Abstract
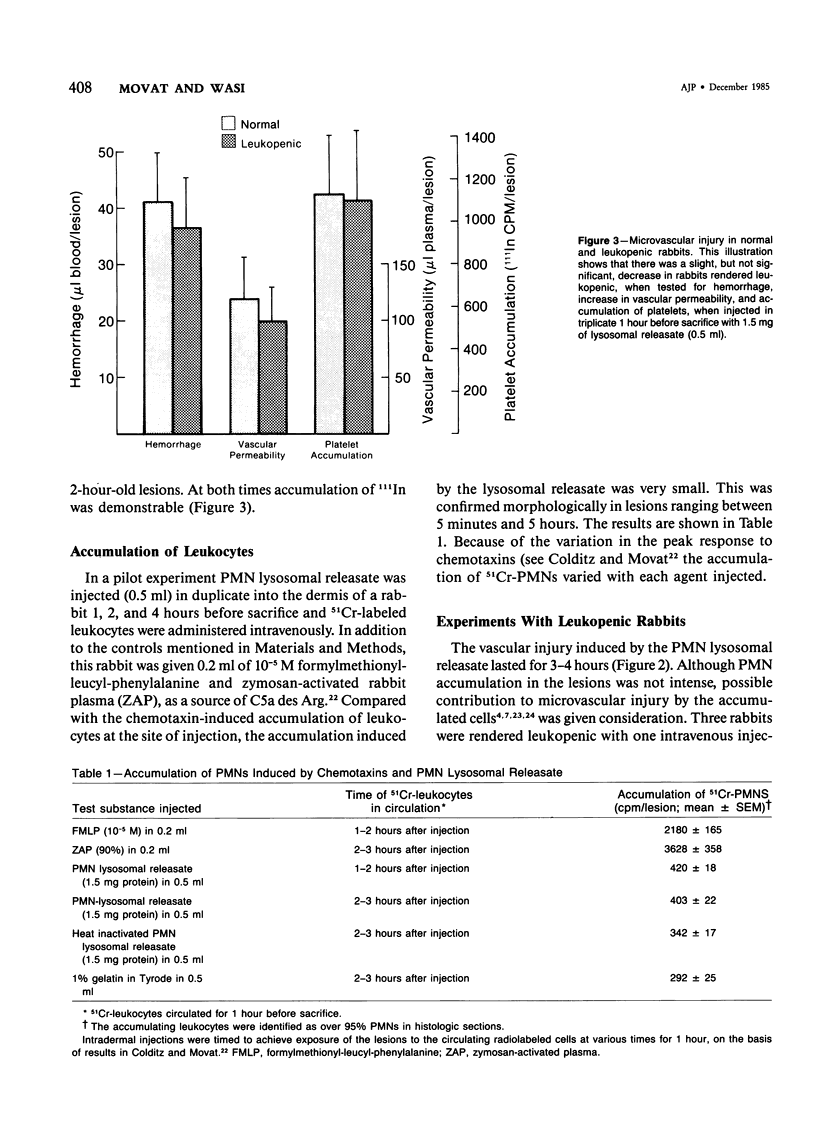
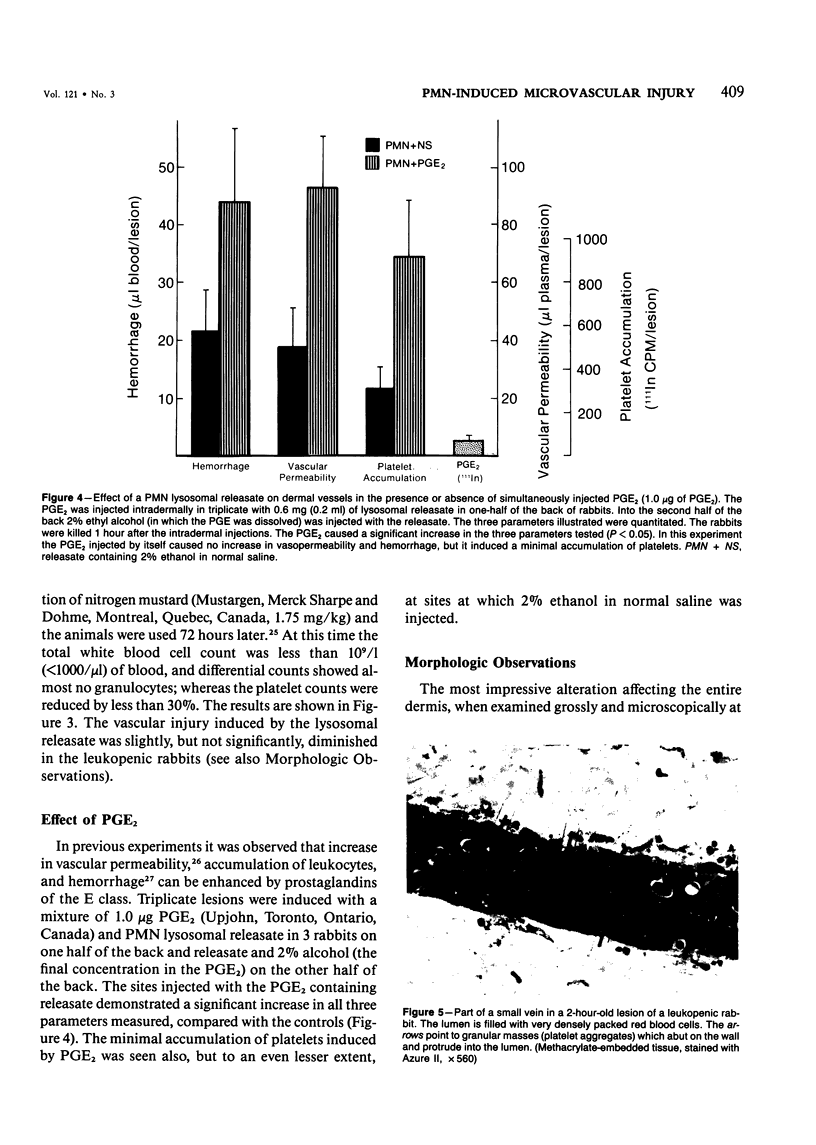
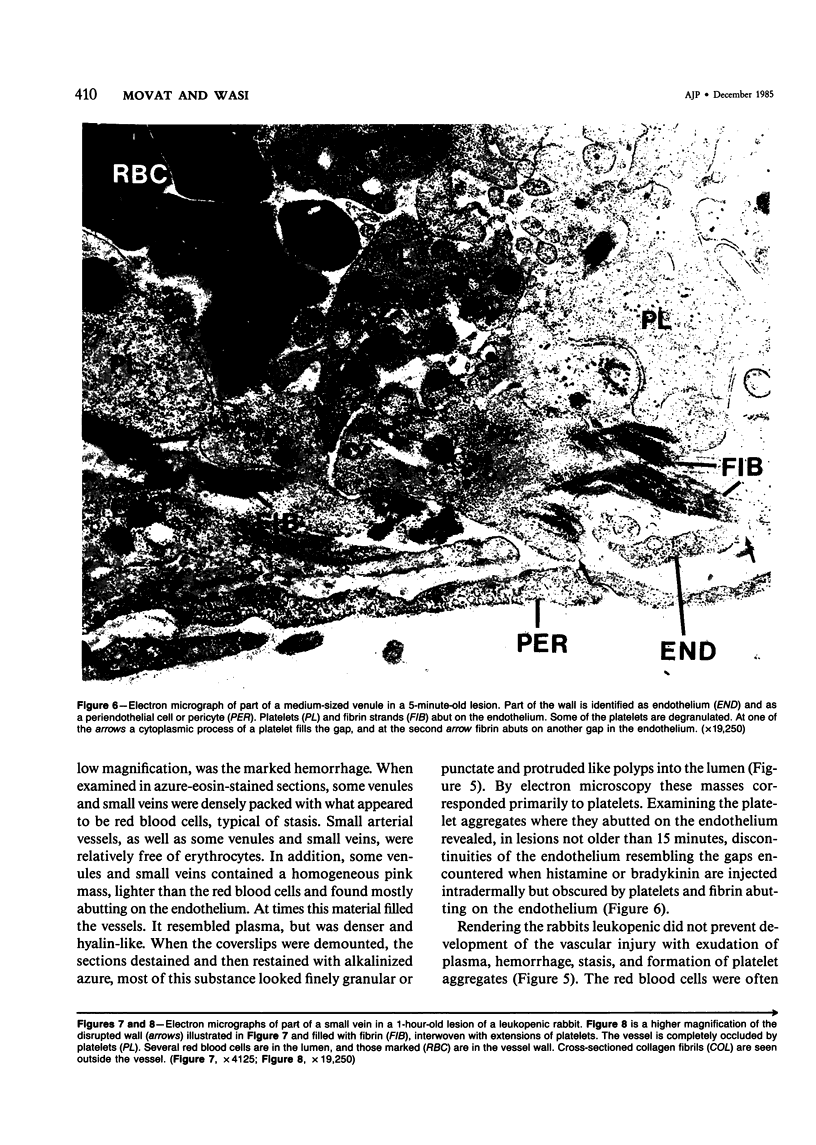
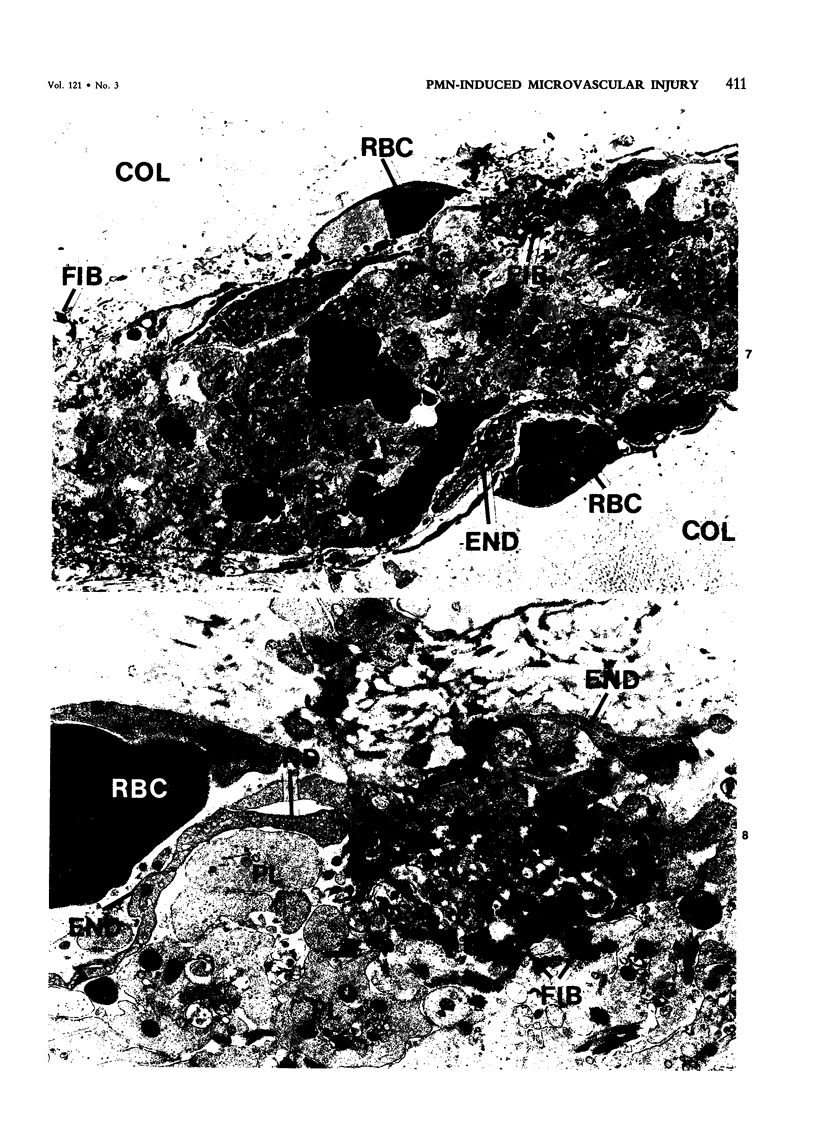
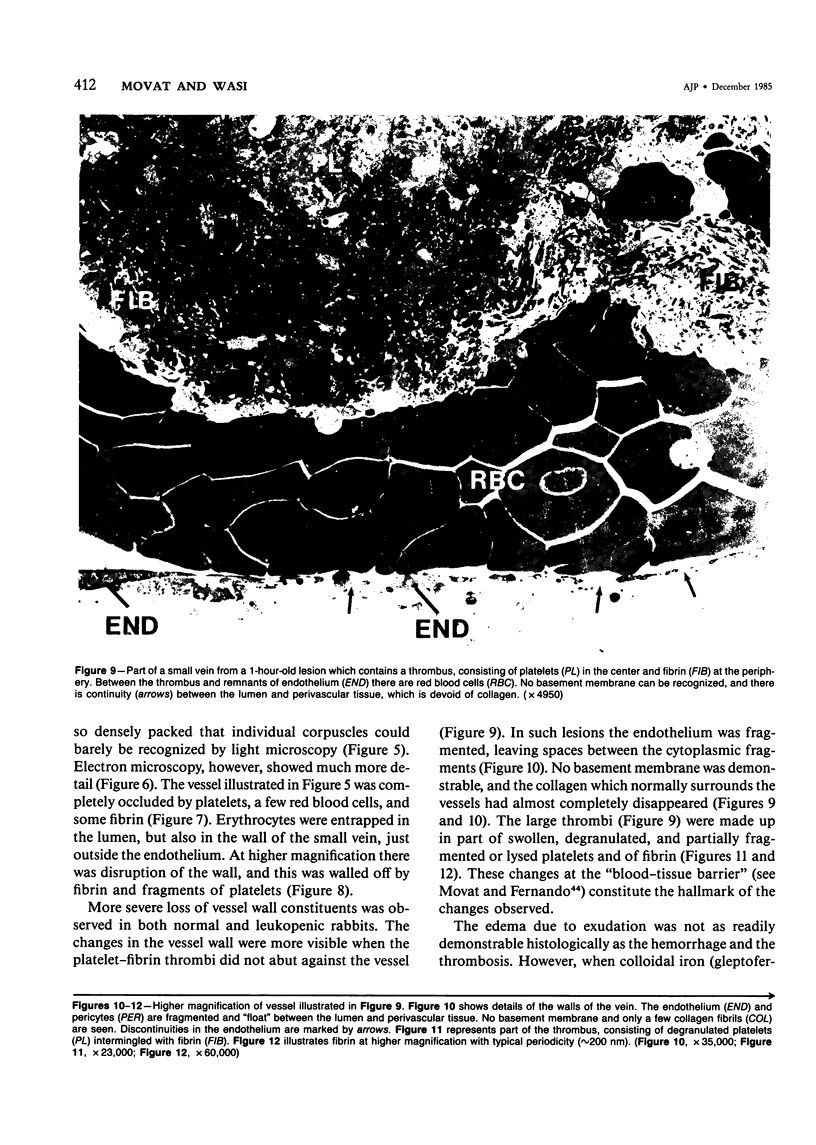
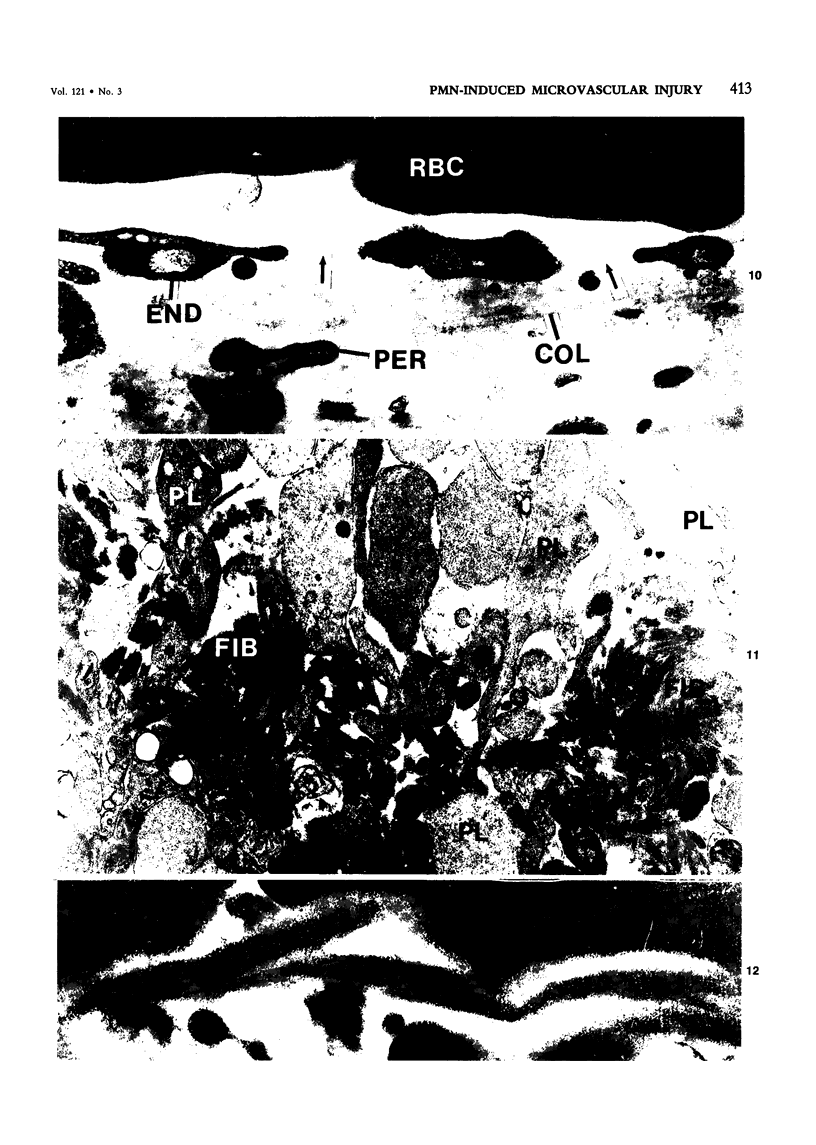
The purpose of this study was to assess the nature of the lesions in the microcirculation of the dermis of rabbits induced with lysosomal releasates of human polymorphonuclear leukocytes (PMNs). No attempt was made in the studies presented in this publication to deal with the offending agent in the releasate. Four parameters of microvascular injury were quantitated: increase in vascular permeability with 125I-labeled serum albumin, hemorrhage with 59Fe-labeled erythrocytes, accumulation (aggregation) of platelets with 111In-labeled platelets. In one experiment accumulation of 51Cr-PMNs was investigated. The lysosomal releasate induced a rapid increase in vasopermeability, but both hemorrhage and exudate formation peaked 1 hour after intradermal injection. Platelet accumulation was also demonstrable in these lesions, and microthrombosis was a very prominent feature. The microvascular injury, including microthrombosis, could be elicited also in animals rendered leukopenic with nitrogen mustard. Simultaneous injection of prostaglandin E2 with the releasate enhanced the microvascular injury. The morphologic changes in the microcirculation of the rabbit's dermis were assessed in lesions 5 minutes to 5 hours old. Several changes were encountered, primarily in the wall of venules and small veins and to a lesser degree in small arteries and capillaries. Ultrastructurally very early lesions (up to 15 minutes) had gaps or spaces in the endothelium, resembling those induced by mediators such as histamine or bradykinin. Older lesions were different, quite characteristic, and represent the hallmark of these lesions. Lysis and disappearance of vascular basement membrane, of perivascular collagen, and of the internal elastic lamina were a frequent finding, best demonstrable when microthrombi did not abut on vessel walls. Cellular components of vessels (endothelium, pericytes, smooth muscle) showed fragmentation, leading to complete disappearance of cellular elements. These lesions were usually walled off by platelet aggregates and fibrin. At times microthrombi occluded an entire vessel. These changes were interpreted as hemostasis. The mild accumulation of PMNs at the site of injury did not contribute significantly to the microvascular injury. The findings indicate that the unique changes in the microcirculation, not described before, may occur quite frequently, when the microvascular injury is elicited primarily by release of lysosomal constituents by phagocytic or nonphagocytic stimuli. One can conclude that the hallmark of this type of injury is disappearance of basement membrane followed secondarily by disintegration of the vascular wall, followed in turn by hemo
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colditz I. G., Movat H. Z. Kinetics of neutrophil accumulation in acute inflammatory lesions induced by chemotaxins and chemotaxinigens. J Immunol. 1984 Oct;133(4):2169–2173. [PubMed] [Google Scholar]
- Cotran R. S. Delayed and prolonged vascular leakage in inflammation. 3. Immediate and delayed vascular reactions in skeletal muscle. Exp Mol Pathol. 1967 Apr;6(2):143–155. doi: 10.1016/0014-4800(67)90052-4. [DOI] [PubMed] [Google Scholar]
- Cotran R. S., Remensnyder J. P. The structural basis of increased vascular permeabiligy after graded thermal injury--light and electron microscopic studies. Ann N Y Acad Sci. 1968 Aug 14;150(3):495–509. doi: 10.1111/j.1749-6632.1968.tb14702.x. [DOI] [PubMed] [Google Scholar]
- Crawford J. P., Movat H. Z., Minta J. O., Opas M. Acute inflammation induced by immune complexes in the microcirculation. Exp Mol Pathol. 1985 Apr;42(2):175–193. doi: 10.1016/0014-4800(85)90026-7. [DOI] [PubMed] [Google Scholar]
- Davies P., Fox R. I., Polyzonis M., Allison A. C., Haswell A. D. The inhibition of phagocytosis and facilitation of exocytosis in rabbit polymorphonuclear leukocytes by cytochalasin B. Lab Invest. 1973 Jan;28(1):16–22. [PubMed] [Google Scholar]
- Dewald B., Bretz U., Baggiolini M. Release of gelatinase from a novel secretory compartment of human neutrophils. J Clin Invest. 1982 Sep;70(3):518–525. doi: 10.1172/JCI110643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub E. S., Spitznagel J. K. The role of lysosomes in hypersensitivity reactions: tissue damage by polymorphonuclear neutrophil lysosomes. J Immunol. 1965 Dec;95(6):1060–1066. [PubMed] [Google Scholar]
- Hawkins D. Biopolymer membrane: a model system for the study of the neutrophilic leukocyte response to immune complexes. J Immunol. 1971 Aug;107(2):344–352. [PubMed] [Google Scholar]
- Henson P. M. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971 Dec;107(6):1535–1546. [PubMed] [Google Scholar]
- Issekutz A. C., Movat H. Z. The effect of vasodilator prostaglandins on polymorphonuclear leukocyte infiltration and vascular injury. Am J Pathol. 1982 Jun;107(3):300–309. [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Movat H. Z. The in vivo quantitation and kinetics of rabbit neutrophil leukocyte accumulation in the skin in response to chemotactic agents and Escherichia coli. Lab Invest. 1980 Mar;42(3):310–317. [PubMed] [Google Scholar]
- Issekutz A. C., Movat K. W., Movat H. Z. Enhanced vascular permeability and haemorrhage-inducing activity of rabbit C5ades arg: probable role of polymorphonuclear leucocyte lysosomes. Clin Exp Immunol. 1980 Sep;41(3):512–520. [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Ripley M., Jackson J. R. Role of neutrophils in the deposition of platelets during acute inflammation. Lab Invest. 1983 Dec;49(6):716–724. [PubMed] [Google Scholar]
- Janoff A. Mediators of tissue damage in leukocyte lysosomes. X. Further studies on human granulocyte elastase. Lab Invest. 1970 Mar;22(3):228–236. [PubMed] [Google Scholar]
- Janoff A., Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968 Nov 1;128(5):1137–1155. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Sloan B., Weinbaum G., Damiano V., Sandhaus R. A., Elias J., Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis. 1977 Mar;115(3):461–478. doi: 10.1164/arrd.1977.115.3.461. [DOI] [PubMed] [Google Scholar]
- Janoff A., Zeligs J. D. Vascular injury and lysis of basement membrane in vitro by neutral protease of human leukocytes. Science. 1968 Aug 16;161(3842):702–704. doi: 10.1126/science.161.3842.702. [DOI] [PubMed] [Google Scholar]
- Jeynes B. J., Issekutz A. C., Issekutz T. B., Movat H. Z. Quantitation of platelets in the microcirculation. Measurement of indium-111 in microthrombi induced in rabbits by inflammatory lesions and related phenomena. Proc Soc Exp Biol Med. 1980 Dec;165(3):445–452. doi: 10.3181/00379727-165-41002. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Acute immunologic pulmonary alveolitis. J Clin Invest. 1974 Aug;54(2):349–357. doi: 10.1172/JCI107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. G., Hay J. B., Movat H. Z. The modulation of enhanced vascular permeability by prostaglandins through alterations in blood flow (hyperemia). Agents Actions. 1976 Nov;6(6):705–711. doi: 10.1007/BF02026092. [DOI] [PubMed] [Google Scholar]
- Keiser H., Greenwald R. A., Feinstein G., Janoff A. Degradation of cartilage proteoglycan by human leukocyte granule neutral proteases--a model of joint injury. II. Degradation of isolated bovine nasal cartilage proteoglycan. J Clin Invest. 1976 Mar;57(3):625–632. doi: 10.1172/JCI108318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopaniak M. M., Issekutz A. C., Burrowes C. E., Movat H. Z. The quantitation of hemorrhage in the skin. Measurement of hemorrhage in the microcirculation in inflammatory lesions and related phenomena. Proc Soc Exp Biol Med. 1980 Jan;163(1):126–131. doi: 10.3181/00379727-163-40733. [DOI] [PubMed] [Google Scholar]
- Kopaniak M. M., Issekutz A. C., Movat H. Z. Kinetics of acute inflammation induced by E coli in rabbits. Quantitation of blood flow, enhanced vascular permeability, hemorrhage, and leukocyte accumulation. Am J Pathol. 1980 Feb;98(2):485–498. [PMC free article] [PubMed] [Google Scholar]
- Kopaniak M. M., Movat H. Z. Kinetics of acute inflammation induced by Escherichia coli in rabbits. II. The effect of hyperimmunization, complement depletion, and depletion of leukocytes. Am J Pathol. 1983 Jan;110(1):13–29. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Intracellular and extracellular degranulation of human polymorphonuclear azurophil and specific granules induced by immune complexes. Infect Immun. 1974 Dec;10(6):1241–1249. doi: 10.1128/iai.10.6.1241-1249.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOVAT H. Z., FERNANDO N. V. Allergic inflammation. I. The earliest fine structural changes at the blood-tissue barrier during antigen-antibody interaction. Am J Pathol. 1963 Jan;42:41–59. [PMC free article] [PubMed] [Google Scholar]
- MOVAT H. Z., URIUHARA T., MACMORINE D. L., BURKE J. S. A PERMEABILITY FACTOR RELEASED FROM LEUKOCYTES AFTER PHAGOCYTOSIS OF IMMUNE COMPLEXES AND ITS POSSIBLE ROLE IN THE ARTHUS REACTION. Life Sci. 1964 Sep;3:1025–1032. doi: 10.1016/0024-3205(64)90115-8. [DOI] [PubMed] [Google Scholar]
- Movat H. Z., Rettl C., Burrowes C. E., Johnston M. G. The in vivo effect of leukotriene B4 on polymorphonuclear leukocytes and the microcirculation. Comparison with activated complement (C5a des Arg) and enhancement by prostaglandin E2. Am J Pathol. 1984 May;115(2):233–244. [PMC free article] [PubMed] [Google Scholar]
- Movat H. Z., Steinberg S. G., Habal F. M., Ranadive N. S. Demonstration of a kinin-generating enzyme in the lysosomes of human polymorphonuclear leukocytes. Lab Invest. 1973 Dec;29(6):669–684. [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The extracellular release of granulocyte collagenase and elastase during phagocytosis and inflammatory processes. Scand J Haematol. 1977 Aug;19(2):145–152. doi: 10.1111/j.1600-0609.1977.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The neutral proteases of human granulocytes. Isolation and partial characterization of granulocyte elastases. Eur J Biochem. 1974 Mar 1;42(2):519–527. doi: 10.1111/j.1432-1033.1974.tb03367.x. [DOI] [PubMed] [Google Scholar]
- Seegers W., Janoff A. Mediators of inflammation in leukocyte lysosomes. VI. Partial purification and characterization of a mast cell-rupturing component. J Exp Med. 1966 Nov 1;124(5):833–849. doi: 10.1084/jem.124.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udaka K., Takeuchi Y., Movat H. Z. Simple method for quantitation of enhanced vascular permeability. Proc Soc Exp Biol Med. 1970 Apr;133(4):1384–1387. doi: 10.3181/00379727-133-34695. [DOI] [PubMed] [Google Scholar]
- Uriuhara T., Movat H. Z. The role of PMN-leukocyte lysosomes in tissue injury, inflammation and hypersensitivity. I. The vascular changes and the role of PMN-leukocytes in the reversed passive Arthus reaction. Exp Mol Pathol. 1966 Dec;5(6):539–558. doi: 10.1016/0014-4800(66)90045-1. [DOI] [PubMed] [Google Scholar]
- WARD P. A., COCHRANE C. G. BOUND COMPLEMENT AND IMMUNOLOGIC INJURY OF BLOOD VESSELS. J Exp Med. 1965 Feb 1;121:215–234. doi: 10.1084/jem.121.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasi S., Movat H. Z. Phlogistic substances in neutrophil leukocyte lyosomes: their possible role in vivo and their in vitro properties. Curr Top Pathol. 1979;68:213–237. doi: 10.1007/978-3-642-67311-5_8. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Regiani S. Neutrophils degrade subendothelial matrices in the presence of alpha-1-proteinase inhibitor. Cooperative use of lysosomal proteinases and oxygen metabolites. J Clin Invest. 1984 May;73(5):1297–1303. doi: 10.1172/JCI111332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. J., Peck M. J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977 Dec 8;270(5637):530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]
- Zurier R. B., Hoffstein S., Weissmann G. Cytochalasin B: effect on lysosomal enzyme release from human leukocytes. Proc Natl Acad Sci U S A. 1973 Mar;70(3):844–848. doi: 10.1073/pnas.70.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]