Abstract
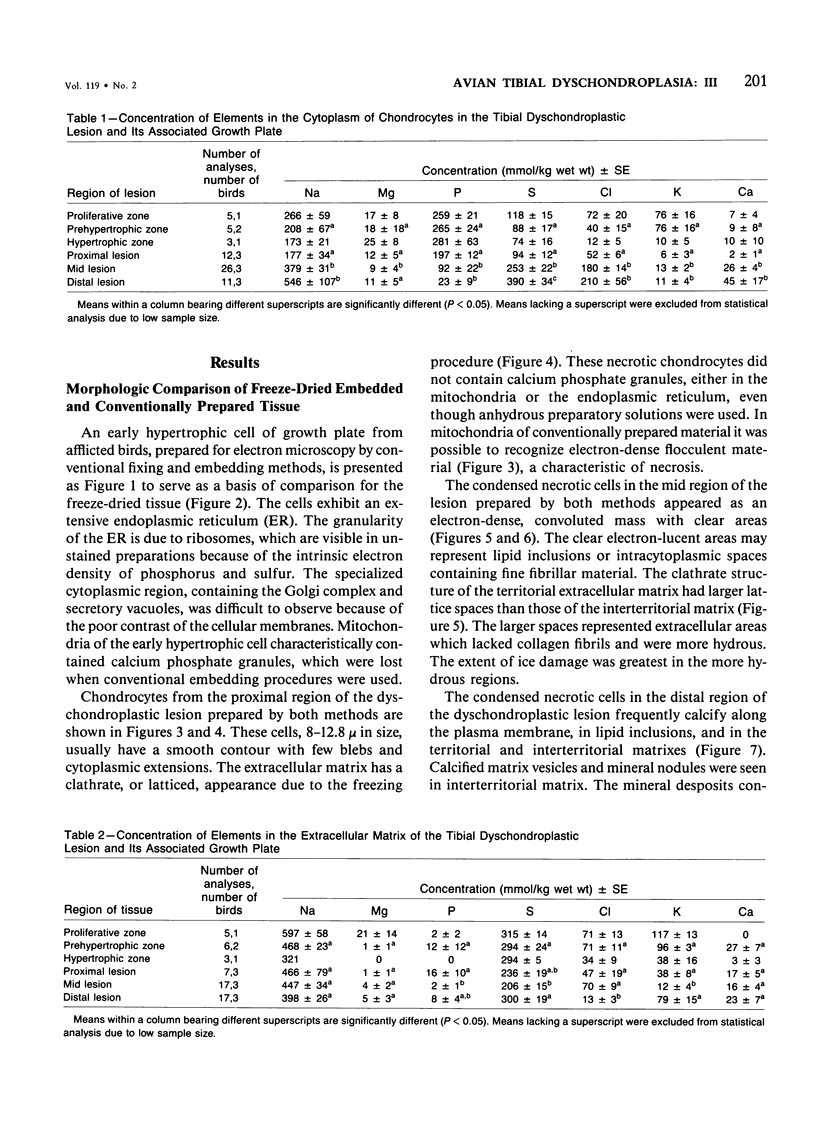
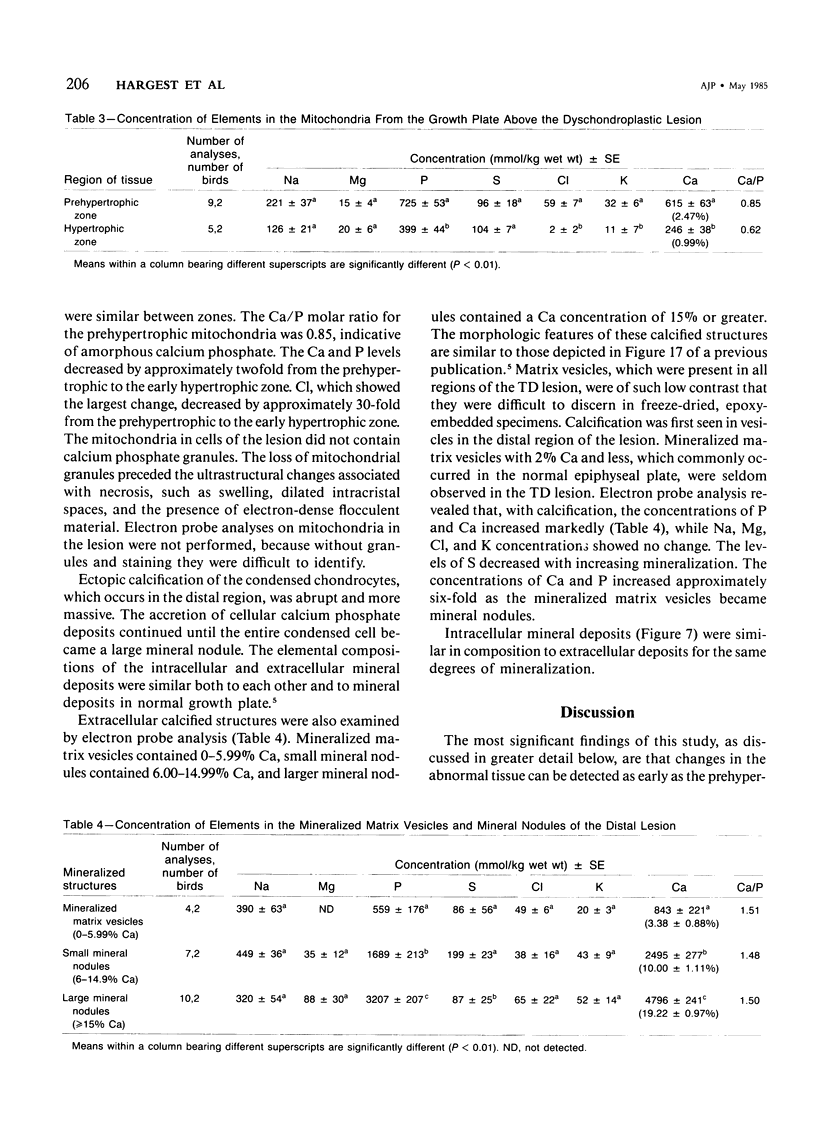
Tibial dyschondroplastic (TD) lesions and their associated growth plates, obtained from chickens, were prepared by freeze-drying and embedding in an anhydrous epoxy resin. Quantitative electron probe analysis was performed on dry, unstained sections. Levels of Na, Mg, P, S, Cl, K, and Ca were determined in cytoplasm (endoplasmic reticulum), mitochondria, and extracellular matrix of the proliferative, prehypertrophic, and early hypertrophic zones of the growth plate and in the proximal, mid, and distal regions of the lesion. A zone of calcification in the growth plate was absent. The concentration of elements in all regions of the TD growth plate was the same as found in an earlier study for normal growth plate. The cytoplasm of proximal lesion chondrocytes was similar to that of early hypertrophic chondrocytes. However, in the remainder of the lesion there was a progressive increase in cellular Na, S, Cl, and Ca and a progressive loss of P. In matrix, there was less S and K than expected in all regions of growth plate and lesion, except in the proliferating zone. Also, in matrix of the distal lesion there was less Na and Cl. The levels of Na, S, Cl, and K in matrix may have been lowered by their adsorption into the condensed masses of dead cells. Mitochondria acquire only half as much Ca and P as normal and release it earlier than usual (ie, early prehypertrophic cells, rather than chondrocytes of the lower hypertrophic zone). There were no granules in mitochondria of the cells at all levels of the lesion, even though anhydrous methods were used. The first sign of the disease appears in the matrix of the growth plate, where it seems that S and K are in abnormally low amounts. Although there are sufficient levels of Ca and P present, the matrix does not calcify. The cartilage remains avascular, and the cells appear to be dying. The event that triggers the chondrocytes of the growth plate to form an abnormal uncalcified matrix is not known.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Althoff J., Quint P., Krefting E. R., Höhling H. J. Morphological studies on the epiphyseal growth plate combined with biochemical and X-ray microprobe analyses. Histochemistry. 1982;74(4):541–552. doi: 10.1007/BF00496668. [DOI] [PubMed] [Google Scholar]
- Boyde A., Shapiro I. M. Energy dispersive X-ray elemental analysis of isolated epiphyseal growth plate chondrocyte fragments. Histochemistry. 1980;69(1):85–94. doi: 10.1007/BF00508369. [DOI] [PubMed] [Google Scholar]
- Cameron I. L., Smith N. K., Pool T. B., Sparks R. L. Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res. 1980 May;40(5):1493–1500. [PubMed] [Google Scholar]
- Davis W. L., Jones R. G., Knight J. P., Hagler H. K. Cartilage calcification: an ultrastructural, histochemical, and analytical x-ray microprobe study of the zone of calcification in the normal avian epiphyseal growth plate. J Histochem Cytochem. 1982 Mar;30(3):221–234. doi: 10.1177/30.3.7061824. [DOI] [PubMed] [Google Scholar]
- Dempster D. W., Elder H. Y., Nicholson W. A., Smith D. A. Microprobe analysis of intracellular mineral deposits in rachitic rat bone [proceedings]. J Physiol. 1979 Jun;291:61P–61P. [PubMed] [Google Scholar]
- Freedman B. D., Gay C. V., Leach R. M. Avian tibial dyschondroplasia. II. Biochemical changes. Am J Pathol. 1985 May;119(2):191–198. [PMC free article] [PubMed] [Google Scholar]
- Goracci G., Porcellati G., Woelk H. Subcellular localization and distribution of phospholipases A in liver and brain tissue. Adv Prostaglandin Thromboxane Res. 1978;3:55–67. [PubMed] [Google Scholar]
- Hargest T. E., Leach R. M., Gay C. V. Avian tibial dyschondroplasia. I. Ultrastructure. Am J Pathol. 1985 May;119(2):175–190. [PMC free article] [PubMed] [Google Scholar]
- Holtrop M. E. The ultrastructure of the epiphyseal plate. I. The flattened chondrocyte. Calcif Tissue Res. 1972;9(2):131–139. doi: 10.1007/BF02061951. [DOI] [PubMed] [Google Scholar]
- Lilburn M. S., Leach R. M., Jr Metabolism of abnormal cartilage cells associated with tibial dyschondroplasia. Poult Sci. 1980 Aug;59(8):1892–1896. doi: 10.3382/ps.0591892. [DOI] [PubMed] [Google Scholar]
- Lowther D. A., Robinson H. C., Dolman J. W., Thomas K. W. Cartilage matrix components in chickens with tibial dyschondroplasia. J Nutr. 1974 Jul;104(7):922–929. doi: 10.1093/jn/104.7.922. [DOI] [PubMed] [Google Scholar]
- Mitchell N., Shepard N., Harrod J. The measurement of proteoglycan in the mineralizing region of the rat growth plate. J Bone Joint Surg Am. 1982 Jan;64(1):32–38. [PubMed] [Google Scholar]
- NEMAN W. F., TORIBARA T. Y., MULRYAN B. J. Synthetic hydroxyapatite crystals. 1. Sodium and potassium fixation. Arch Biochem Biophys. 1962 Sep;98:384–390. doi: 10.1016/0003-9861(62)90202-3. [DOI] [PubMed] [Google Scholar]
- Ozawa H., Yamamoto T. An application of energy-dispersive X-ray microanalysis for the study of biological calcification. J Histochem Cytochem. 1983 Jan;31(1A):210–213. [PubMed] [Google Scholar]
- Poulos P. W., Jr Tibial dyschondroplasia (osteochondrosis) in the turkey. A morphologic investigation. Acta Radiol Suppl. 1978;358:197–227. [PubMed] [Google Scholar]
- Reddi A. H., Kuettner K. E. Vascular invasion of cartilage: correlation of morphology with lysozyme, glycosaminoglycans, protease, and protease-inhibitory activity during endochondral bone development. Dev Biol. 1981 Mar;82(2):217–223. doi: 10.1016/0012-1606(81)90447-4. [DOI] [PubMed] [Google Scholar]
- Shuman H., Somlyo A. V., Somlyo A. P. Quantitative electron probe microanalysis of biological thin sections: methods and validity. Ultramicroscopy. 1976 Sep-Oct;1(4):317–339. doi: 10.1016/0304-3991(76)90049-8. [DOI] [PubMed] [Google Scholar]
- Silberberg R. Ultrastructure of cartilage in chondrodystrophies. Birth Defects Orig Artic Ser. 1974;10(12):306–313. [PubMed] [Google Scholar]
- Termine J. D. Mineral chemistry and skeletal biology. Clin Orthop Relat Res. 1972;85:207–239. doi: 10.1097/00003086-197206000-00036. [DOI] [PubMed] [Google Scholar]
- Terracio L., Bankston P. W., McAteer J. A. Ultrastructural observations on tissues processed by a quick-freezing, rapid-drying method: comparison with conventional specimen preparation. Cryobiology. 1981 Feb;18(1):55–71. doi: 10.1016/0011-2240(81)90006-7. [DOI] [PubMed] [Google Scholar]
- Terracio L., Schwabe K. G. Freezing and drying of biological tissues for electron microscopy. J Histochem Cytochem. 1981 Sep;29(9):1021–1028. doi: 10.1177/29.9.7026665. [DOI] [PubMed] [Google Scholar]
- Vogel J. J., Boyan-Salyers B. D. Acidic lipids associated with the local mechanism of calcificaiton: a review. Clin Orthop Relat Res. 1976 Jul-Aug;(118):231–241. [PubMed] [Google Scholar]
- Weakley B. S., Weakley T. J., James J. L. Phosphorus standards for the electron probe X-ray microanalysis of ultrathin tissue sections. J Microsc. 1980 Apr;118(4):471–476. doi: 10.1111/j.1365-2818.1980.tb00297.x. [DOI] [PubMed] [Google Scholar]
- Wergedal J. E., Baylink D. J. Electron microprobe measurements of bone mineralization rate in vivo. Am J Physiol. 1974 Feb;226(2):345–352. doi: 10.1152/ajplegacy.1974.226.2.345. [DOI] [PubMed] [Google Scholar]