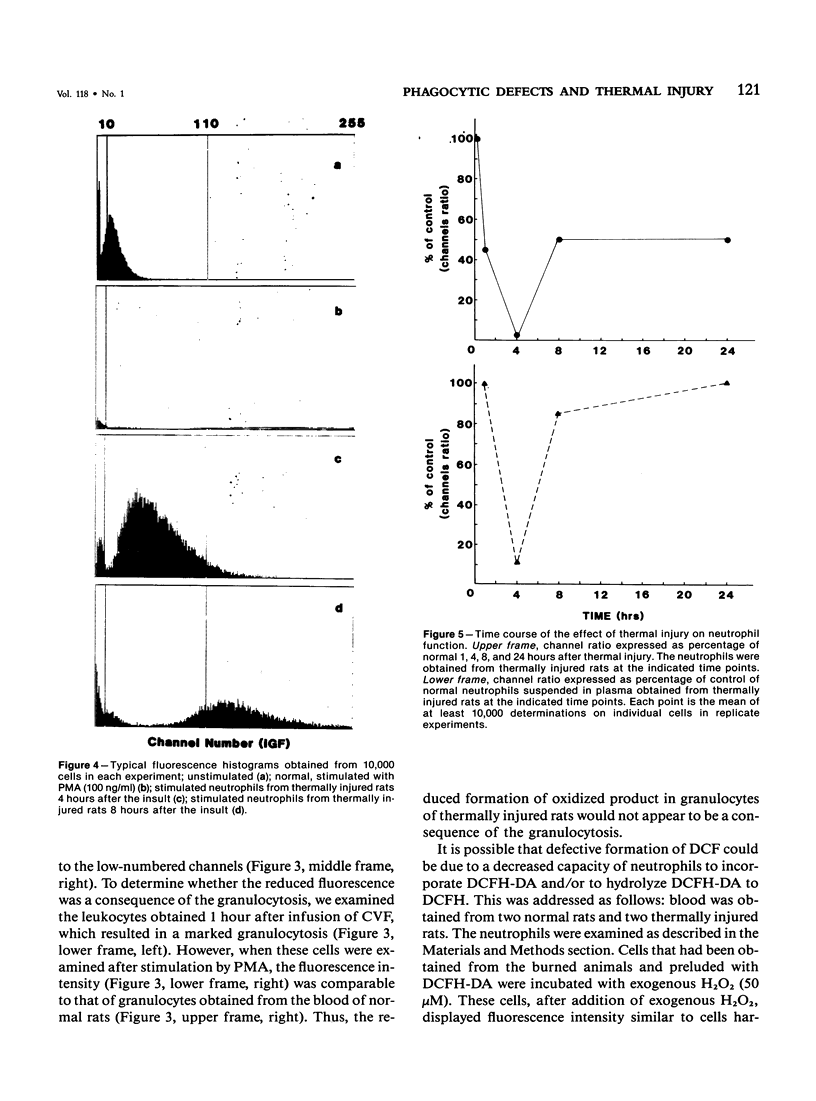


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Becker E. L., Sigman M., Oliver J. M. Superoxide production induced in rabbit polymorphonuclear leukocytes by synthetic chemotactic peptides and A23187. Am J Pathol. 1979 Apr;95(1):81–97. [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Altemeier W. A., Bjornson H. S. Changes in humoral components of host defense following burn trauma. Ann Surg. 1977 Jul;186(1):88–96. doi: 10.1097/00000658-197707000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Bjornson H. S., Altemeier W. A. Serum-mediated inhibition of polymorphonuclear leukocyte function following burn injury. Ann Surg. 1981 Nov;194(5):568–575. doi: 10.1097/00000658-198111000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottone E. J., Sheehan D. J. Yersinia enterocolitica: guidelines for serologic diagnosis of human infections. Rev Infect Dis. 1983 Sep-Oct;5(5):898–906. doi: 10.1093/clinids/5.5.898. [DOI] [PubMed] [Google Scholar]
- Chio K. S., Tappel A. L. Inactivation of ribonuclease and other enzymes by peroxidizing lipids and by malonaldehyde. Biochemistry. 1969 Jul;8(7):2827–2832. doi: 10.1021/bi00835a020. [DOI] [PubMed] [Google Scholar]
- Curreri P. W., Heck E. L., Browne L., Baxter C. R. Stimulated nitroblue tetrazolium test to assess neutrophil antibacterial function: prediction of wound sepsis in burned patients. Surgery. 1973 Jul;74(1):6–13. [PubMed] [Google Scholar]
- Davis J. M., Dineen P., Gallin J. I. Neutrophil degranulation and abnormal chemotaxis after thermal injury. J Immunol. 1980 Mar;124(3):1467–1471. [PubMed] [Google Scholar]
- Deitch E. A., Gelder F., McDonald J. C. The relationship between CIG depletion and peripheral neutrophil function in rabbits and man. J Trauma. 1982 Jun;22(6):469–475. doi: 10.1097/00005373-198206000-00005. [DOI] [PubMed] [Google Scholar]
- Duque R. E., Phan S. H., Sulavik M. C., Ward P. A. Inhibition by tosyl-L-phenylalanyl chloromethyl ketone of membrane potential changes in rat neutrophils. Correlation with the inhibition of biological activity. J Biol Chem. 1983 Jul 10;258(13):8123–8128. [PubMed] [Google Scholar]
- Fikrig S. M., Karl S. C., Suntharalingam K. Neutrophil chemotaxis in patients with burns. Ann Surg. 1977 Dec;186(6):746–748. doi: 10.1097/00000658-197712000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogan J. B. Altered neutrophil phagocytic function in burn patients. J Trauma. 1976 Sep;16(9):734–738. doi: 10.1097/00005373-197609000-00009. [DOI] [PubMed] [Google Scholar]
- Grogan J. B., Miller R. C. Impaired function of polymorphonuclear leukocytes in patients with burns and other trauma. Surg Gynecol Obstet. 1973 Nov;137(5):784–788. [PubMed] [Google Scholar]
- Grogan J. B. Suppressed in vitro chemotaxis of burn neutrophils. J Trauma. 1976 Dec;16(12):985–988. doi: 10.1097/00005373-197612000-00008. [DOI] [PubMed] [Google Scholar]
- Heck E. L., Browne L., Curreri P. W., Baxter C. R. Evaluation of leukocyte function in burned individuals by in vitro oxygen consumption. J Trauma. 1975 Jun;15(6):486–489. doi: 10.1097/00005373-197506000-00005. [DOI] [PubMed] [Google Scholar]
- Heck E. L., Edgar M. A., Masters B. S., Baxter C. R. The role of NADH-NADPH oxidase activity in the leukocyte function of burned patients. J Trauma. 1979 Jan;19(1):49–51. doi: 10.1097/00005373-197901000-00009. [DOI] [PubMed] [Google Scholar]
- Loose L. D., Turinsky J. Depression of the respiratory burst in alveolar and peritoneal macrophages after thermal injury. Infect Immun. 1980 Dec;30(3):718–722. doi: 10.1128/iai.30.3.718-722.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunec J., Halloran S. P., White A. G., Dormandy T. L. Free-radical oxidation (peroxidation) products in serum and synovial fluid in rheumatoid arthritis. J Rheumatol. 1981 Mar-Apr;8(2):233–245. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Ninnemann J. L., Fisher J. C., Wachtel T. L. Thermal injury-associated immunosuppression: occurrence and in vitro blocking effect of post recovery serum. J Immunol. 1979 May;122(5):1736–1741. [PubMed] [Google Scholar]
- Nishigaki I., Hagihara M., Hiramatsu M., Izawa Y., Yagi K. Effect of thermal injury on lipid peroxide levels of rat. Biochem Med. 1980 Oct;24(2):185–189. doi: 10.1016/0006-2944(80)90010-1. [DOI] [PubMed] [Google Scholar]
- Northrop J., Slepecky R. A. Sporulation mutations induced by heat in Bacillus subtilis. Science. 1967 Feb 17;155(3764):838–839. doi: 10.1126/science.155.3764.838. [DOI] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Bruchelt G., Kistler D., Koslowski L. Phagocytic activity of granulocytes and alveolar macrophages after burn injury measured by chemiluminescence. Burns Incl Therm Inj. 1983 Nov;10(2):79–85. doi: 10.1016/0305-4179(83)90002-5. [DOI] [PubMed] [Google Scholar]
- Seligmann B. E., Gallin J. I. Use of lipophilic probes of membrane potential to assess human neutrophil activation. Abnormality in chronic granulomatous disease. J Clin Invest. 1980 Sep;66(3):493–503. doi: 10.1172/JCI109880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till G. O., Beauchamp C., Menapace D., Tourtellotte W., Jr, Kunkel R., Johnson K. J., Ward P. A. Oxygen radical dependent lung damage following thermal injury of rat skin. J Trauma. 1983 Apr;23(4):269–277. doi: 10.1097/00005373-198304000-00001. [DOI] [PubMed] [Google Scholar]
- Till G. O. Cellular and humoral defense systems and inflammatory mechanisms in thermal injury. Clin Lab Med. 1983 Dec;3(4):801–815. [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker H. L., Mason A. D., Jr A standard animal burn. J Trauma. 1968 Nov;8(6):1049–1051. doi: 10.1097/00005373-196811000-00006. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980 Jul 3;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- Whitin J. C., Chapman C. E., Simons E. R., Chovaniec M. E., Cohen H. J. Correlation between membrane potential changes and superoxide production in human granulocytes stimulated by phorbol myristate acetate. Evidence for defective activation in chronic granulomatous disease. J Biol Chem. 1980 Mar 10;255(5):1874–1878. [PubMed] [Google Scholar]
- Wickens D., Wilkins M. H., Lunec J., Ball G., Dormandy T. L. Free radical oxidation (peroxidation)products in plasma in normal and abnormal pregnancy. Ann Clin Biochem. 1981 May;18(Pt 3):158–162. doi: 10.1177/000456328101800306. [DOI] [PubMed] [Google Scholar]



