Abstract
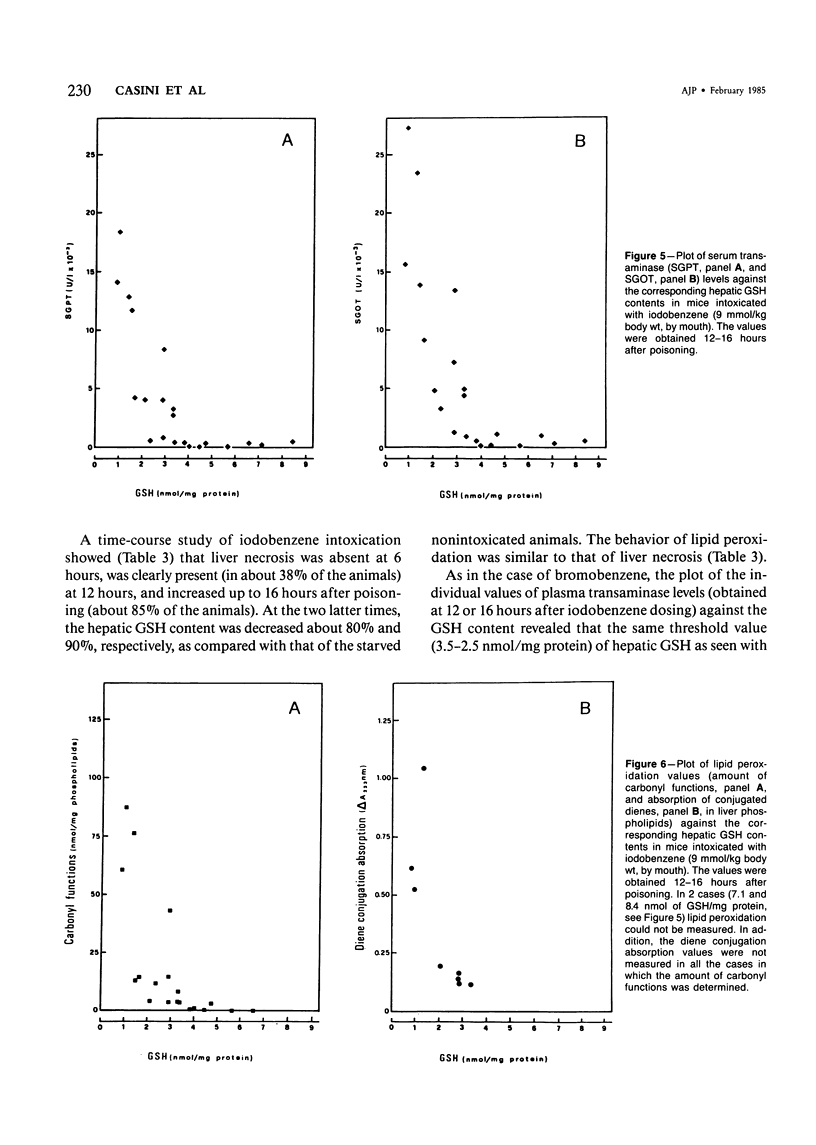
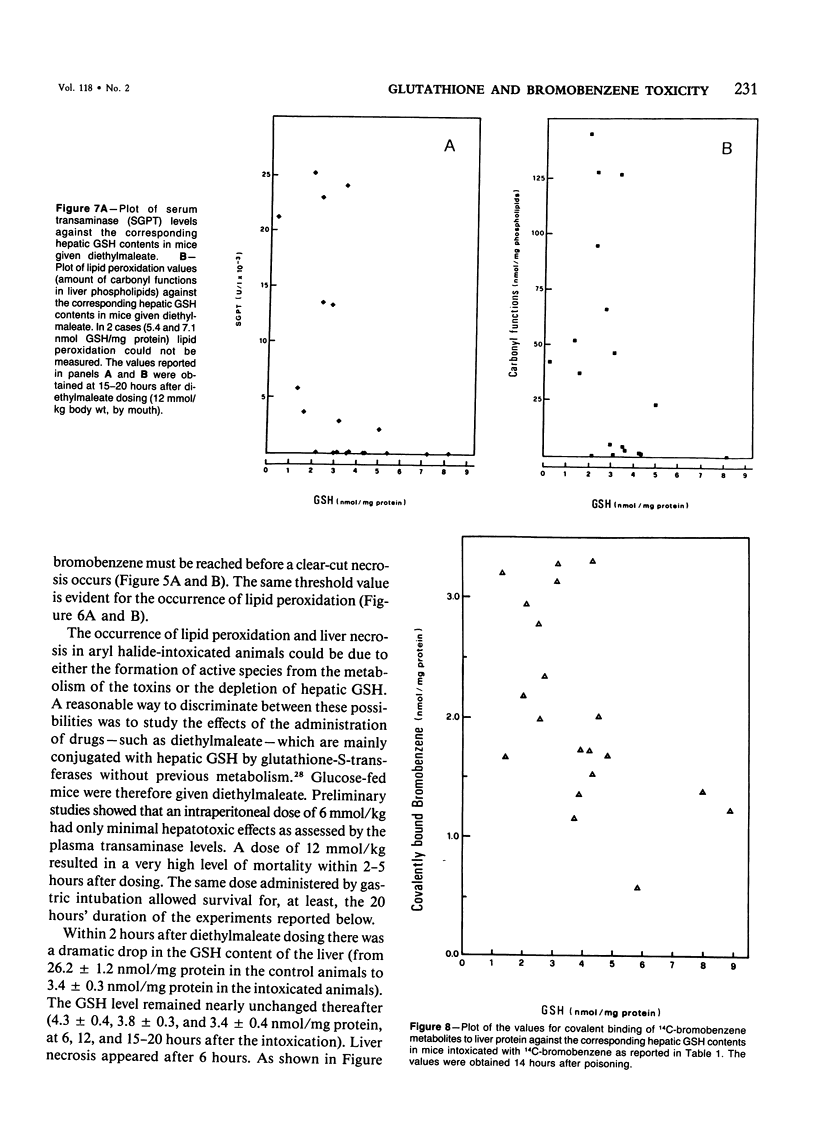
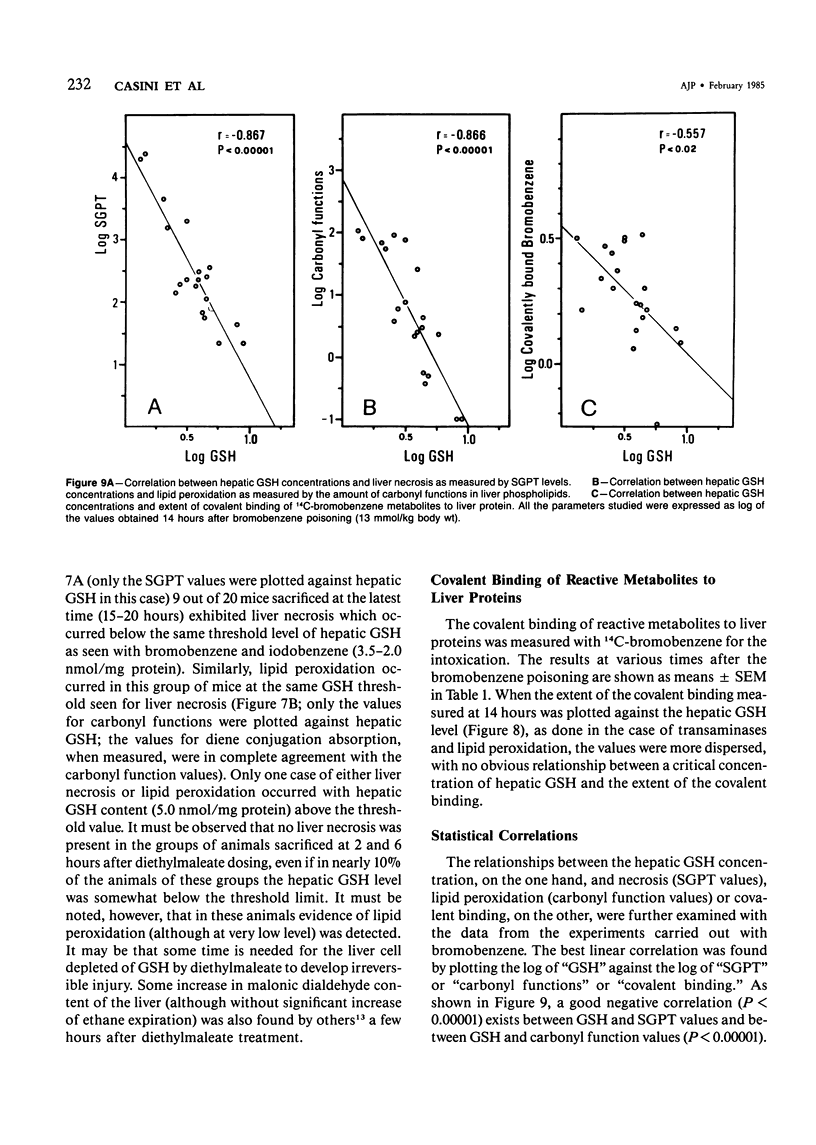
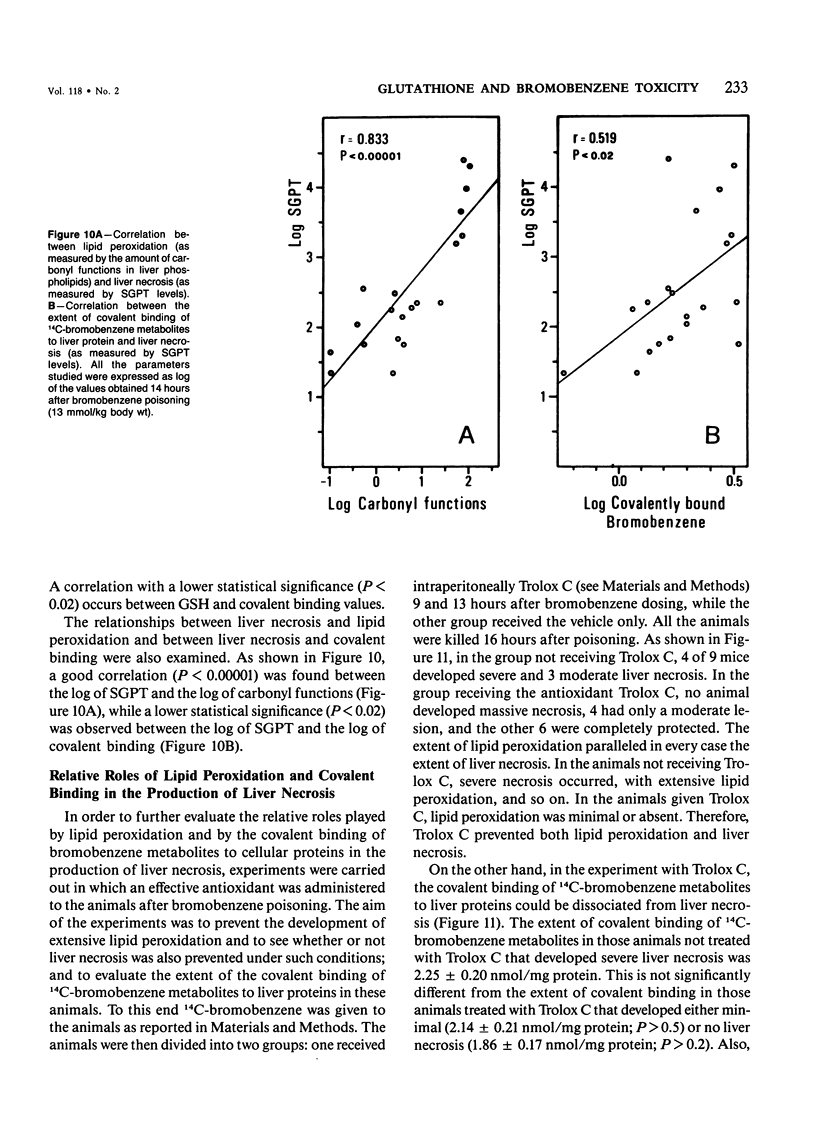
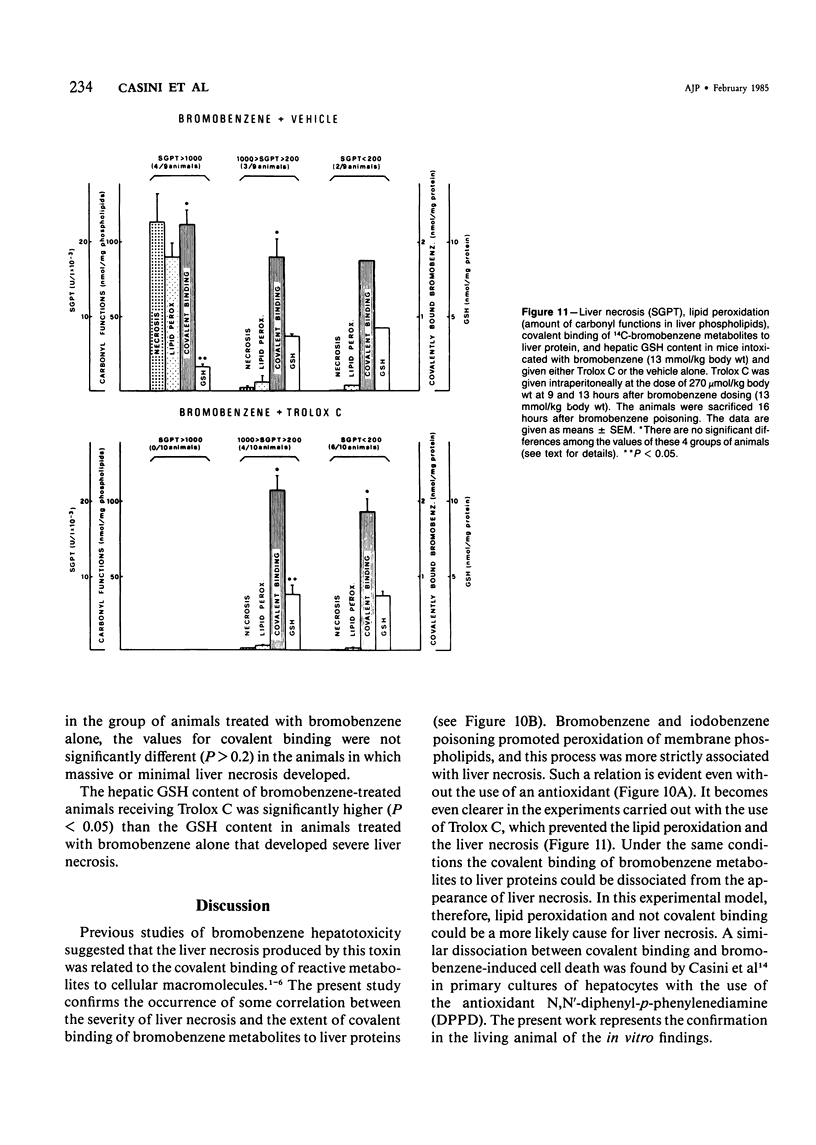
The mechanisms of bromobenzene and iodobenzene hepatotoxicity in vivo were studied in mice. Both the intoxications caused a progressive decrease in hepatic glutathione content. In both instances liver necrosis was evident only when the hepatic glutathione depletion reached a threshold value (3.5-2.5 nmol/mg protein). The same threshold value was evident for the occurrence of lipid peroxidation. Similar results were obtained in a group of mice sacrificed 15-20 hours after the administration of diethylmaleate, a drug which is mainly conjugated with hepatic glutathione without previous metabolism. The correlation between lipid peroxidation and liver necrosis was much more significant than that between covalent binding and liver necrosis. This fact supports the view that lipid peroxidation is the major candidate for the liver cell death produced by bromobenzene intoxication. Moreover, a dissociation of liver necrosis from covalent binding was observed in experiments in which Trolox C (a lower homolog of vitamin E) was administered after bromobenzene poisoning. The treatment with Trolox C, in fact, almost completely prevented both liver necrosis and lipid peroxidation, while not changing at all the extent of the covalent binding of bromobenzene metabolites to liver protein.
Full text
PDF
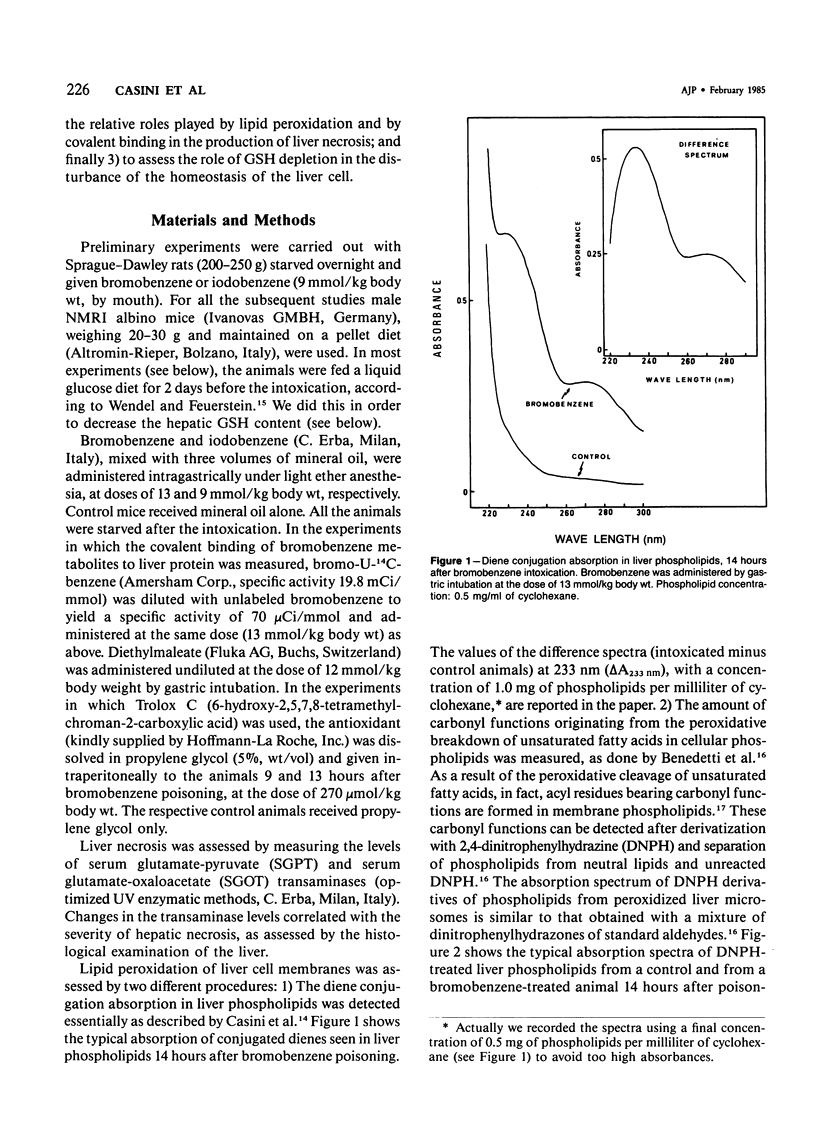
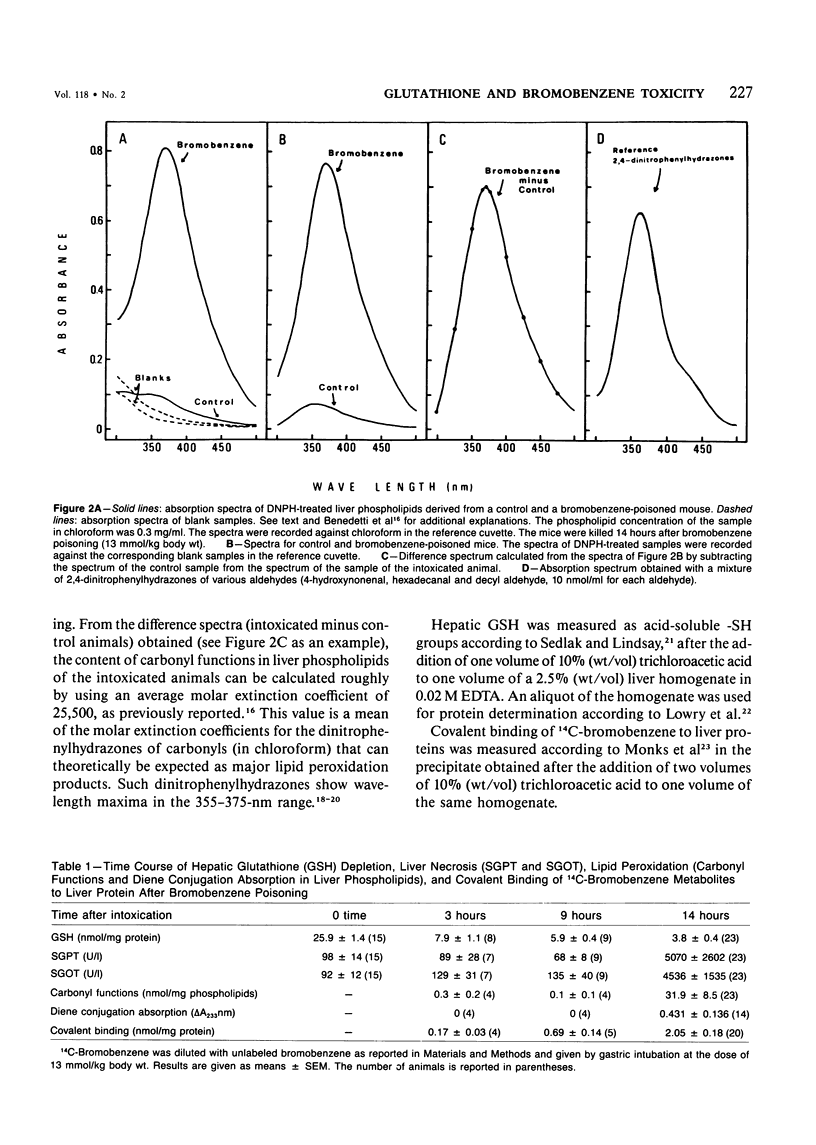
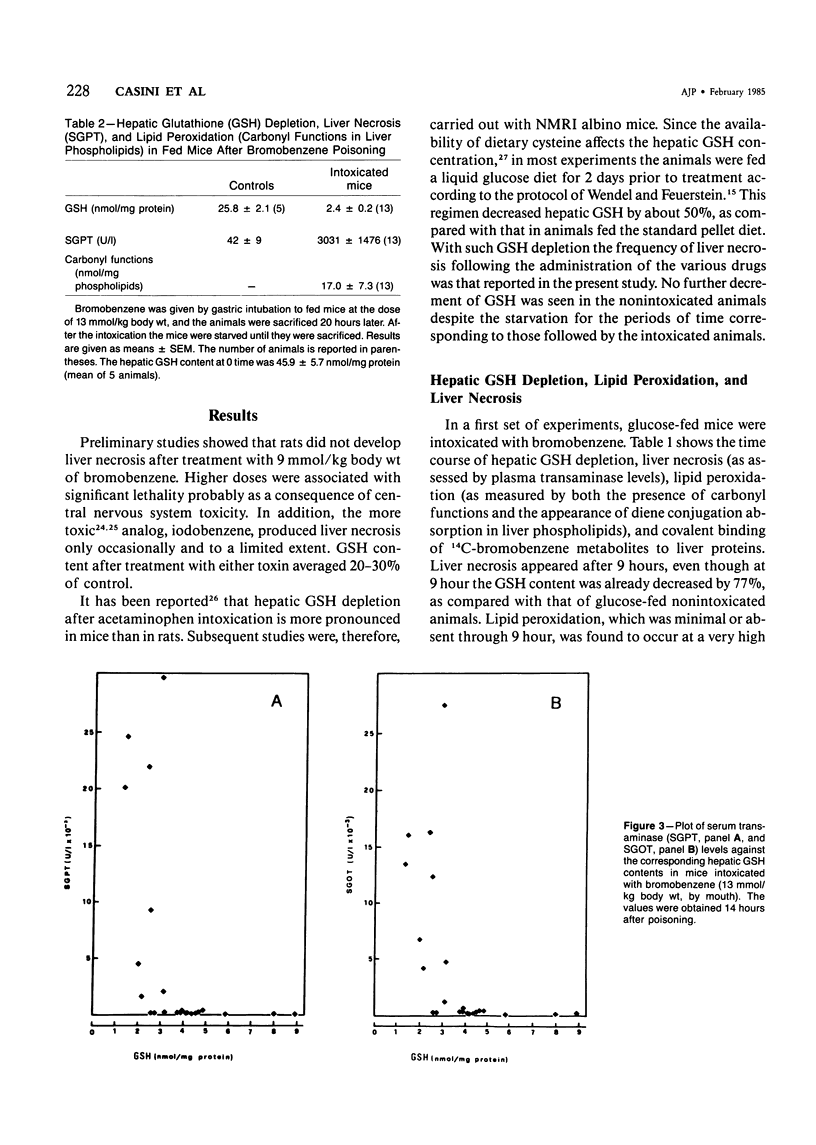
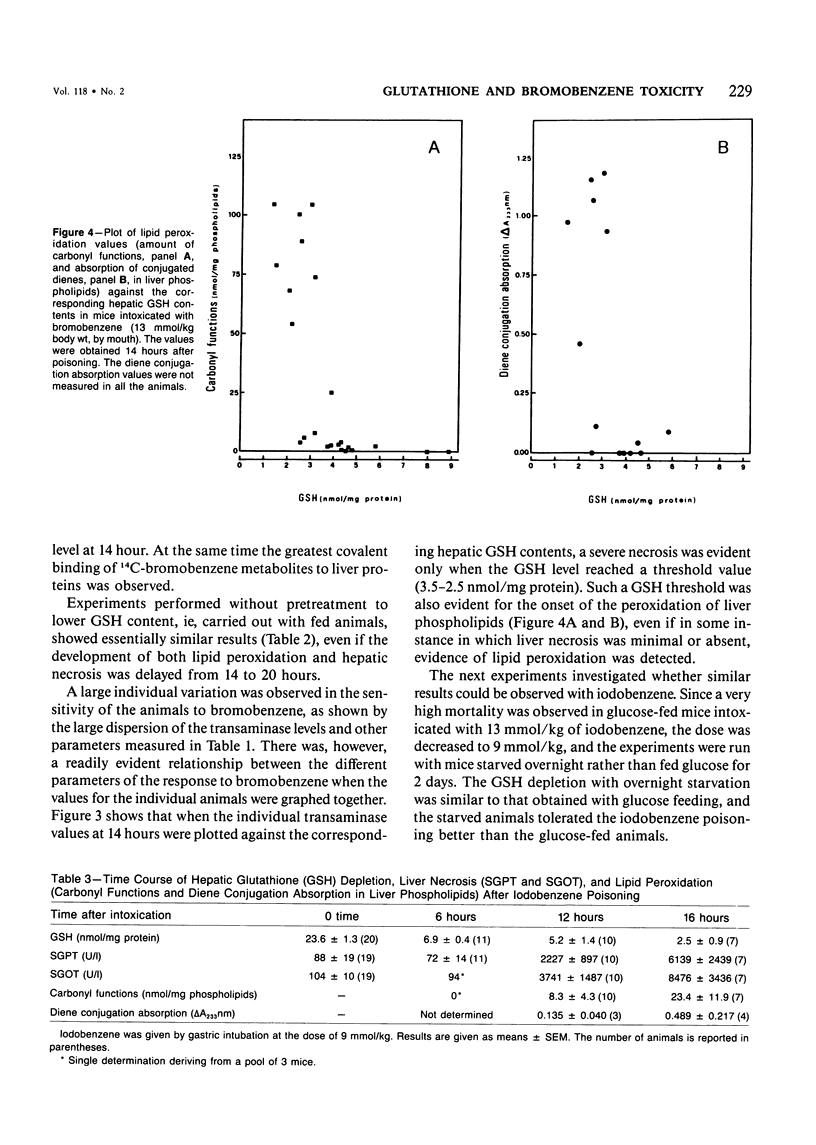



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anundi I., Högberg J., Stead A. H. Glutathione depletion in isolated hepatocytes: its relation to lipid peroxidation and cell damage. Acta Pharmacol Toxicol (Copenh) 1979 Jul;45(1):45–51. doi: 10.1111/j.1600-0773.1979.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Fulceri R., Ferrali M., Ciccoli L., Esterbauer H., Comporti M. Detection of carbonyl functions in phospholipids of liver microsomes in CCl4- and BrCCl3-poisoned rats. Biochim Biophys Acta. 1982 Sep 14;712(3):628–638. doi: 10.1016/0005-2760(82)90292-2. [DOI] [PubMed] [Google Scholar]
- Boyland E., Chasseaud L. F. Enzyme-catalysed conjugations of glutathione with unsaturated compounds. Biochem J. 1967 Jul;104(1):95–102. doi: 10.1042/bj1040095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie B. B., Reid W. D., Cho A. K., Sipes G., Krishna G., Gillette J. R. Possible mechanism of liver necrosis caused by aromatic organic compounds. Proc Natl Acad Sci U S A. 1971 Jan;68(1):160–164. doi: 10.1073/pnas.68.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini A., Giorli M., Hyland R. J., Serroni A., Gilfor D., Farber J. L. Mechanisms of cell injury in the killing of cultured hepatocytes by bromobenzene. J Biol Chem. 1982 Jun 25;257(12):6721–6728. [PubMed] [Google Scholar]
- Davis D. C., Potter W. Z., Jollow D. J., Mitchell J. R. Species differences in hepatic glutathione depletion, covalent binding and hepatic necrosis after acetaminophen. Life Sci. 1974 Jun 1;14(11):2099–2109. doi: 10.1016/0024-3205(74)90092-7. [DOI] [PubMed] [Google Scholar]
- Devalia J. L., Ogilvie R. C., McLean A. E. Dissociation of cell death from covalent binding of paracetamol by flavones in a hepatocyte system. Biochem Pharmacol. 1982 Dec 1;31(23):3745–3749. doi: 10.1016/0006-2952(82)90287-8. [DOI] [PubMed] [Google Scholar]
- Gerber J. G., MacDonald J. S., Harbison R. D., Villeneuve J. P., Wood A. J., Nies A. S. Effect of N-acetylcysteine on hepatic covalent binding of paracetamol (acetaminophen) Lancet. 1977 Mar 19;1(8012):657–658. doi: 10.1016/s0140-6736(77)92100-6. [DOI] [PubMed] [Google Scholar]
- Gillette J. R. Commentary. A perspective on the role of chemically reactive metabolites of foreign compounds in toxicity. I. Correlation of changes in covalent binding of reactivity metabolites with changes in the incidence and severity of toxicity. Biochem Pharmacol. 1974 Oct 15;23(20):2785–2794. doi: 10.1016/0006-2952(74)90052-5. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A. G., Speck M., Roots I. Possible control of hydrogen peroxide production and degradation in microsomes during mixed function oxidation reaction. Biochem Biophys Res Commun. 1973 Oct 1;54(3):968–975. doi: 10.1016/0006-291x(73)90789-4. [DOI] [PubMed] [Google Scholar]
- Jollow D. J., Mitchell J. R., Potter W. Z., Davis D. C., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973 Oct;187(1):195–202. [PubMed] [Google Scholar]
- Jollow D. J., Mitchell J. R., Zampaglione N., Gillette J. R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labadarios D., Davis M., Portmann B., Williams R. Paracetamol-induced hepatic necrosis in the mouse-relationship between covalent binding, hepatic glutathione depletion and the protective effect of alpha-mercaptopropionylglycine. Biochem Pharmacol. 1977 Jan 1;26(1):31–35. doi: 10.1016/0006-2952(77)90126-5. [DOI] [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973 Oct;187(1):211–217. [PubMed] [Google Scholar]
- Mitchell J. R., Jollows D. J. Progress in hepatology. Metabolic activation of drugs to toxic substances. Gastroenterology. 1975 Feb;68(2):392–410. [PubMed] [Google Scholar]
- Monks T. J., Hinson J. A., Gillette J. R. Bromobenzene and p-bromophenol toxicity and covalent binding in vivo. Life Sci. 1982 Mar 8;30(10):841–848. doi: 10.1016/0024-3205(82)90598-7. [DOI] [PubMed] [Google Scholar]
- Nordblom G. D., Coon M. J. Hydrogen peroxide formation and stoichiometry of hydroxylation reactions catalyzed by highly purified liver microsomal cytochrome P-450. Arch Biochem Biophys. 1977 Apr 30;180(2):343–347. doi: 10.1016/0003-9861(77)90047-9. [DOI] [PubMed] [Google Scholar]
- Reid W. D., Krishna G., Gillette R., Brodie B. B. Biochemical mechanism of hepatic necrosis induced by aromatic hydrocarbons. Pharmacology. 1973;10(4):193–214. doi: 10.1159/000136440. [DOI] [PubMed] [Google Scholar]
- Sedlak J., Lindsay R. H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968 Oct 24;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Smith M. T., Thor H., Hartizell P., Orrenius S. The measurement of lipid peroxidation in isolated hepatocytes. Biochem Pharmacol. 1982 Jan 1;31(1):19–26. doi: 10.1016/0006-2952(82)90230-1. [DOI] [PubMed] [Google Scholar]
- Tam B. K., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. 3. Transient formation of phospholipid peroxides. J Biol Chem. 1970 May 10;245(9):2295–2300. [PubMed] [Google Scholar]
- Tateishi N., Higashi T., Shinya S., Naruse A., Sakamoto Y. Studies on the regulation of glutathione level in rat liver. J Biochem. 1974 Jan;75(1):93–103. doi: 10.1093/oxfordjournals.jbchem.a130387. [DOI] [PubMed] [Google Scholar]
- Wendel A., Feuerstein S. Drug-induced lipid peroxidation in mice--I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem Pharmacol. 1981 Sep 15;30(18):2513–2520. doi: 10.1016/0006-2952(81)90576-1. [DOI] [PubMed] [Google Scholar]
- Wendel A., Feuerstein S., Konz K. H. Acute paracetamol intoxication of starved mice leads to lipid peroxidation in vivo. Biochem Pharmacol. 1979 Jul 1;28(13):2051–2055. doi: 10.1016/0006-2952(79)90223-5. [DOI] [PubMed] [Google Scholar]
- Wiley R. A., Hanzlik R. P., Gillesse T. Effect of substituents on in vitro metabolism and covalent binding of substituted bromobenzenes. Toxicol Appl Pharmacol. 1979 Jun 30;49(2):249–255. doi: 10.1016/0041-008x(79)90248-5. [DOI] [PubMed] [Google Scholar]
- Younes M., Siegers C. P. Mechanistic aspects of enhanced lipid peroxidation following glutathione depletion in vivo. Chem Biol Interact. 1981 Mar 15;34(3):257–266. doi: 10.1016/0009-2797(81)90098-3. [DOI] [PubMed] [Google Scholar]



