Abstract
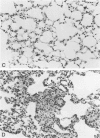
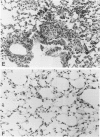
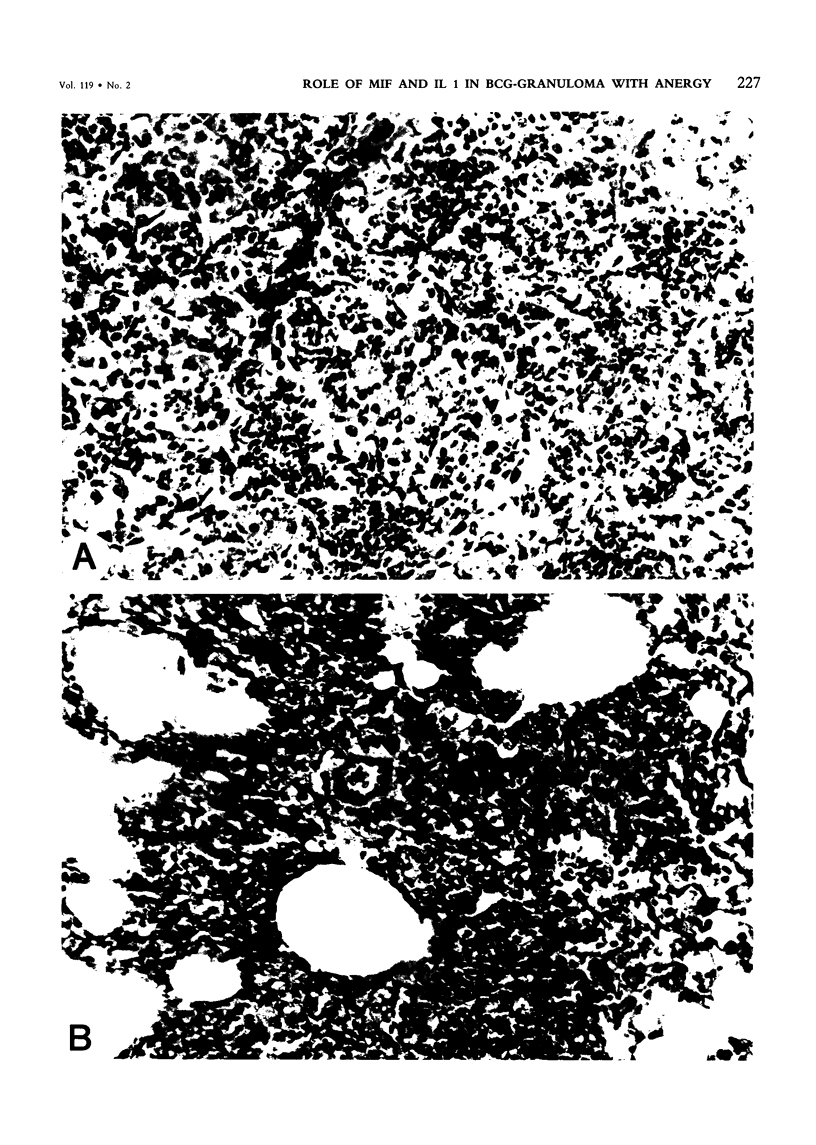
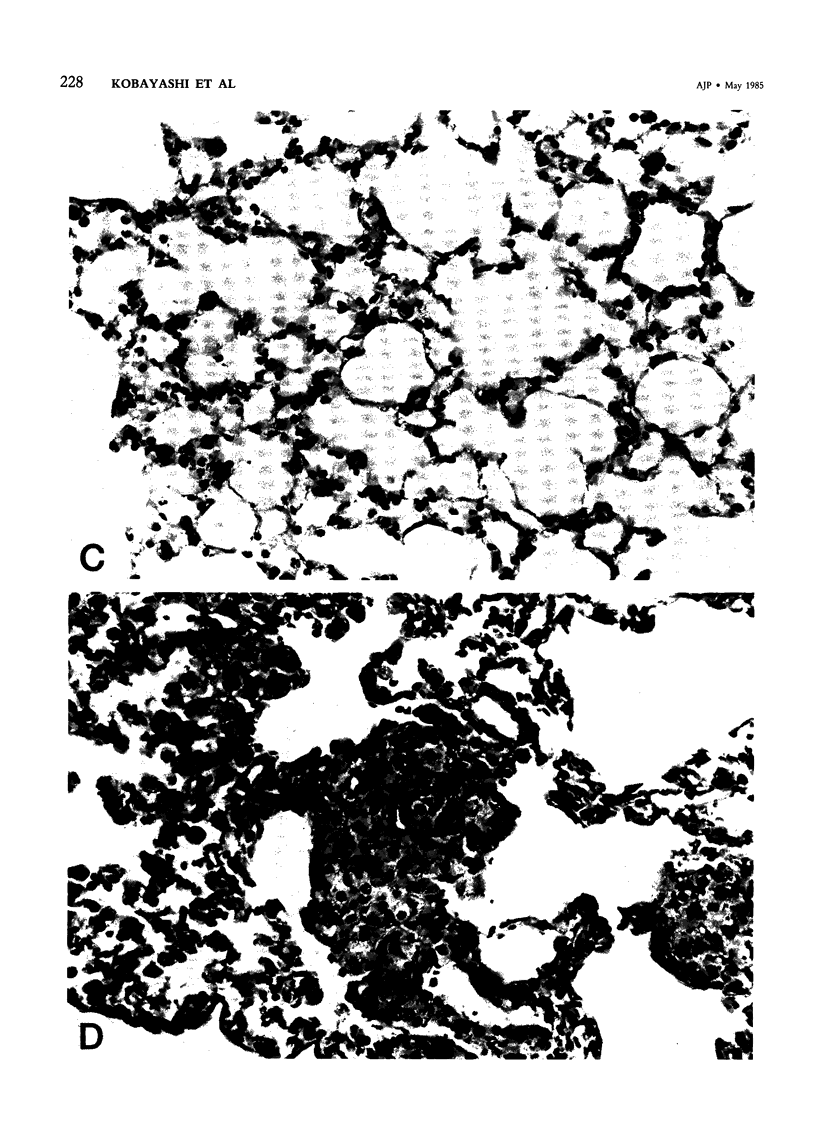
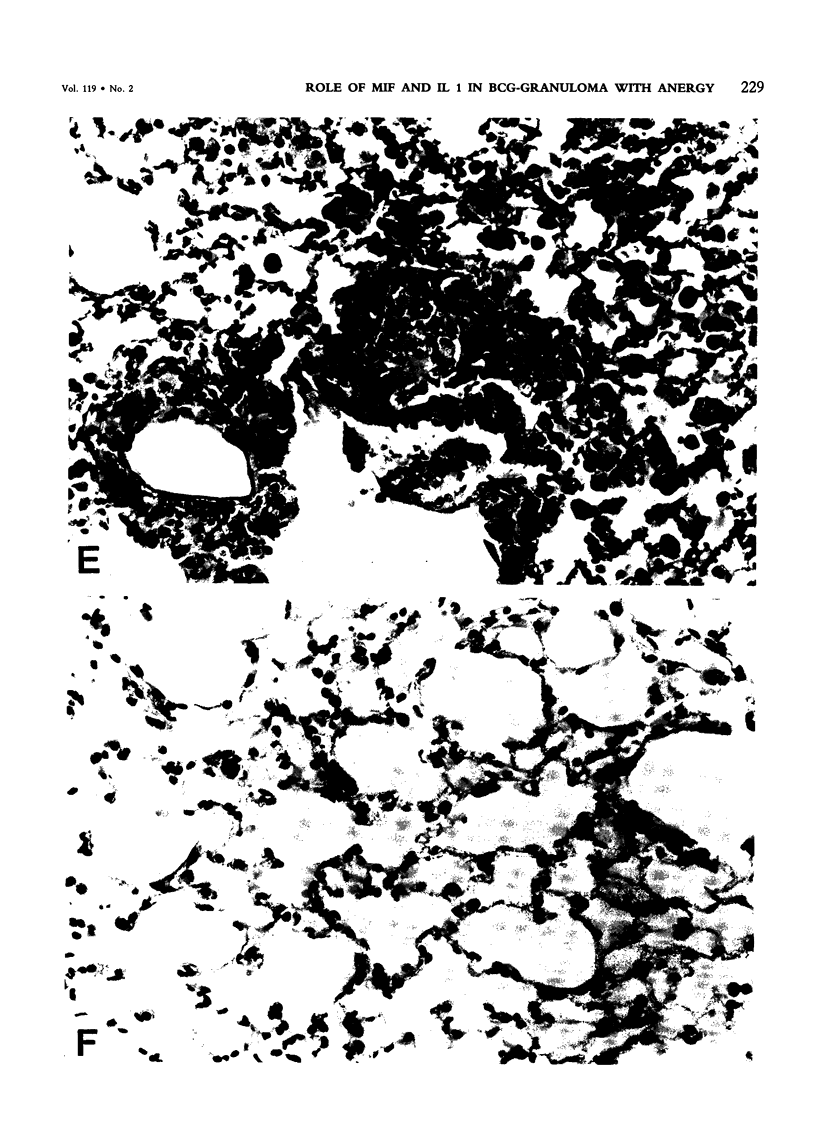
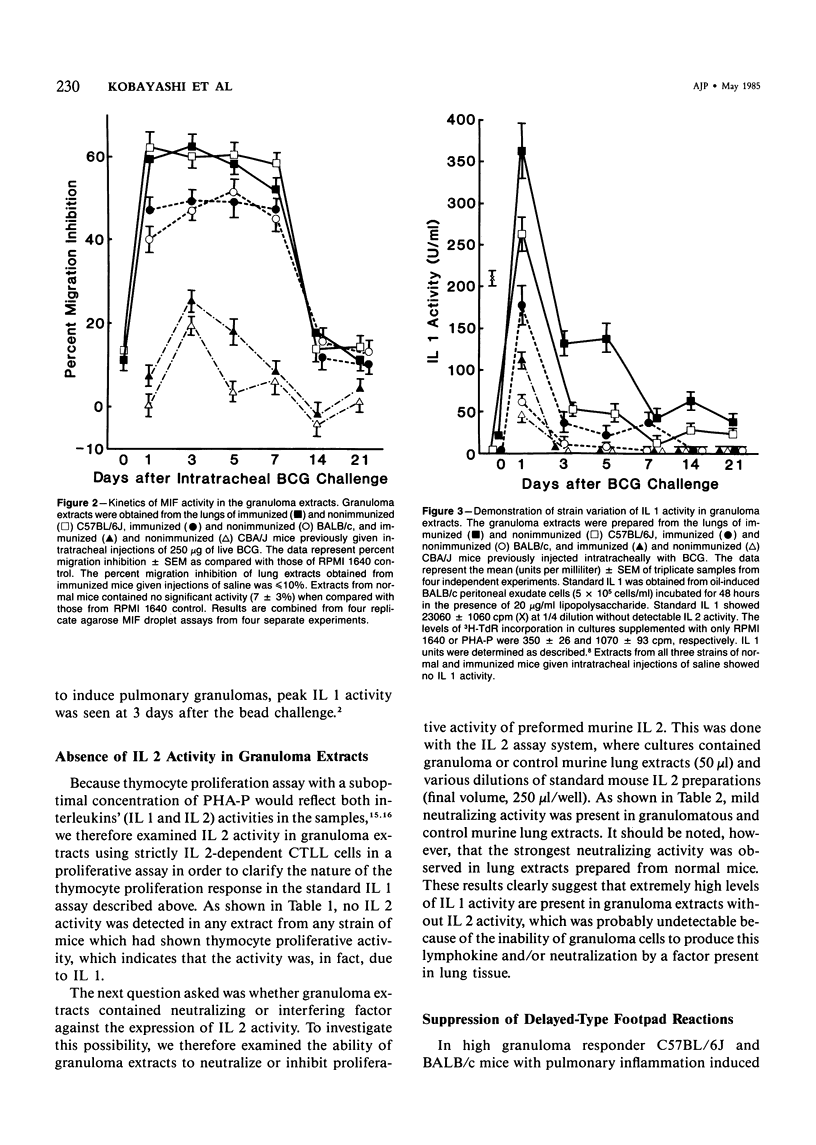
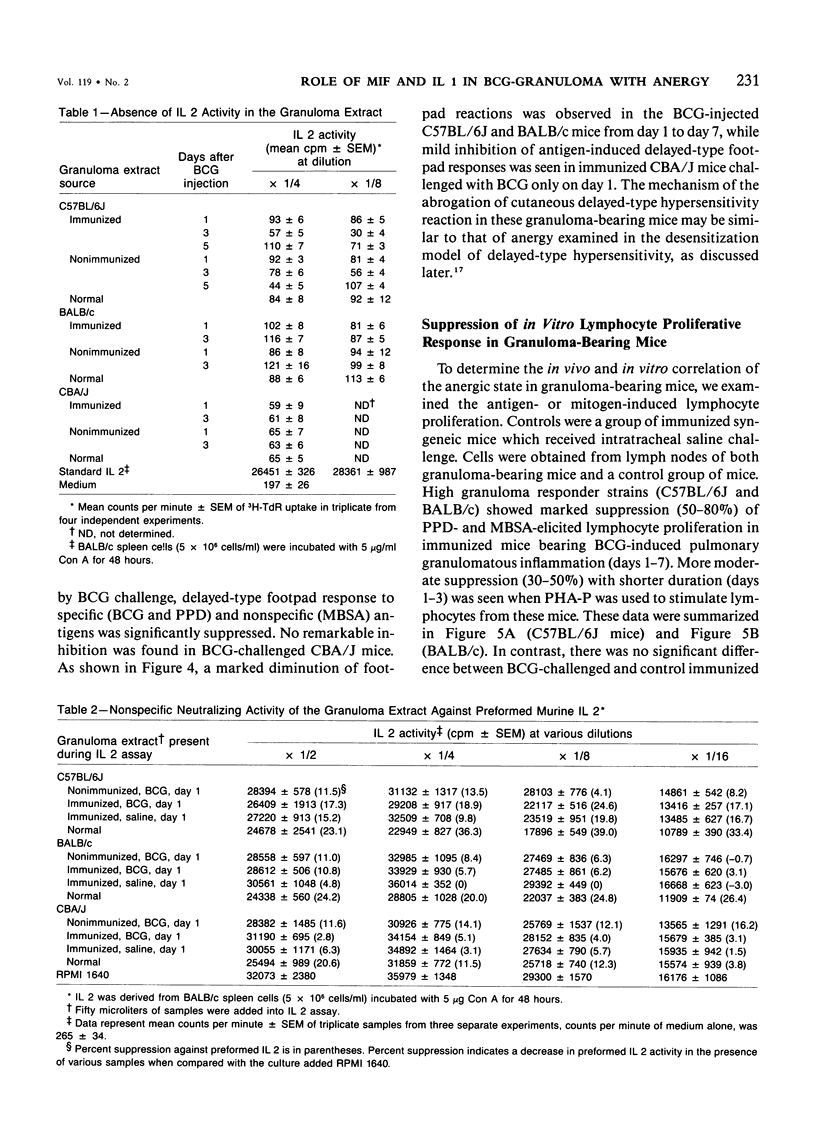
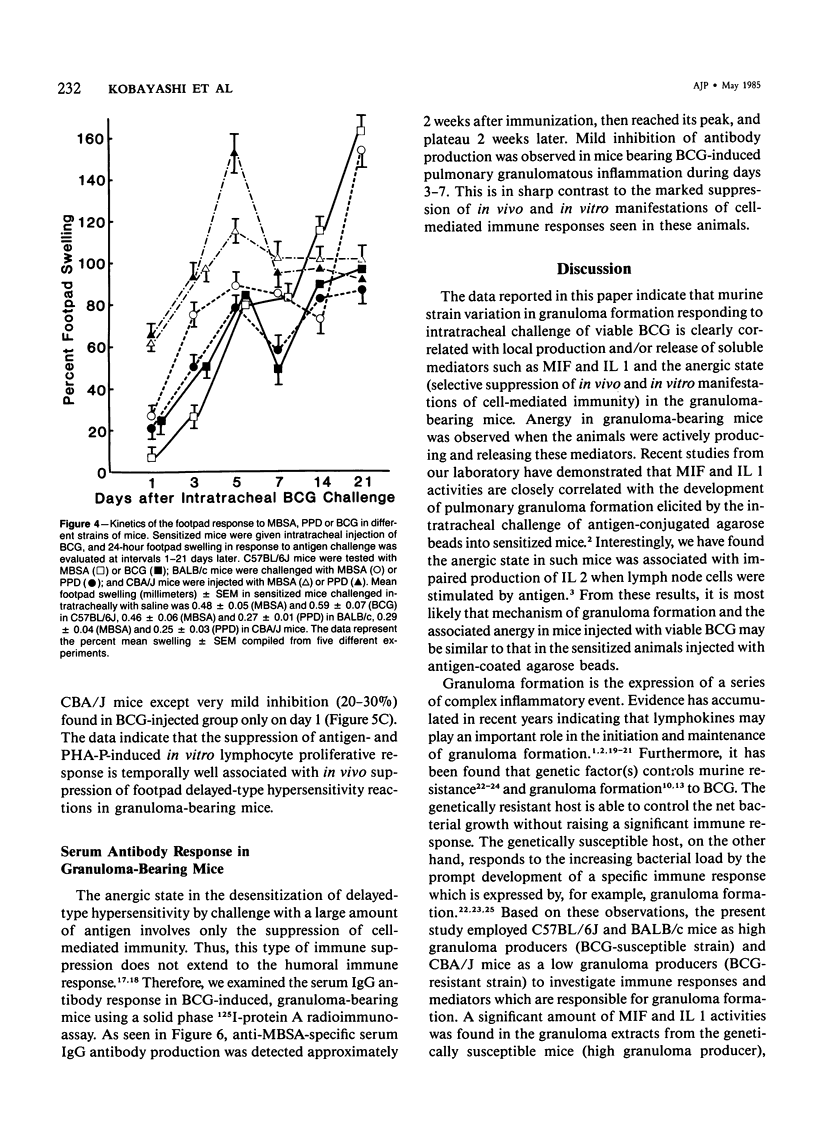
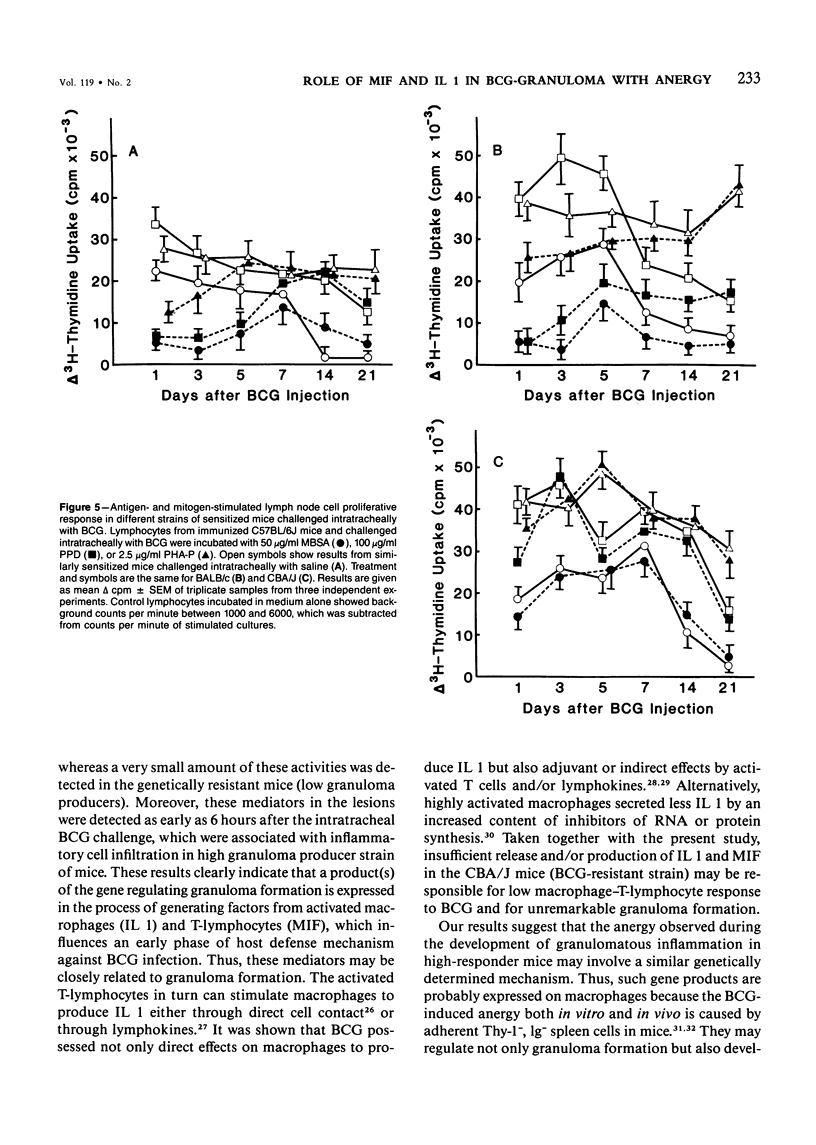
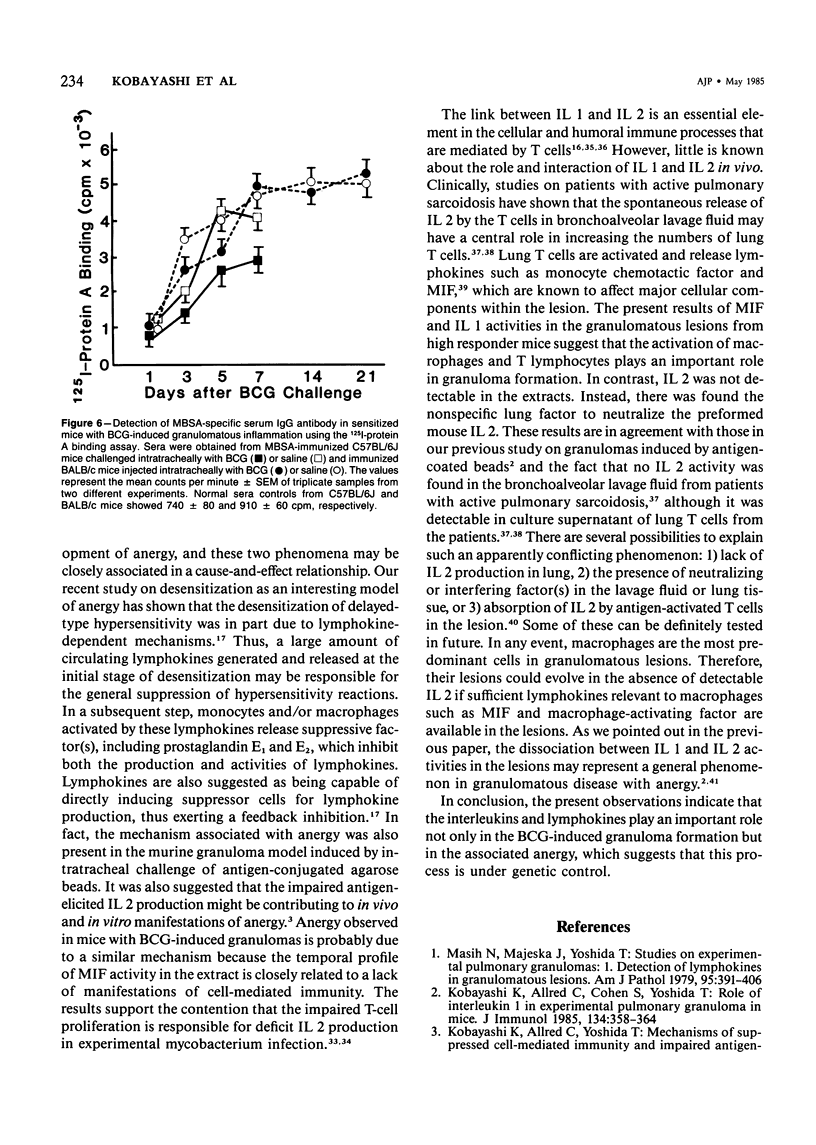
Pulmonary granulomatous inflammation was induced by the intratracheal injection of viable bacillus Calmette-Guerin (BCG) into genetically high granuloma responder (C57BL/6J and BALB/c) and low responder (CBA/J) mice with and without immunization by methylated bovine serum albumin in complete Freund's adjuvant. Significant migration inhibition factor (MIF) and interleukin 1 (IL 1) activities were detected in aqueous lung granuloma extracts prepared from high responder mice bearing BCG-induced granulomatous inflammation. Interleukin 2 activity was not detected. Very low MIF and IL 1 activities were detected in extracts from low responder mice. Furthermore, high responder, but not low responder, mice showed marked suppression of in vivo and in vitro manifestations of cell-mediated immunity to both specific and nonspecific antigens. In contrast, humoral antibody response was not affected significantly. The kinetics of anergy in granuloma-bearing mice correlated closely with the appearance of MIF and IL 1 activities in the lesions. Thus, genetically determined granuloma response to BCG and the expression of anergy in various strains of mice were well associated with in vivo release of MIF and IL 1. These results indicate that the genetic ability or inability to mount a granulomatous inflammatory response to BCG may extend to the capacity of cells within the lesions to generate soluble mediator(s) which is also responsible for anergy in granuloma-bearing mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. M., Moore V. L., Stevens J. O. Strain variation in BCG-induced chronic pulmonary inflammation in mice. I. Basic model and possible genetic control by non-H-2 genes. J Immunol. 1977 Jul;119(1):343–347. [PubMed] [Google Scholar]
- Allen E. M., Moore V. L. Suppression of phytohemagglutinin and lipopolysaccharide responses in mouse spleen cells by Bacillus Calmette-Guerin. J Reticuloendothel Soc. 1979 Oct;26(4):349–356. [PubMed] [Google Scholar]
- BRENER Z., PELLEGRINO J. Method for isolating schistosome granulomas from mouse liver. J Parasitol. 1956 Dec;42(6):564–564. [PubMed] [Google Scholar]
- Boros D. L., Warren K. S., Pelley R. P. The secretion of migration inhibitory factor by intact schistosome egg granulomas maintained in vitro. Nature. 1973 Nov 23;246(5430):224–226. doi: 10.1038/246224a0. [DOI] [PubMed] [Google Scholar]
- Carrick L., Jr, Boros D. L. The artificial granuloma 1: in vitro lymphokine production by pulmonary artificial hypersensitivity granulomas. Clin Immunol Immunopathol. 1980 Nov;17(3):415–426. doi: 10.1016/0090-1229(80)90113-0. [DOI] [PubMed] [Google Scholar]
- Colizzi V., Ferluga J., Garreau F., Malkovsky M., Asherson G. L. Suppressor cells induced by BCG release non-specific factors in vitro which inhibit DNA synthesis and interleukin-2 production. Immunology. 1984 Jan;51(1):65–71. [PMC free article] [PubMed] [Google Scholar]
- Corradin G., Etlinger H. M., Chiller J. M. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977 Sep;119(3):1048–1053. [PubMed] [Google Scholar]
- Falkoff R. J., Muraguchi A., Hong J. X., Butler J. L., Dinarello C. A., Fauci A. S. The effects of interleukin 1 on human B cell activation and proliferation. J Immunol. 1983 Aug;131(2):801–805. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Goding J. W. Use of staphylococcal protein A as an immunological reagent. J Immunol Methods. 1978;20:241–253. doi: 10.1016/0022-1759(78)90259-4. [DOI] [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Cellular mechanisms of genetically controlled host resistance to Mycobacterium bovis (BCG). J Immunol. 1983 Oct;131(4):1966–1972. [PubMed] [Google Scholar]
- Harrington J. T., Jr, Stastny P. Macrophage migration from an agarose droplet: development of a micromethod for assay of delayed hypersensitivity. J Immunol. 1973 Mar;110(3):752–759. [PubMed] [Google Scholar]
- Hoffenbach A., Lagrange P. H., Bach M. A. Deficit of interleukin 2 production associated with impaired T-cell proliferative responses in Mycobacterium lepraemurium infection. Infect Immun. 1983 Jan;39(1):109–116. doi: 10.1128/iai.39.1.109-116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunninghake G. W., Bedell G. N., Zavala D. C., Monick M., Brady M. Role of interleukin-2 release by lung T-cells in active pulmonary sarcoidosis. Am Rev Respir Dis. 1983 Oct;128(4):634–638. doi: 10.1164/arrd.1983.128.4.634. [DOI] [PubMed] [Google Scholar]
- James S. L., Colley D. G. Eosinophils and immune mechanisms: production of the lymphokine eosinophil stimulation promoter (ESP) in vitro by isolated intact granulomas. J Reticuloendothel Soc. 1975 Nov;18(5):283–293. [PubMed] [Google Scholar]
- Kantor F. S. Infection, anergy and cell-mediated immunity. N Engl J Med. 1975 Mar 20;292(12):629–634. doi: 10.1056/NEJM197503202921210. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Allred C., Cohen S., Yoshida T. Role of interleukin 1 in experimental pulmonary granuloma in mice. J Immunol. 1985 Jan;134(1):358–364. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masih N., Majeska J., Yoshida T. Studies on experimental pulmonary granulomas. I. Detection of lymphokines in granulomatous lesions. Am J Pathol. 1979 May;95(2):391–406. [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Oppenheim J. J. Bidirectional amplification of macrophage-lymphocyte interactions: enhanced lymphocyte activation factor production by activated adherent mouse peritoneal cells. J Immunol. 1977 Jan;118(1):77–82. [PubMed] [Google Scholar]
- Mitchell M. S., Kirkpatrick D., Mokyr M. B., Gery I. On the mode of action of BCG. Nat New Biol. 1973 Jun 13;243(128):216–218. doi: 10.1038/newbio243216a0. [DOI] [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Modlin R. L., Hofman F. M., Horwitz D. A., Husmann L. A., Gillis S., Taylor C. R., Rea T. H. In situ identification of cells in human leprosy granulomas with monoclonal antibodies to interleukin 2 and its receptor. J Immunol. 1984 Jun;132(6):3085–3090. [PubMed] [Google Scholar]
- Mokyr M. B., Mitchell M. S. Activation of lymphoid cells by BCG in vitro. Cell Immunol. 1975 Feb;15(2):264–273. doi: 10.1016/0008-8749(75)90005-2. [DOI] [PubMed] [Google Scholar]
- Neta R., Salvin S. B. In vivo release of lymphokines in different strains of mice. Cell Immunol. 1980 Apr;51(1):173–178. doi: 10.1016/0008-8749(80)90247-6. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Shneyour A., Kook A. I. Enhancement of DNA synthesis and cAMP content of mouse thymocytes by mediator(s) derived from adherent cells. J Immunol. 1976 May;116(5):1466–1472. [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Resistance of various strains of mycobacteria to killing by activated macrophages in vivo. J Immunol. 1983 Sep;131(3):1452–1454. [PubMed] [Google Scholar]
- Pelletier M., Forget A., Bourassa D., Gros P., Skamene E. Immunopathology of BCG infection in genetically resistant and susceptible mouse strains. J Immunol. 1982 Nov;129(5):2179–2185. [PubMed] [Google Scholar]
- Pinkston P., Bitterman P. B., Crystal R. G. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983 Apr 7;308(14):793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- Schrier D. J., Allen E. M., Moore V. L. BCG-induced macrophage suppression in mice: suppression of specific and nonspecific antibody-medicated and cellular immunologic responses. Cell Immunol. 1980 Dec;56(2):347–356. doi: 10.1016/0008-8749(80)90110-0. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Baker P. E., Gillis S., Ruscetti F. W. Functional and molecular characteristics of T-cell growth factor. Mol Immunol. 1980 May;17(5):579–589. doi: 10.1016/0161-5890(80)90156-x. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Ruscetti F. W. T-cell growth factor and the culture of cloned functional T cells. Adv Immunol. 1981;31:137–175. doi: 10.1016/s0065-2776(08)60920-7. [DOI] [PubMed] [Google Scholar]
- Stach J. L., Gros P., Forget A., Skamene E. Phenotypic expression of genetically-controlled natural resistance to Mycobacterium bovis (BCG). J Immunol. 1984 Feb;132(2):888–892. [PubMed] [Google Scholar]
- Unanue E. R. Cooperation between mononuclear phagocytes and lymphocytes in immunity. N Engl J Med. 1980 Oct 23;303(17):977–985. doi: 10.1056/NEJM198010233031706. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Watson J., Gillis S., Marbrook J., Mochizuki D., Smith K. A. Biochemical and biological characterization of lymphocyte regulatory molecules. I. Purification of a class of murine lymphokines. J Exp Med. 1979 Oct 1;150(4):849–861. doi: 10.1084/jem.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Karinuma M. Genetic control of granuloma response to oil-associated BCG cell wall vaccine in mice. Microbiol Immunol. 1978;22(6):335–348. doi: 10.1111/j.1348-0421.1978.tb00378.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Cohen S. Biological control of lymphokine function. Fed Proc. 1982 Jun;41(8):2480–2483. [PubMed] [Google Scholar]