Abstract
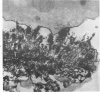
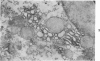
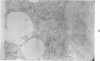
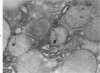
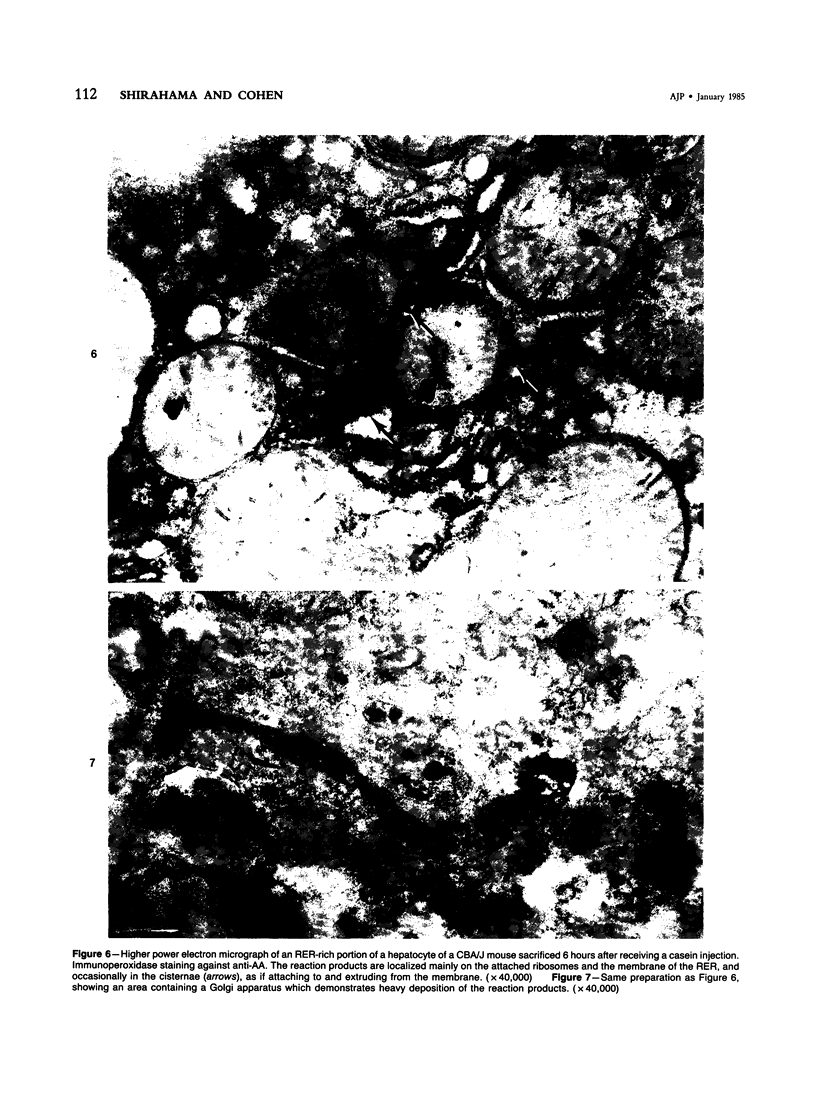
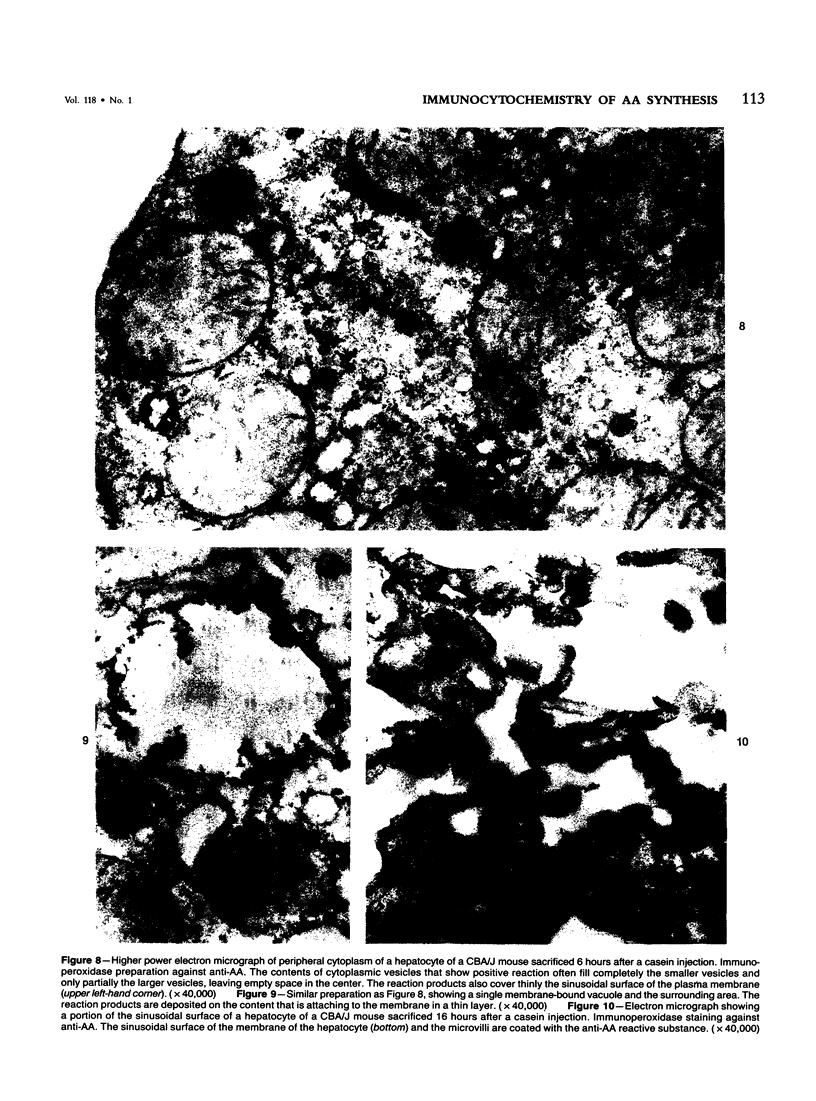
For determination of the intracellular site of synthesis and the pathways followed by amyloid protein AA, immunocytochemical localization of the anti-AA reactive substance was investigated in the livers of CBA/J mice in an acute-phase response evoked by a single subcutaneous injection of 0.5 ml of 10% casein. In the cytoplasm of the hepatocytes, the positive reaction was localized on and/or in the rough endoplasmic reticulum and the single membrane-bound vesicles, vacuoles and lamellae including the Golgi apparatus, confirming that amyloid protein AA follows the common routes of synthesis and secretion established for other proteins. The anti-AA-reactive substance was also localized on the free surface of the hepatocyte membrane, including the microvilli. The latter reaction appeared as early as but lasted at least several hours longer than its cytoplasmic counterpart, suggesting that a certain retention period exists before the release of the AA-reactive substance from the cellular surface to the free blood plasma.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Hoffman J. S., Eriksen N., Parmelee D. C., Walsh K. A. SAA, an apoprotein of HDL: its structure and function. Ann N Y Acad Sci. 1982;389:183–189. doi: 10.1111/j.1749-6632.1982.tb22136.x. [DOI] [PubMed] [Google Scholar]
- Benson M. D., Kleiner E. Synthesis and secretion of serum amyloid protein A (SAA) by hepatocytes in mice treated with casein. J Immunol. 1980 Feb;124(2):495–499. [PubMed] [Google Scholar]
- Benson M. D., Scheinberg M. A., Shirahama T., Cathcart E. S., Skinner M. Kinetics of serum amyloid protein A in casein-induced murine amyloidosis. J Clin Invest. 1977 Mar;59(3):412–417. doi: 10.1172/JCI108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein D. L. Leukocytic pyrogen: a major mediator of the acute phase reaction. Ann N Y Acad Sci. 1982;389:323–337. doi: 10.1111/j.1749-6632.1982.tb22147.x. [DOI] [PubMed] [Google Scholar]
- Cohen A. S., Shirahama T., Sipe J. D., Skinner M. Amyloid proteins, precursors, mediator, and enhancer. Lab Invest. 1983 Jan;48(1):1–4. [PubMed] [Google Scholar]
- Eldred W. D., Zucker C., Karten H. J., Yazulla S. Comparison of fixation and penetration enhancement techniques for use in ultrastructural immunocytochemistry. J Histochem Cytochem. 1983 Feb;31(2):285–292. doi: 10.1177/31.2.6339606. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Changes in high density lipoprotein content following endotoxin administration in the mouse. Formation of serum amyloid protein-rich subfractions. J Biol Chem. 1982 Sep 10;257(17):10510–10517. [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Secretion of serum amyloid protein and assembly of serum amyloid protein-rich high density lipoprotein in primary mouse hepatocyte culture. J Biol Chem. 1982 Sep 10;257(17):10518–10522. [PubMed] [Google Scholar]
- Hoffman J. S., Ericsson L. H., Eriksen N., Walsh K. A., Benditt E. P. Murine tissue amyloid protein AA. NH2-terminal sequence identity with only one of two serum amyloid protein (ApoSAA) gene products. J Exp Med. 1984 Feb 1;159(2):641–646. doi: 10.1084/jem.159.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampschmidt R. F., Upchurch H. F., Pulliam L. A. Characterization of a leukocyte-derived endogenous mediator responsible for increased plasma fibrinogen. Ann N Y Acad Sci. 1982;389:338–353. doi: 10.1111/j.1749-6632.1982.tb22148.x. [DOI] [PubMed] [Google Scholar]
- Kushner I., Feldmann G. Control of the acute phase response. Demonstration of C-reactive protein synthesis and secretion by hepatocytes during acute inflammation in the rabbit. J Exp Med. 1978 Aug 1;148(2):466–477. doi: 10.1084/jem.148.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M., Pras M., Franklin E. C. Immunologic studies of the major nonimmunoglobulin protein of amyloid. I. Identification and partial characterization of a related serum component. J Exp Med. 1973 Aug 1;138(2):373–380. doi: 10.1084/jem.138.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre S. S., Schultz D., Kushner I. Biosynthesis of C-reactive protein. Ann N Y Acad Sci. 1982;389:76–87. doi: 10.1111/j.1749-6632.1982.tb22126.x. [DOI] [PubMed] [Google Scholar]
- McAdam K. P., Elin R. J., Sipe J. D., Wolff S. M. Changes in human serum amyloid A and C-reactive protein after etiocholanolone-induced inflammation. J Clin Invest. 1978 Feb;61(2):390–394. doi: 10.1172/JCI108949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam K. P., Li J., Knowles J., Foss N. T., Dinarello C. A., Rosenwasser L. J., Selinger M. J., Kaplan M. M., Goodman R., Herbert P. N. The biology of SAA: identification of the inducer, in vitro synthesis, and heterogeneity demonstrated with monoclonal antibodies. Ann N Y Acad Sci. 1982;389:126–136. doi: 10.1111/j.1749-6632.1982.tb22131.x. [DOI] [PubMed] [Google Scholar]
- McAdam K. P., Sipe J. D. Murine model for human secondary amyloidosis: genetic variability of the acute-phase serum protein SAA response to endotoxins and casein. J Exp Med. 1976 Oct 1;144(4):1121–1127. doi: 10.1084/jem.144.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Stearman R. S., Peltzman C. G., Potter D. A. Induction of hepatic synthesis of serum amyloid A protein and actin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4718–4722. doi: 10.1073/pnas.78.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Rosenthal C. J., Franklin E. C. Variation with age and disease of an amyloid A protein-related serum component. J Clin Invest. 1975 Apr;55(4):746–753. doi: 10.1172/JCI107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. Intralysosomal formation of amyloid fibrils. Am J Pathol. 1975 Oct;81(1):101–116. [PMC free article] [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. Redistribution of amyloid deposits. Am J Pathol. 1980 Jun;99(3):539–550. [PMC free article] [PubMed] [Google Scholar]
- Shirahama T., Skinner M., Cohen A. S. Immunocytochemical identification of amyloid in formalin-fixed paraffin sections. Histochemistry. 1981;72(2):161–171. doi: 10.1007/BF00517130. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., Vogel S. N., Sztein M. B., Skinner M., Cohen A. S. The role of interleukin 1 in acute phase serum amyloid A (SAA) and serum amyloid P (SAP) biosynthesis. Ann N Y Acad Sci. 1982;389:137–150. doi: 10.1111/j.1749-6632.1982.tb22132.x. [DOI] [PubMed] [Google Scholar]
- Skinner M., Shirahama T., Benson M. D., Cohen A. S. Murine amyloid protein AA in casein-induced experimental amyloidosis. Lab Invest. 1977 Apr;36(4):420–427. [PubMed] [Google Scholar]
- TEILUM G. PATHOGENESIS OF AMYLOIDOSIS. Acta Pathol Microbiol Scand. 1964;61:21–45. doi: 10.1111/apm.1964.61.1.21. [DOI] [PubMed] [Google Scholar]
- Tatsuta E., Sipe J. D., Shirahama T., Skinner M., Cohen A. S. Different regulatory mechanisms for serum amyloid A and serum amyloid P synthesis by cultured mouse hepatocytes. J Biol Chem. 1983 May 10;258(9):5414–5418. [PubMed] [Google Scholar]
- Weinstein P. S., Skinner M., Sipe J. D., Lokich J. J., Zamcheck N., Cohen A. S. Acute-phase proteins or tumour markers: the role of SAA, SAP, CRP and CEA as indicators of metastasis in a broad spectrum of neoplastic diseases. Scand J Immunol. 1984 Mar;19(3):193–198. doi: 10.1111/j.1365-3083.1984.tb00919.x. [DOI] [PubMed] [Google Scholar]