Abstract
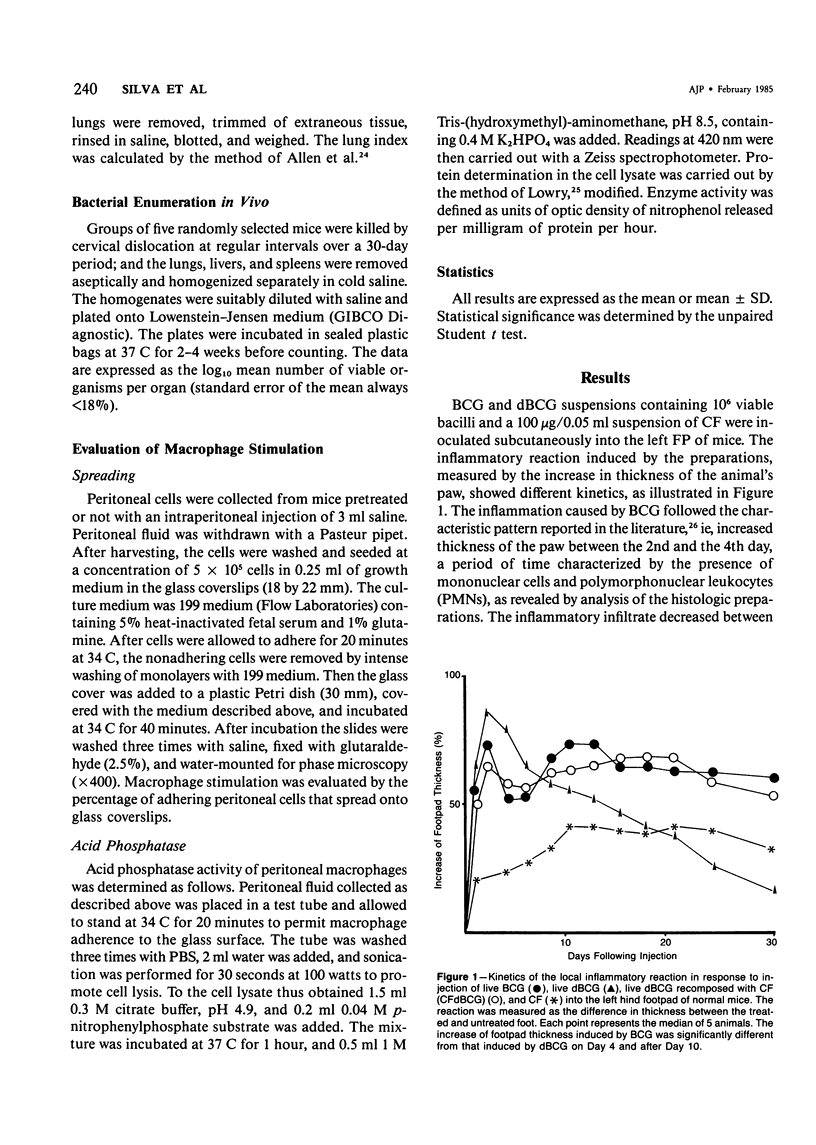
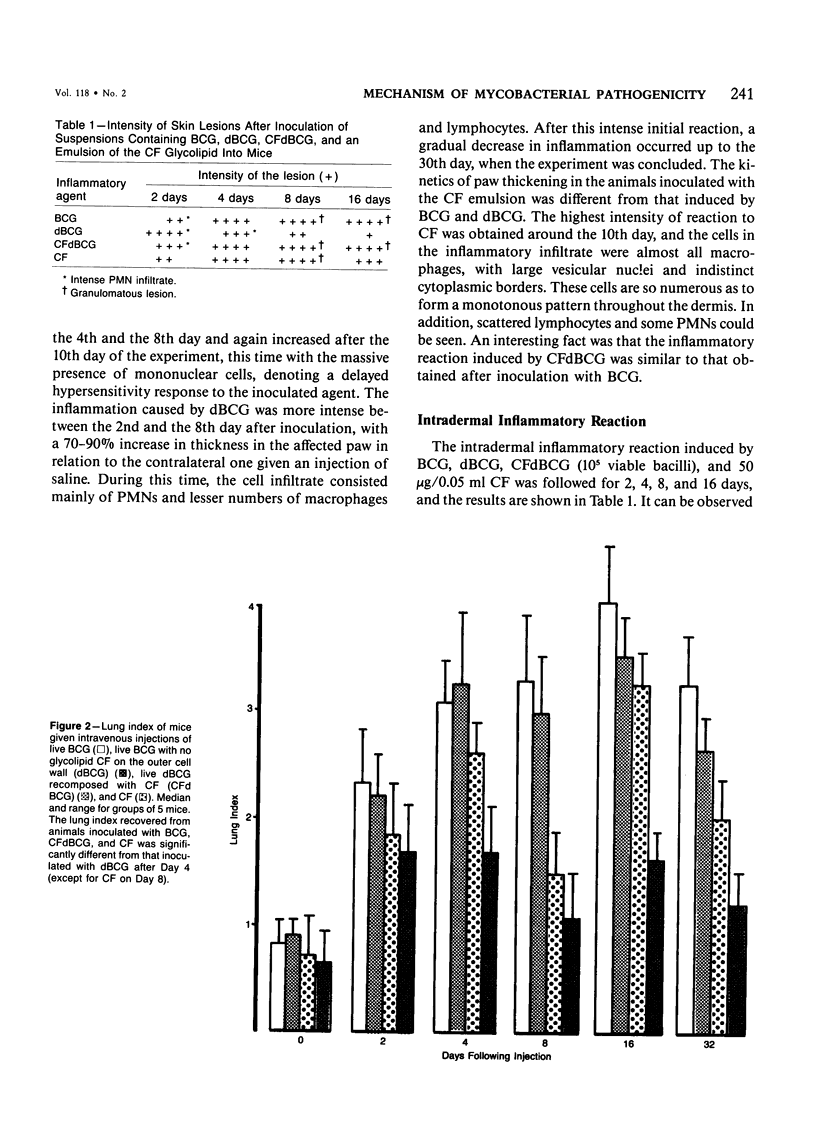
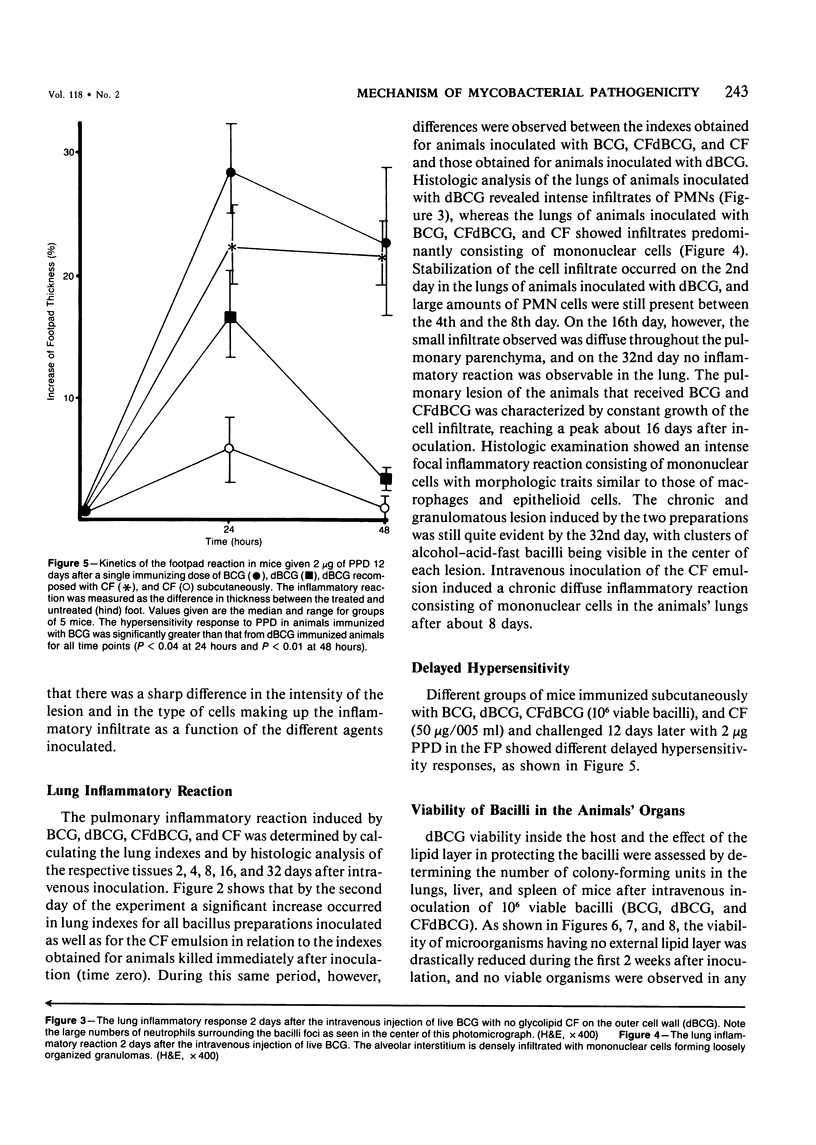
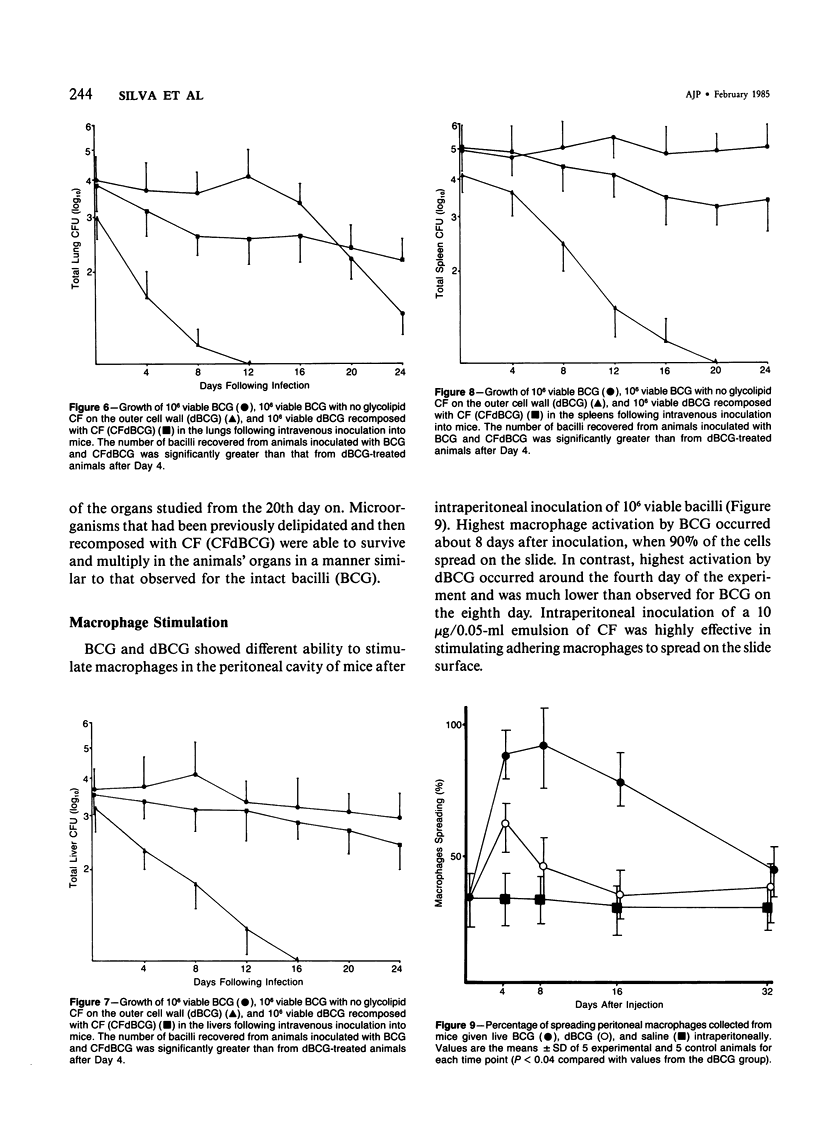
The subcutaneous, intradermal, and pulmonary inflammatory lesions induced in mice by viable Mycobacterium bovis (BCG) with no glycolipid cord factor (CF) on the outer cell wall (delipidated BCG, dBCG) was drastically different from that induced by inoculation with intact bacteria. The reaction caused by dBCG was of an acute nature: the cells making up the inflammatory infiltrate exhibited polymorphonuclear-like (PMNs) morphologic characteristics, there was a decrease on delayed hypersensitivity response, and the lesion was resolved around the 16th day after inoculation. Complete disappearance of viable organisms from the lungs, liver, and spleen of these animals occurred in parallel with the dissipation of the dBCG-induced inflammatory infiltrate, showing that CF plays an important role in the host-parasite relationship that takes place in infections caused by mycobacteria. In addition, when deprived of this glycolipid component, bacilli lose their immunostimulant ability.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. M., Moore V. L., Stevens J. O. Strain variation in BCG-induced chronic pulmonary inflammation in mice. I. Basic model and possible genetic control by non-H-2 genes. J Immunol. 1977 Jul;119(1):343–347. [PubMed] [Google Scholar]
- Azuma I., Ribi E. E., Meyer T. J., Zbar B. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J Natl Cancer Inst. 1974 Jan;52(1):95–101. doi: 10.1093/jnci/52.1.95. [DOI] [PubMed] [Google Scholar]
- BLOCH H. Studies on the virulence of tubercle bacilli; isolation and biological properties of a constituent of virulent organisms. J Exp Med. 1950 Feb;91(2):197-218, pl. doi: 10.1084/jem.91.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barksdale L., Kim K. S. Mycobacterium. Bacteriol Rev. 1977 Mar;41(1):217–372. doi: 10.1128/br.41.1.217-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A. Acute granulomatous response produced in mice by trehalose-6,6-dimycolate. J Bacteriol. 1968 Oct;96(4):958–961. doi: 10.1128/jb.96.4.958-961.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A., Levij I. S., Yarkoni E., Vilkas E., Adam A., Lederer E. Granuloma formation induced in mice by chemically defined mycobacterial fractions. J Bacteriol. 1969 Oct;100(1):95–102. doi: 10.1128/jb.100.1.95-102.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekierkunst A., Yarkoni E., Flechner I., Morecki S., Vilkas E., Lederer E. Immune response to sheep red blood cells in mice pretreated with mycobacterial fractions. Infect Immun. 1971 Sep;4(3):256–263. doi: 10.1128/iai.4.3.256-263.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos R. J. CELLULAR STRUCTURES AND FUNCTIONS CONCERNED IN PARASITISM. Bacteriol Rev. 1948 Sep;12(3):173–194. doi: 10.1128/br.12.3.173-194.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget A., Skamene E., Gros P., Miailhe A. C., Turcotte R. Differences in response among inbred mouse strains to infection with small doses of Mycobacterium bovis BCG. Infect Immun. 1981 Apr;32(1):42–47. doi: 10.1128/iai.32.1.42-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B., Cernich M., Brokl O. Some observations of mycobacterial acid-fastness. Am Rev Respir Dis. 1978 Jul;118(1):151–154. doi: 10.1164/arrd.1978.118.1.151. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Kato M. Antibody formation to trehalose-6,6'-dimycolate (cord factor) of Mycobacterium tuberculosis. Infect Immun. 1972 Feb;5(2):203–212. doi: 10.1128/iai.5.2.203-212.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Asselineau J. Chemical structure and biochemical activity of cord factor analogs. 6,6'-Dimycoloyl sucrose and methyl 6-mycoloyl- -D-glucoside. Eur J Biochem. 1971 Oct 14;22(3):364–370. doi: 10.1111/j.1432-1033.1971.tb01553.x. [DOI] [PubMed] [Google Scholar]
- Kato M. Effect of anti-cord factor antibody on experimental tuberculosis in mice. Infect Immun. 1973 Jan;7(1):14–21. doi: 10.1128/iai.7.1.14-21.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Induction and expression of immunity after BCG immunization. Infect Immun. 1977 Dec;18(3):646–653. doi: 10.1128/iai.18.3.646-653.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Macrophage activation and resistance to pulmonary tuberculosis. Infect Immun. 1980 May;28(2):508–515. doi: 10.1128/iai.28.2.508-515.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnikin D. E., Minnikin S. M., Dobson G., Goodfellow M., Portaels F., van den Breen L., Sesardic D. Mycolic acid patterns of four vaccine strains of Mycobacterium bovis BCG. J Gen Microbiol. 1983 Mar;129(3):889–891. doi: 10.1099/00221287-129-3-889. [DOI] [PubMed] [Google Scholar]
- NOLL H., BLOCH H., ASSELINEAU J., LEDERER E. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochim Biophys Acta. 1956 May;20(2):299–309. doi: 10.1016/0006-3002(56)90289-x. [DOI] [PubMed] [Google Scholar]
- Parant M., Audibert F., Parant F., Chedid L., Soler E., Polonsky J., Lederer E. Nonspecific immunostimulant activities of synthetic trehalose-6,6'-diesters (lower homologs of cord factor). Infect Immun. 1978 Apr;20(1):12–19. doi: 10.1128/iai.20.1.12-19.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]





